ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2009; 5(3):276-285. doi:10.7150/ijbs.5.276 This issue Cite
Research Paper
Correlated alterations in prostate basal cell layer and basement membrane
1. Department of Pathology, Chinese PLA General Hospital, Beijing, China
2. Mammalian Genetics Section, GDDB, NIDDK, National Institutes of Health, Bethesda, MD, USA
3. Armed Forces Institute of Pathology and American Registry of Pathology, Washington DC
Abstract
Our recent studies revealed that focal basal cell layer disruption (FBCLD) induced auto-immunoreactions represented a contributing factor for human prostate tumor progression and invasion. As the basement membrane surrounds and attaches to the basal cell layer, our current study assessed whether FBCLD would impact the physical integrity of the associated basement membrane. Paraffin sections from 25-human prostate tumors were subjected to double immunohistochemistry to simultaneously elucidate the basal cell layer and the basement membrane with corresponding biomarkers. The physical integrity of the basement membrane overlying FBCLD was examined to determine the extent of correlated alterations. Of a total of 89 FBCLD encountered, 76 (85 %) showed correlated alterations in the overlying basement membrane, which included distinct focal disruptions or fragmentations. In the remaining 13 (15%) FBCLD, the overlying basement membrane showed significant attenuation or reduction of the immunostaining intensity. The basement membrane in all or nearly all ducts or acini with p63 positive basal cells was substantially thicker and more uniform than that in ducts or acini without p63 positive basal cells, and also, a vast majority of the focal disruptions occurred near basal cells that lack p63 expression. These findings suggest that focal disruptions in the basal cell layer and alterations in the basement membrane are correlated events and that the physical and functional status of the basal cells could significantly impact the physical integrity of the overlying basement membrane. As the degradation of both the basal cell layer and the basement membrane is a pre-requisite for prostate tumor invasion or progression, ducts or acini with focally disrupted basal cell layer and basement membrane are likely at greater risk to develop invasive lesions. Thus, further elucidation of the specific molecules and mechanism associated with these events may lead to the development of a more effective alternative for repeat biopsy to monitor tumor progression and invasion.
Keywords: focal basal cell layer disruption, prostate tumors, basement membrane
Introduction
The normal prostate luminal cells, which are the histological origin of most prostate malignancies, are physically separated from the stroma by the basal cells and basement membrane (BM). Basal cells are joined by intercellular junctions and adhesion molecules, constituting a continuous sheet encircling luminal cells [1-2]. The BM is composed of type IV collagen, laminins, and other molecules, forming a continuous lining surrounding and attaching to the basal cell layer [3-4]. The epithelium is normally devoid of blood vessels and lymphatic ducts, and totally relies on the stroma for its metabolic and even survival needs. Due to these structural relationships, the disruption of both the basal cell layer and the BM is a pre-requisite for prostate tumor invasion.
It is a commonly held belief that human prostate tumor invasion is a multistage process, progressing sequentially from normal to hyperplasia, to prostatic intraepithelial neoplasia (PIN), and to invasive or metastatic stages [5-8]. Progression from PIN to invasion is believed to be triggered by cancer cells that increasingly produce proteolytic enzymes with tumor progression, which cause degradation of the BM [9-10]. These theories are consistent with results of studies in tissue cultures and animal models, whereas are hard to interpret the following critical facts: (1) Our previous studies revealed that some healthy men between 19 and 29 years old had a spectrum of proliferative lesions, including hyperplasia, PIN, and incipient adenocarcinoma [11-13], (2) Recent studies detected a DNA phenotype that is identical to that of invasive prostate cancer in some “healthy” men, and in morphologically normal prostate tissues adjacent to prostate cancer [14-17], (3) A vast majority of PIN express high levels of proteolytic enzymes, while only 10-30% of untreated PIN progress to invasive lesions during patients' lifetime [18-21]. Unfortunately, none of the current approaches could predict which PIN lesions will progress [22-25]. The only established approach to monitor PIN progression is repeat biopsy [22-25], which is costly and painful, and (4) Results from all at clinical trials of prostate cancer treatment or prevention with corresponding proteolytic enzyme inhibitors have been very disappointing [26-28].
Together, these facts argue that alternative pathways of prostate tumor progression and invasion may exist or even play more direct roles. Since over 90% of prostate cancer related mortality result from invasion-related illness, and the incidence of PIN could be up to 16.5%-25% prostate biopsies [24-28], there is an urgent need to uncover the intrinsic mechanism of tumor invasion. Promoted by the fact that the basal cell layer is the sole source of tumor suppressor p63 and maspin [29-32], and that degradation of basal cell layers is a pre-requisite for tumor invasion, our recent studies have attempted to identify early signs of basal cell degradation. Our initial study examined the physical integrity of basal cell layers in 50 patients with co-existing pre-invasive and invasive prostate tumors. Of 2,047 ducts and acini examined, 197 were found to harbor focal disruptions (the absence of basal cells resulting in a gap greater than the combined size of at least 3 basal cells) in their basal cell layers. The frequency of focal basal cell layer disruptions (FBCLD) varied from none in 22 cases to over 1/3 of the ducts or acini with FBCLD in 17 cases [33].
