ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2015; 11(6):633-642. doi:10.7150/ijbs.11127 This issue Cite
Research Paper
The Xanthine Derivative KMUP-1 Attenuates Serotonin-Induced Vasoconstriction and K+-Channel Inhibitory Activity via the PKC Pathway in Pulmonary Arteries
1. Department of Pediatrics, Division of Pediatric Pulmonology and Cardiology, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
2. Department of Pharmacology, Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
Abstract
Serotonin (5-hydroxytryptamine, 5-HT) is a potent pulmonary vasoconstrictor that promotes pulmonary artery smooth muscle cell (PASMC) proliferation. 5-HT-induced K+ channel inhibition increases [Ca2+]i in PASMCs, which is a major trigger for pulmonary vasoconstriction and development of pulmonary arterial hypertension (PAH). This study investigated whether KMUP-1 reduces pulmonary vasoconstriction in isolated pulmonary arteries (PAs) and attenuates 5-HT-inhibited K+ channel activities in PASMCs. In endothelium-denuded PA rings, KMUP-1 (1 μM) dose-dependently reduced 5-HT (100 μM) mediated contractile responses. Responses to KMUP-1 were reversed by K+ channel inhibitors (TEA, 10 mM, 4-aminopyridine, 5 mM, and paxilline, 10 μM). In primary PASMCs, KMUP-1 also dose-dependently restored 5-HT-inhibited voltage-gated K+-channel (Kv1.5 and Kv2.1) and large-conductance Ca2+-activated K+-channel (BKCa) proteins, as confirmed by immunofluorescent staining. Furthermore, 5-HT (10 μM)-inhibited Kv1.5 protein was unaffected by the PKA inhibitor KT5720 (1 μM) and the PKC activator PMA (1 μM), but these effects were reversed by KMUP-1 (1 μM), 8-Br-cAMP (100 μM), chelerythrine (1 μM), and KMUP-1 combined with a PKA/PKC activator or inhibitor. Notably, KMUP-1 reversed 5-HT-inhibited Kv1.5 protein and this response was significantly attenuated by co-incubation with the PKC activator PMA, suggesting that 5-HT-mediated PKC signaling can be modulated by KMUP-1. In conclusion, KMUP-1 ameliorates 5-HT-induced vasoconstriction and K+-channel inhibition through the PKC pathway, which could be valuable to prevent the development of PAH.
Keywords: Serotonin, pulmonary vasoconstriction, K+-channel inhibition, Kv1.5 protein, PKC signaling, pulmonary artery smooth muscle cells
Introduction
Pulmonary arterial hypertension (PAH) is characterized by increased pulmonary vascular tone, adventitial and medial hypertrophy, neointimal hyperplasia and fibrosis [1]. Pulmonary vasoconstriction is considered to be an early component of the pulmonary hypertensive process. Its pathogenesis is suggested by the findings of increased production of thromboxane A2 (TXA2), reduction of lung endothelial nitric oxide synthase (eNOS) and increased RhoA/Rho kinase (ROCK) in the pulmonary artery (PA) [2]. A xanthine derivative KMUP-1 (7-[2-[4-(2-chlorobenzene) piperazinyl]ethyl]-1,3-dimethylxanthine) has been established to increase NO/cGMP levels, inhibit phosphodiesterases (PDEs) and stimulate K+ channels resulting in relaxations of aortic smooth muscles [3]. In recent years, KMUP-1 was demonstrated to inhibit TXA2-mimetic-induced acute or monocrotaline (MCT) induced chronic PAH [4-7] in 2 animal models useful for studying the development of PAH. We reported that KMUP-1 attenuates MCT-induced PAH attributed to the alternation of Ca2+ sensitivity and K+-channel function [5]. Similarly, KMUP-1 prevented MCT-induced PAH and endothelin-1 release [4]. KMUP-1 also inhibited MCT-induced PA proliferation by binding to serotonin (5-hydroxytryptamine, 5-HT) receptors, increasing eNOS expression and NO release, and inhibiting RhoA/ROCK expression and AKT/ERK phosphorylation [6]. However, the biochemical mechanisms by which KMUP-1 alleviates 5-HT-induced vasoconstriction and K+ channel-mediated hyperpolarization remain undetermined.