Compared to their non-disrupted counterparts, focally disrupted basal cell layers showed a significantly lower frequency of tumor suppressor expression and proliferation, but a significantly higher rate of degeneration and leukocyte infiltration [33]. In contrast, epithelial cells overlying focally disrupted basal cell layers had a significantly higher rate of proliferation and expression of tumor invasion related genes [33-34]. Based on these and other findings, we have proposed that prostate tumor invasion or progression is triggered by FBCLD induced auto-immunoreactions, which facilitate formation of more aggressive cell clones or monoclonal proliferation of tumor stem cells overlying focally disrupted basal cell layers. Our hypothesis and supporting data have been recently published in multiple peer-reviewed journals [33-36]. As the basement membrane surrounds and attaches to the basal cell layer, our current study attempted to assess whether FBCLD would impact the physical integrity of the associated basement membrane.
Materials and Methods
Formalin-fixed and paraffin embedded tissue blocks from 25-human prostate tumors with both pre-invasive and invasive components were selected from our previous studies [33-36]. Consecutive sections at 4 μm thickness were prepared and placed on positively charged slides. The first and last sections from each case were stained with hematoxylin and eosin (H&E) for morphological classification, based on our published criteria [11].
To identify focal basal cell layer disruptions (FBCLD), two sections from each case were subjected to double immunohistochemistry with basal cell phenotypic markers p63 (clone: 4A4; Cell Marque, Foster City, CA) at a 1:50 dilution and cytokeratin (CK) 34βE12 (clone: M0630; Dako, Carpinteria, CA) at a 1:50 dilution according to the manufacturers' protocols. Immunostained sections were examined independently by two investigators. A FBCLD was defined as the focal absence of basal cells resulting in a gap larger than the combined size of at least three basal cells in at least two immediate adjacent sections.
To identify the potential impact of FBCLD on the physical integrity of the associated basement membrane, two sections immediate adjacent to double immunostained ones that harbored FBCLD from each case were subjected to double immunohistochemistry to simultaneously elucidate the basal cell layer and the basement membrane using a previously published protocol. Briefly, deparaffinized sections were incubated in 1X antigen retrieval solution (Cat #: RV1000M; Biocare Medical, Concord, CA) overnight (?) at 70℃ in a regular oven. After incubation, the sections were washed in tap water and PBS (pH 7.4), each for 5-10 minutes, and then, incubated with antibodies to p63 (at a 1:50 dilution) and CK 34βE12 (at a 1:50 dilution) for 2-3 hours at room temperature. After the incubation, the sections were washed in three changes of PBS, each for 2-3 minutes, and then, incubated with the corresponding secondary antibody. The antigen and antibody complex was detected with an ABC detection kit and a DAB chromogen kit (Vector Laboratories, Burlingame, CA), according to the instructions provided by the manufacturer. After chromogen reaction, the sections were washed in tap water and PBS, each for 5-10 minutes. Then, the sections were incubated with proteinase K ready-to-use solution (Cat #: S3020; Dako, Carpinteria, CA) at room temperature for 3-5 minutes. After the proteinase K digestion, sections were incubated with mouse monoclonal antibodies to collagen IV (clone: CIV 22; Dako; Carpinteria, CA) at a 1:50 dilution or laminin (clone: VP-L551; Vector Laboratories, Burlingame, CA) at a 1:25 dilution at room temperature for 2-3 hours. After the incubation, the sections were washed in three changes of PBS, each for 2-3 minutes, and then, incubated with the corresponding secondary antibody. The antigen and antibody complex was detected with an ABC detection kit and an AP red-chromogen kit (Cat #: 00-2203; Zymad, South San Francisco, CA) according to the instructions provided by the manufacturers.
To assess the specificity of the immunostaining, three technical approaches were used. First, different negative controls were used, which included (1) the substitution of the primary antibody with normal serum, (2) the omission of the secondary antibody from the immunostaining sequence, (3) serial dilutions of the primary antibody, and (4) the inclusion of sections from normal lymph-nodes in the normal immunostaining process. Second, the same immunostaining protocol was used on the same cases, but substituting with different detection system and substrates. Third, the immunostaining procedure was repeated at least twice using the same protocol and under the same condition and immunostained sections were independently evaluated by at least two investigators.
Using p63 and CKβE12 double immunostained sections as references, the corresponding sites of FBCLD in sections double immunostained for both basal cell phenotypic and basement membrane markers were photographed, and large prints were made and examined, to determine whether alterations in the basal cell layer and the basement membrane are correlated events. Correlated alterations of the basal cell layer and basement membrane were defined as a simultaneous focal loss of both structures on the same sites.
Results
Distinct immunoreactivities to p63 or CKβE12 were exclusively seen in basal cells. Distinct immunoreactivities to collagen or laminin were preferentially seen in the basement membrane, but were also seen in the stroma and blood vessels, which contain abundant collagen and laminin as structural elements. All negative controls completely lacked distinct immunoreactivities to any of the markers used.
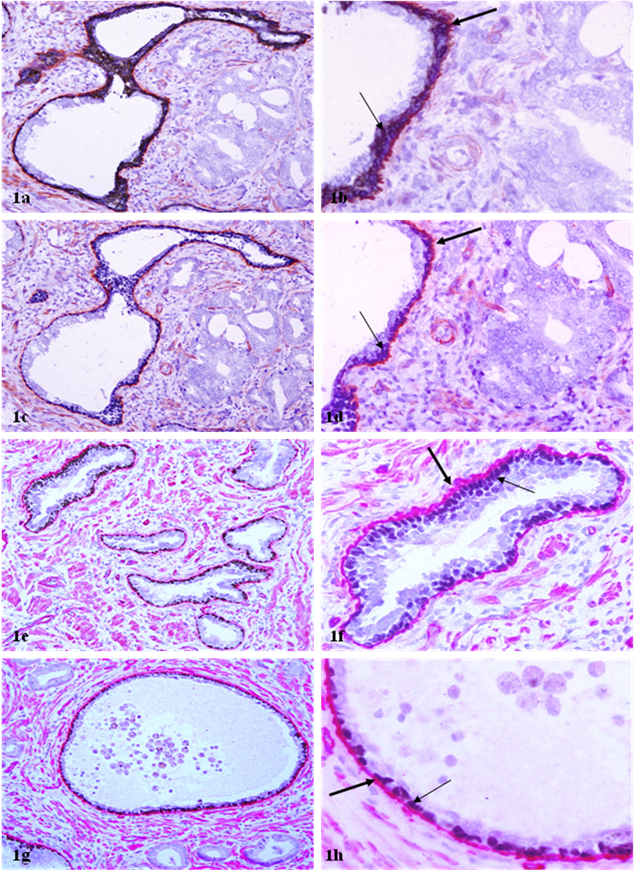
In sections double immunostained for basal cell phenotypic and basement membrane markers, both p63 and CKβE12 could elucidate the basal cell layer, while p63 appeared to be able to better differentiate between the basal cell layer from the basement membrane (Fig 1). A vast majority of the ducts and acini with normal morphology or with hyperplastic or PIN lesions contained a non-disrupted basal cell layer and a continuous basement membrane, whereas their adjacent invasive lesions laced both (Fig 1). The physical integrity of the basal cell layer and basement membrane appeared to be largely independent of the ductal or acinar lumen or tumor size (Fig 1e-1h).