The 5-HT system in PAH has attracted a lot of interest. Serotonin has vasocontractile and mitogenic properties. 5-HT is a potent pulmonary vasoconstrictor whose high circulating concentration is clinically associated with PAH. In animal models of PAH, 5-HT-induced hyperreactivity and mitogenic effects have been reported in PAs [8, 9]. 5-HT also acts as a mitogen in pulmonary artery smooth muscle cells (PASMCs) and is important in PA remodeling. The mitogenic effect of 5-HT is initiated by binding to one or more 5-HT receptors, including 5-HT1B, 5-HT2A and 5-HT2B, and by activating 5-HT transporter (5-HTT) internalized into the cell [10, 11]. It is well known that 5-HT evokes PA remodeling in the form of neointimal thickening of PA, leading to PA obstruction and PAH. Plasma levels of 5-HT are elevated in hypoxia-induced PAH. A correlation between high plasma 5-HT levels and pulmonary resistance has been reported [12].
Serotonin binding to 5-HT receptors modulates a number of ion channels via several biochemical pathways in PASMCs. The effects of serotonin on PASMC ion channels are not well understood. A previous report showed that 5-HT has both a fast direct effect on voltage-gated K+ (Kv) channels as well as a slower indirect effect via internalization of membrane proteins on ionic currents [13]. In addition to the effects of 5-HT receptors on ion channels, they also activate PKC, which phosphorylates tyrosine kinases, which in turn phosphorylate cell membrane-associated caveolins. Caveolins facilitate internalization of membrane proteins such as Kv channels and the transient receptor potential canonical (TRPC) family of ion channels [14]. This interesting mechanism seems to play an important role in the 5-HT-induced changes in K+ currents in PASMCs described by Cogolludo et al. (2006).
K+ channels play a central role in regulating resting membrane potential, intracellular calcium concentration, and contraction of vascular smooth muscles [15, 16]. Kv channels present in PASMCs are inhibited by hypoxia, endothelin-1, thromboxane A2, and anorectic drugs [13, 15]. Decreased expression or function of Kv channels in PASMC has been involved in the pathogenesis of primary and anorexigen-induced pulmonary hypertension [17]. Kv1.5 subunits are believed to be major contributors of the native Kv currents in PA [15], and contribute to the pathogenesis of PAH [13, 18]. Both 5-HT and Kv channels may have an etiological role in PAH. Although 5-HT inhibition of Kv channels in PASMC has been explored, so far no K+ channel opener has ever used to control PAH. In the present study, we further established the functional relationship between 5-HT and K+ channels in PAs and sought to determine if the K+ channel opener KMUP-1 [16, 19] could be beneficial in PAH.
Materials and Methods
Animal procedure and tissue preparation
All procedures and protocols were approved by the Animal Care and Use Committee at Kaohsiung Medical University and complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Briefly, male Sprague Dawley rats (13-15 weeks of age) were euthanized by pentobarbital sodium (130 mg/kg, i.p.) overdose. The heart and lungs were removed en bloc and placed in cold physiological salt solution containing (in mM): 119 NaCl, 4.8 KCl, 1.7 KH2PO4, 20 NaHCO3, 10 Glucose, 1.2 CaCl2, and 1.2 MgSO4 (pH 7.4). Extralobar PAs were dissected free of the surrounding tissue.
Contractile tension recording
Extralobar PA rings were quickly isolated and cut into 2-3 mm rings. Endothelium was removed and the rings were suspended under isometric conditions and connected to a force transducer (Ugo Basile, Model 7004, Comerio-VA, Italy) as previously described [6, 7] to measure tension changes. Endothelium-denuded PA rings were equilibrated in organ chambers with a resting tension of 1 g for 90 min and the bath solution was replaced every 15 min. Contractile responses in PA rings were recorded in the presence of KCl (16 mM) and once the contractions reached a plateau, which was considered as 100%. After washout, 5-HT (1-100 μM) was added concentration-dependently to induce the maximal contraction of PA rings compared to 16 mM KCl, and KMUP-1 was applied to observe the vasorelaxant responses. To investigate the underlying mechanism of PA relaxation by KMUP-1, K+ channel blockers (nonselective: TEA, 10 mM; BKCa: paxilline, 10 μM; Kv: 4-AP, 5 mM) were used as pretreatments before KMUP-1 (1 μM) application.