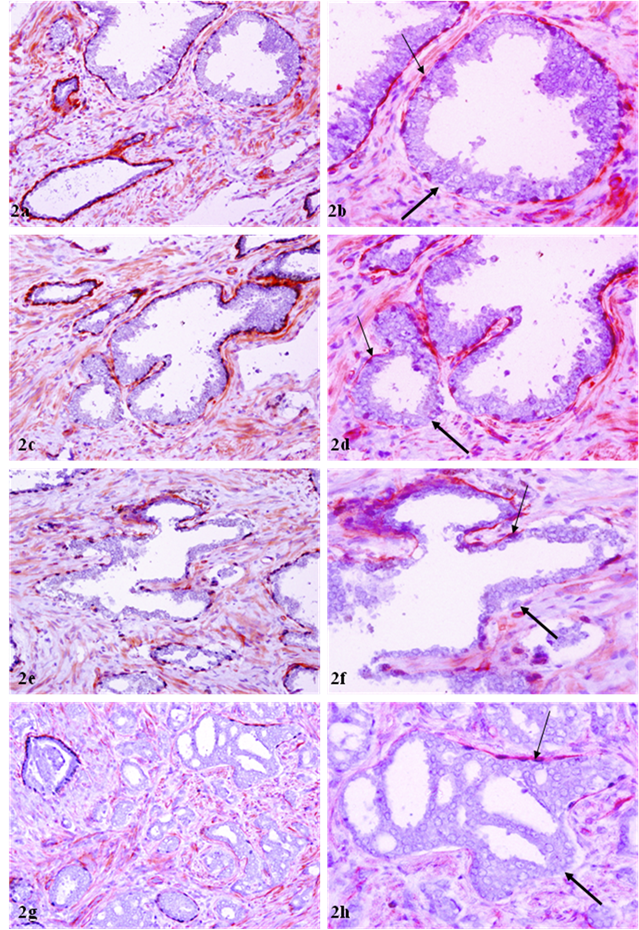
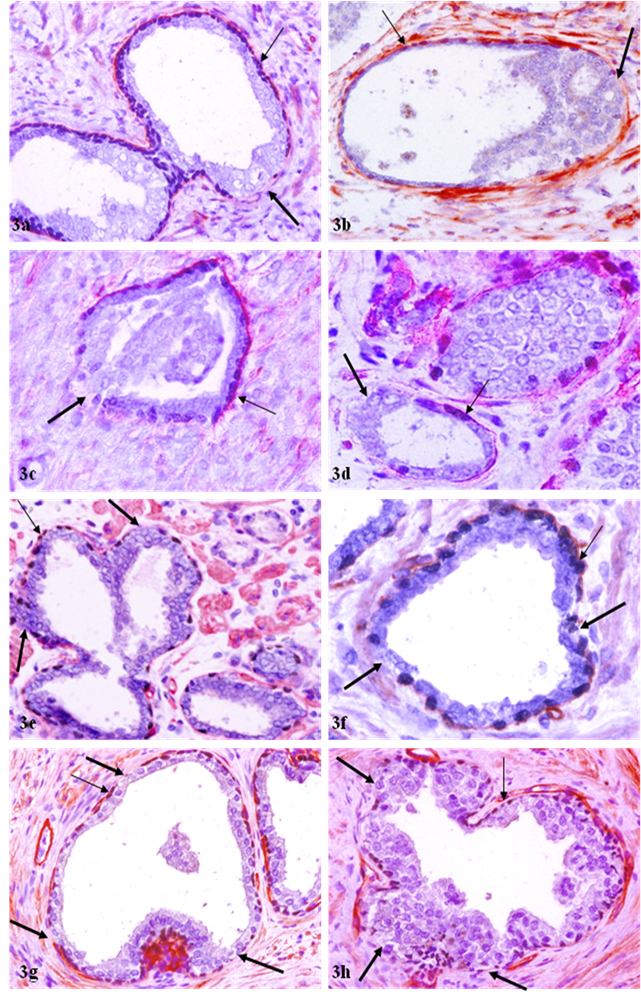
Of a total of 89 FBCLD encountered, 76 (85 %) showed focal disruption or fragmentations in the overlying basement membrane (Table 1), whereas none showed the integrity of basement membrane is impaired while the basal cell layer is normal. Over 60% of the focal disruptions in both the basal cell layer and the overlying basement membrane were seen in PIN (Fig 2), while about 30% of these focal disruptions were seen in ducts or acini with benign morphology (Fig 3). The size of these focal disruptions varied substantially, from a few cells (Fig 2a-2d) to more than a half of the entire basal cell layer and the basement membrane (Fig 2e-2h). The size of these focal disruptions in normal or hyperplastic lesions was generally small and varied in numbers (Fig 3).
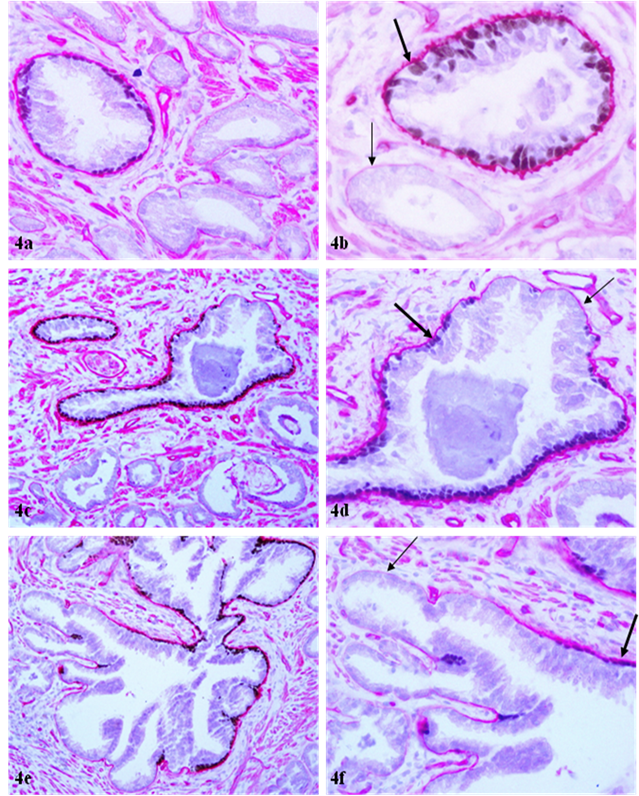
The basement membrane overlying the remaining 13 (15%) FBCLD showed significant attenuation or reduction of the immunostaining intensity, compared to its adjacent counterpart overlying the non-disrupted basal cell layer (Fig 4). The basement membrane in all or nearly all ducts or acini with p63 positive basal cells was substantially thicker and more uniform than that in ducts or acini without p63 positive basal cells (Fig 4a-4b), and also, a vast majority of the focal disruptions occurred near basal cells that lack p63 expression (not shown).
Correlated alterations in basal cell layer and overlying basement membrane
| Total FBCLD | With loss of BM | Without loss of BM | p |
|---|---|---|---|
| 89 | 76 (85%) | 13 (15%) | < 0.01 |
Basal cell layer and basement membrane in benign prostatic ducts and acini. Sections were double immunostained with basal cell phenotypic and basement membrane specific markers. Note that the luminal cells of most benign ducts or acini are surrounded by a non-disrupted basal cell layer (thin arrows) and a continuous basement membrane (thick arrows). a, c, e, and g: 100X; b, d, f, and h: a higher (400X) magnification of a, c, e, and g, respectively.
Correlated focal loss of basal cell layer and basement membrane in PIN. Sections were double immunostained with basal cell phenotypic and basement membrane specific markers. Thick arrows identify correlated focal disruption in the basal cell layer and the overlying basement membrane. Thin arrows identify the residual basal cell layer and basement membrane. Note that the size of focal disruptions varies from a few cells (a-d) to over a half of the entire basal cell layer and the basement membrane (e-h). a, c, e, and g: 100X; b, d, f, and h: a higher (400X) magnification of a, c, e, and g, respectively.
Correlated focal loss of basal cell layer and basement membrane in PIN. Sections were double immunostained with basal cell phenotypic and basement membrane specific markers. Thick arrows identify correlated focal disruption in the basal cell layer and the overlying basement membrane. Thin arrows identify the residual basal cell layer and basement membrane. Note that although most ducts or acini harbor only one small focal disruption (a-b), while some contain multiple focal disruptions (g-h). a, c, e, and g: 100X; b, d, f, and h: a higher (400X) magnification of a, c, e, and g, respectively.
Attenuation of basement membrane in areas lacking p63 expressing basal cells. Sections were double immunostained with basal cell phenotypic and basement membrane specific markers. Thin arrows identify the attenuated basement membrane in a small duct (b) and areas lacking p63 expressing cells. Thick arrows identify the basement membrane adjacent to p63 expressing cells. a, c, and e: 100X; b, d, and f: a higher (400X) magnification of a, c, and e, respectively.
Discussion
The pattern and frequency of FBCLD seen in our current study are in total agreement with those of our previous studies, and also those from other groups. Our findings of the loss or fragmentations of the basement membrane are also in line with previous reports. To our best knowledge, our finding of correlated alterations in the basal cell layer and the underlying basement membrane, and malignancy-associated morphologic alterations (focal disruptions in both the basal cell layer and basement membrane) in morphologically normal or hyperplastic duct or acinar clusters, however, have not been previously reported.
Since the epithelium is normally devoid of both blood vessels and lymphatic ducts, and the basal cell layer is the sole source of several tumor suppressors [29-32], a focal disruption in the basal cell layer and its underlying basement membrane could potentially have a number of consequences, including: (1) a loss or reduction of tumor suppressors and the paracrine inhibitory functions, which allow the luminal cells to undergo elevated proliferation [37-41], (2) alterations in the permeability for oxygen or growth factors, which selectively triggers the exit of stem or progenitor cells from quiescence, and favor proliferation of cells overlying FBCLD [42-44], (3) the exposure of luminal cells to different cytokines, which facilitates vasculogenic mimicry and tumor angiogenesis [45-46], (4) the physical contact between luminal and stromal cells, which augments the expression of stromal MMP and facilitates epithelial-mesenchymal transition and cell motility [47-49], and (5) the physical contact between luminal and immunoreactive cells, which directly causes genomic or cellular damages that trigger a cascade reaction of malignant transformation [50-55]. These alterations could individually or collectively trigger elevated proliferation in luminal cells near FBCLD, which leads to the enlargement of FBCLD and stretching-out of the residual basal cell layer and basement membrane. Eventually, the entire basal cell layer and basement membrane becomes dissociated or degenerated (as those shown in Fig 2e-2h), which facilitates invasion or progression of the overlying tumor cells. Thus, ducts or acini with focal disruptions in both the basal cell layer and the underlying basement membrane are very likely at greater risk to develop invasive prostate lesions. Consequently, the development of more practical and quantitative methods to assess the physical and functional integrity of the basal cell layer and basement membrane may lead to the development of a more effective alternative for repeat biopsy to monitor tumor progression and invasion. More importantly, our findings suggest that in addition to the multistage model, in which prostate carcinogenesis is believed to be sequentially progressing from normal, to hyperplasia, to high grade PIN, and to invasive lesions, prostate tumor invasion could potentially take place at any stage, if a focal disruption of the basal cell layer and the basement membrane happens to occur near a tumor progenitor [34-36].