Pulmonary artery smooth muscle cell culture
Extralobar PAs were carefully dissected and prepared for tissue culture. Explant cultures were prepared as in our previous reports [6, 7]. Briefly, the endothelium was removed by gentle rubbing with a sterile cotton swab. The tunica adventitia was carefully removed, together with the most superficial part of the tunica media. The remaining part of the media was cut into small pieces that were explanted in culture flasks. The explants were incubated in Dulbecco's Modified Eagle Medium (DMEM; Gibco Laboratories, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin amphotericin B (Biological Industries, Kibbutz Beit Haemek, Israel) at 37ºC in a humidified 5% CO2 atmosphere. Pulmonary artery smooth muscle cells (PASMCs) began to proliferate from explants after 7 days in culture. Cell growth was arrested by replacing the media with FBS-free DMEM for 24 h and then the cells were incubated with low-serum DMEM as a control (2% FBS) or test agents for 24 h. Primary cultures of 2-5 passages were used in the experiments. PASMCs were examined by immunofluorescent staining of α-actin to confirm the purity of PASMCs.
Double immunofluorescent staining forKv1.5, Kv2.1 and BKCa channels
Cultured PASMCs were washed in PBS three times for 5 min each and then rapidly fixed in acetone at -20oC for 10 min. Fixed cells were rehydrated by washing in PBS twice for 10 min and thus permeabilized using PBS containing 0.5% Triton X-100, washed with PBS, blocked in 1% bovine serum albumin (BSA) in PBS at room temperature for 1.5 hr, and incubated overnight at 4oC in the appropriate primary antibody for Kv1.5, Kv2.1 and BKCa (Alomone Labs, Jerusalem, Israel) diluted 1:100 in 1% BSA in PBS. Cells were washed with PBS before being incubated at room temperature with an appropriate secondary antibody conjugated with Alexa Fluor 555 (red; Invitrogen, Carlsbad, CA) diluted 1:100 in 1% BSA in PBS for 2 hr in the dark. For double labeling with α-smooth muscle actin (α-SMA) antibody, sections were then incubated at room temperature with the appropriate primary anti-α-SMA direct-fluorescence antibody (green; Sigma-Aldrich, St. Louis, MO) diluted 1:100 in 1% BSA in PBS for 2 hr in the dark. Subsequently, cells were washed three times with PBS and coverslipped using mounting medium (Fluoromount-GTM; eBioscience Inc., San Diego, CA). Images were taken using a confocal laser-scanning microscope (Olympus Fluoview FV500, Tokyo, Japan).
Protein extraction and Western blot analysis
Confluent PASMCs were collected, homogenized and centrifuged at 10,000 g at 4°C for 30 min. The protein concentrations of supernatants were determined using bovine serum albumin as the standard. PASMC extracts were then boiled in ratio of 4:1 with sample buffer (100 mM Tris, pH 6.8, 20% glycerol, 4% SDS, and 0.2% bromophenol blue). Electrophoresis was performed using 10% SDS-polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes (Millipore Corp., Billerica, MA). The membrane was blocked with Tris-buffered saline (TBS; 20 mM Tris and 137 mM NaCl, pH 7.6) containing 0.1% Tween 20 (TTBS) and 5% nonfat milk at room temperature for 1 h, washed with TTBS, and then incubated overnight at 4°C in the appropriate primary antibody for Kv1.5, Kv2.1 and BKCa (Alomone Labs, Jerusalem, Israel). The membranes were washed in TTBS before being incubated with horseradish peroxidase-conjugated antibody against mouse, goat, or rabbit IgG for 1 hr. The membrane was then washed in TTBS and developed with enhanced chemiluminescence for the detection of the specific antigen. The intensity of the bands was quantitated by densitometry.