The underling mechanism for the correlated alterations in the basal cell layer and the underlying basement membrane is unknown, but is likely to result from focal degeneration of aged or injured basal cells and resultant auto-immunoreactions. The basal cell belongs to a self-renewal population that has to consistently undergo proliferation and differentiation to replace aged or injured cells. A number of external or internal insults, such as radiation, carcinogens, localized trauma, inflammation, or other factors, could cause the inactivation of, or defects, in basal cell renewal-related genes, which impair the basal cell replenishment process to replace the aged or injured basal cells, resulting in a “senesced” basal cell population. These “senesced” basal cells may have significantly reduced functions to produce the major building blocks of the basement membrane or may have significantly reduced affinity in their surface to attract the deposition of collagen, laminin, and other building elements of the basement membrane. Consistent with this possibility is the fact that the basement membrane in all or nearly all ducts or acini with p63 positive basal cells was substantially thicker and more uniform than that in ducts or acini without p63 positive basal cells (Fig 4a-4b), and also, a vast majority of the focal disruptions occurred near basal cells that lack p63 expression.
In summary, our current study reveals for the first time that the basement membrane underlying all focally disrupted basal cell layers encountered showed either correlated focal disruptions (85%) or substantial attenuation (15%), suggesting that the functional or physical status of the basal cells significantly impact the physical integrity of the associated basement membrane. As the degradation of both the basal cell layer and the basement membrane is a pre-requisite for prostate tumor invasion or progression, ducts or acini with focally disrupted basal cell layer and basement membrane are likely at greater risk to develop invasive lesions. Thus, further elucidation of the specific molecules and mechanism associated with these events may lead to the development of a more effective alternative for repeat biopsy to monitor tumor progression and invasion.
Acknowledgements
This study was supported in part by grant 2006CB910505 from the Ministry of Chinese Science and Technology Department to Drs. Xichen Zhang and Yan-gao Man, and also by grants DAMD17-01-1-0129, DAMD17-01-1-0130, and PC051308 from the US Congressionally Directed Medical Research Programs, grant BCTR0706983 from The Susan G. Komen Breast Cancer Foundation, and grant 05AA from the AFIP/ARP joint research initiative project to Dr. Yan-gao Man.
The opinions and assertions contained herein represent the personal views of the authors and are not to be construed as official or as representing the views of the Department of the Army or the Department of Defense.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Carruba G, Stefano R, Cocciaeliferro L. Intercellular communication and human prostate carcinogenesis. Ann NY Acad Sci. 2002;963:156-68
2. Goldstein NS, Underhiel J, Roszka N, Neill JS. Cytokeratin 34 beta E-12 immunoreactivity in benign prostate acini. Quantitation, pattern assessment, and electron microscopic study. Am J Clin Pathol. 1999;112:69-74
3. Bonkhoff H, Wernert N, Dhom G, Remberger K. Basement membranes in fetal, adult normal, hyperplastic and neoplastic human prostate. Virchows Arch A Pathol Anat Histopathol. 1991;418:375-81
4. Kosir MA, Wang W, Zukowski KL, Tromp G. Degradation of basement membrane by prostate tumor heparanase. J Surg Res. 1999;81:42-7
5. Bonkhoff H, Remberger K. Morphogenesis of benign prostatic hyperplasia and prostatic carcinoma. Pathology. 1998;19:12-20
6. Bostwick DG. Prospective origins of prostate carcinoma. Prostate intraepithelial neoplasia and atypical adenomatous hyperplasia. Cancer. 1996;78:330-6
7. Haggman MJ, Macoska JA, Wojno KJ, Oesterling JE. The relationship between prostate intraepithelial neoplasia and prostate cancer: critical issues. J Urol. 1997;58:12-22
8. Bonkhoff H, Remberger K. Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model. Prostate. 1996;28:98-106
9. Barsky SH, Siegal GP, Jannotta F, Liotta LA. Loss of basement membrane components by invasive tumors but not by the benign counterparts. Lab Invest. 1983;49:140-7
10. Goldfarb RH, Liotta LA. Proteolytic enzymes in cancer invasion and metastasis. Semin Thromb Hemost. 1986;12:294-307
11. Gardner WA, Culberson DE. Atrophy and proliferation in the young adult Prostate. J Urol. 1987;137(1):53-6
12. Gardner WA. Hypothesis: Pediatric Origins of Prostate Cancer. Hum Path. 1995;26:1291-1292
13. Bennett BD, Gardner WA. Embryonal hyperplasia of the prostate. Prostate. 1985;3(7):411-7
14. Malins DC, Polissar NL, Su Y, Gardner HS, Gunselman SJ. A new structural analysis of DNA using statistical models of infrared sepctra. Nat Med. 1997;3:927-30
15. Malins DC, Johnson PM, Barker EA. et al. Cancer-related changes in prostate DNA as men age and early identification of metastasis in prostate tumors. Proc Natl Acad Sci USA. 2003;100:5401-6
16. Malins DC, Anderson KM, Gilman NK. et al. Development of a cancer DNA phenotype prior to tumor formation. Proc Natl Acad Sci Sci USA. 2004;101:10721-5
17. Malins DC, Gilman NK, Green VM. et al. A DNA phenotype in healthy prostates, conserved in tumors and adjacent normal cells, implies a relationship to carcinogenesis. Proc Natl Acad Sci USA. 2005;102:19093-6
18. Ashida S, Nakagawa H, Katagiri T, Furihata M, Liizumi M, Anazawa Y. et al. Molecular features of the transition from prostate intraepithelial neoplasia (PIN) to prostate cancer: genome-wide gene-expression profiles of prostate cancers and PINs. Cancer Res. 2004;64:5963-72
19. Dawkins HJ, Sellner LN, Turbett GR, Thompson CA, Redmond SL, MeNeal JE, Cohen RJ. Distinction between intraductal carcinoma of the prostate (IDC-P), high-grade dysplasia (PIN), and invasive prostatic adecarcinoma, using molecular markers of cancer progression. Prostate. 2000;44:265-70
20. Harvei S, Skijorten FJ, Robsahm TE, Berner A, Tretli S. Is prostaticibtraepithelial neoplasi in the transitio/central zone a true presursor of cancer? A long-tern retrospectivestudy in Norway. Br J Cancer. 1998;78:46-9
21. Goeman L, Joniau S, Ponette D, Van der Aa F, Roskams T, Oyen R, Van Poppel H. Is low-grade prostatic intraepithelial neoplasia a risk factor for cancer. Prostate Cancer Prostatic Dis. 2003;6:305-10
22. Mostofi FK, Sesterhenn IA, Davis CJJr. Prostatic intraepithelial neoplasia (PIN): morphological clinical significance. Prostate Suppl. 1992;4:71-7
23. Kasahara Y, Tsukada Y. New insights and future advances in cancer diagnostics: Limitations of conventional tumor markers. In: (ed.) Nakarnura RM, Grody WW, Wu JT, Nagle RB. Cancer Diagnostics: Current and future trends. Totowa, NJ: Humanna Press. 2004
24. Joniau S, Goeman L, Pennings J, Van Poppel H. Prostatic intraepithelial neoplasia (PIN): importance and clinical managment. Eur Urol. 2005;48:379-85
25. Haggman MJ, Adolfsson J, Khoury S, Montie JE, Norlen J. Clinical managment of premalignant lesions of the prostate. WHO Collaborative Project and Consensus Conference on Public Health and Clinical Significance of Premalignant Alterations in the Genitourinary Tract. Scand J Urol Nephol Suppl. 2000;205:44-9
26. Parker SL, Tong T, Bolders S, Wingo PA. Cancer statistics. Cancer J Clin. 1997;47:5-27
27. Bostwick DG. Prostatic intraepithelial neoplasia is a risk factor for cancer. Semin Urol Oncol. 1999;17:187-9
28. Bostwick DG, Qian J, Frankel K. The incidence of high grade prostatic intraepithelial neoplasia in needle biopsies. J Urol. 1985;154:1791-4
29. Signoretti S, Waltregny D, Dilks J. et al. p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol. 2000;157:1769-75
30. Kurita T, Medina RT, Mills AA, Cunha GR. Role of p63 and basal cells in prostate. Development. 2004;131:4955-64
31. Zou Z, Zhang W, Young D. et al. Maspin expression profile in human prostate cancer (caP) and in vitro induction of maspin expression by androgen ablation. Clin Cancer Res. 2002;8(5):1172-7
32. Cher ML, Biliran HR jr, Bhangat S. et al. Maspin expression inhibits osteolysis, tumor growth, and angiogenesis in animal model of prostate cancer bone metastasis. Proc Natl Acad Sci USA. 2003;100(13):7847-52
33. Man YG, Shen T, Zhao YG, Sang QXA. Focal prostate basal cell layer disruptions and leukocyte infiltration are correlated events: A potential mechanism for basal cell layer degradations and tumor invasion. Cancer Detect Prev. 2005;29:161-9
34. Man YG, Zhao CQ, Wang J, XL Chen. A subset of prostate basal cells lacks corresponding phenotypic markers. Pathology-Research & Practice. 2006;202(9):651-62
35. Man YG, Gardner WA. Focal degeneration of basal cells and the resultant auto-immunoreactions: a novel mechanism for prostate tumor progression and invasion. Med Hypoth. 2008;70:387-408
36. Man YG, Gardner WA. Bad seeds produce bad crops: a single step-process of prostate carcinogenesis and Progression. Int J Biol Sci. 2008;4:246-258
37. Verona EV, Elkahloum AG, Yang J, Bandyopadhyay A, Yeh IT, Sun LZ. Transforming growth factor-beta signaling in prostate stromal cells supports prostate carcinoma growth by up-regulating stromal genes related to tissue remodeling. Cancer Res. 2007;67(12):5737-46
38. Zhou W, Grandis JR, Wells A. STAT3 is required but not sufficient for EGF receptor-mediated migration and invasion of human prostate carcinoma cell lines. Br J Cancer. 2006;95(2):164-71
39. Chung IW, Baseman A, Assikis V, Zhau HE. Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol. 2005;173(1):10-20
40. Boulikas T. Control of DNA replication by protein phosphorylation. Anticancer Res. 1994;14:2465-72
41. Boulikas T. Phosphorylation of transcription factors and control of the cell cycle. Crit Rev Eukaryot Gene Expr. 1995;5:1-77
42. Chakravarthy MV, Spangenhurg EE, Booth FW. Culture in low levels of oxygen enhances in vitro proliferation potential of satellite cells from old skeletal muscles. Cell Mol Life Sci. 2001;58:1150-8
43. Csete M, Walikonis J, Slawny N. et al. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogrnesis in culture. J Cell Physical. 2001;189:189-96
44. Studer L, Csete M, Lee SH. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowed pxygen. J Neurosci. 2000;20:7377-83
45. Klos KS, Wyszomierski SL, Sun M. et al. c-erbB2 increases vascular endothelial growth factor protein synthesis via activation of mammalian target of rapamycin/p70S6K leading to increased angiogenesis and spontaneous metastasis of human breast cancer cells. Cancer Res. 2006;66(4):2028-37
46. Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumor-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3(6):411-21
47. Kang Y, Massague J. Epithelial-mesenchymal transition: twist in development and metastasis. Cell. 2004;118:277-9
48. Sato T, Sakai T, Noguchi Y, Takita M, Hirakawa S, Ito A. Tumor-stromal cell contact promotes invasion of human uterine cervical carcinoma cells by augmenting the expression and activation of stromal matrix metalloproteinases. Gynecol Oncol. 2004;92:47-56
49. Strizzi L, Bianco C, Normanno N. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV-Criptol-1 transgenic mice. J Cell Physiol. 2004;201:266-76
50. Gardner WA. "Pathology Paradigms". In: (ed.) Coffey D, Bruchovsky N, Gardner W, Resnick M, Karr J. Current Concepts and Approaches to the Study of Prostate Cancer. NY: A R Liss. 1987
51. Smith CJ, Gardner WAJr. Inflammation - Proliferation: Possible Relationships in the Prostate. In: (ed.) Coffey D, Bruchovsky N, Gardner W, Resnick M, Karr J. Current Concepts and Approaches to the Study of Prostate Cancer. NY: A R Liss. 1987
52. Nelson G, Culberson DE, Gardner WA. Intraprostatic Spermatozoa. Hum Path. 1988;19(1):119-20
53. Gardner WA, Bennett BD. The Prostate - Overview: Recent Insights and Speculations. WA. Gardner and RS Weinstein (Eds.) Pathology and Pathobiology of Urinary Bladder and Prostate. Williams and Wilkins, Baltimore, MD. 1992
54. Peek RM Jr, Mohla S, DuBois RN. Inflammation in the genesis and perpetuation of cancer: summary and recommendations from a national cancer institute-sponsored meeting. Cancer Res. 2005;65:8583-6
55. MacLennan GT, Eisenberg R, Fleshman RL. et al. The influence of chronic inflammation in prostatic carcinogenesis: a 5-year follow-up study. J Urol. 2006;176:1012-6
Author contact
![]() Correspondence to: Yan-gao Man, MD., PhD, Director of Gynecologic and Breast Research Laboratory, Armed Forces Institute of Pathology and American Registry of Pathology, Washington DC 20306-6000. Telephone: 202-782-1612; Fax: 202-782-3939; E-mail:manosd.mil
Correspondence to: Yan-gao Man, MD., PhD, Director of Gynecologic and Breast Research Laboratory, Armed Forces Institute of Pathology and American Registry of Pathology, Washington DC 20306-6000. Telephone: 202-782-1612; Fax: 202-782-3939; E-mail:manosd.mil
Received 2009-2-27
Accepted 2009-3-26
Published 2009-3-29