Chemicals
Buffer reagents, 4-aminopyridine (4-AP), 8-Br-cAMP, chelerythrine, 5-HT, KT5720, KT5823, paxilline, phorbol 12-myristate 13-acetate (PMA) and tetraethylammonium (TEA) were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO). All drugs and reagents were dissolved in distilled water unless otherwise noted. Chelerythrine, paxilline and PMA were dissolved in DMSO at 10 mM. KMUP-1 was dissolved in 10% absolute alcohol, 10% propylene glycol and 2% 1N HCl at 10 mM. Serial dilutions were made in phosphate buffer solution to a final solvent concentration of ≤ 0.01%.
Statistical analyses
All data are expressed as the mean ± S.E., n=6-8. One-way analysis of variance (ANOVA) was used to analyze differences among multiple comparisons. When appropriate, a Tukey-Kramer pairwise comparison was used for post hoc analysis. A p value <0.05 was considered significant in all experiments.
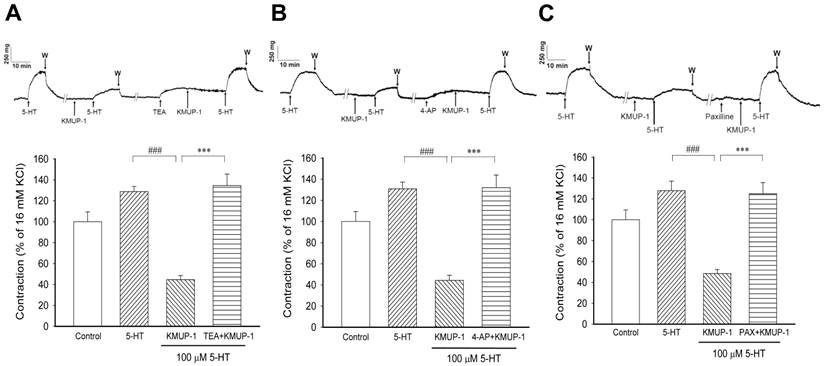
KMUP-1 (1 μM) effects on 5-HT (100 μM) induced PA contractions in the absence and presence of various K+ channel inhibitors. (A) The nonselective K+ channel inhibitor TEA (10 mM), (B) the Kv channel inhibitor 4-AP (5 mM) and (C) the BKCa channel inhibitor paxilline (10 μM) were applied. The top panel shows the typical trace of muscle force in the PA of each group. Bar chart summarizes the results from the top panel. Data are means ± SE, n=8. ###P< 0.001 compared with 5-HT group; ***P < 0.001 compared with KMUP-1 group. W: Washout.
Results
Effects of KMUP-1 on 5-HT-induced vasoconstrictions
In endothelium-denuded PA rings were contracted with 16 mM KCl to serve as a reference at the beginning and end of each experiment. In a series experiments, the reproducibility of 5-HT induced contractions was performed in the same PA preparation (Fig 1). In 16 mM KCl-precontracted PA rings (100 ± 9.4%), pretreatment with KMUP-1 (0.1, 1, 10 μM) reduced 5-HT (100 μM, 128.7 ± 8.3%) induced contractile responses in a dose-dependent manner (91.6 ± 5.6%, 47.8 ± 12.4%, 19.6 ± 5.9%). The effect of KMUP-1 (1 μM) on 5-HT (100 μM) contractile responses was reversed by the nonselective K+ channel inhibitor TEA (10 mM, Fig 1A). Likewise, KMUP-1 attenuation of 5-HT contractile responses was also reversed by the Kv channel inhibitor 4-AP (5 mM, Fig 1B) or by the BKCa inhibitor paxilline (10 μM, Fig 1C). Notably, the basal tension of PA rings was altered by pretreatment with various K+ channel inhibitors that are attributed to membrane depolarization. For this reason, the magnitude of 5-HT-induced contractile responses was adjusted under the same basal tension in each separate experiment.
Effects of KMUP-1 on 5-HT-inhibited Kv and BKCa channel immunoreactivity
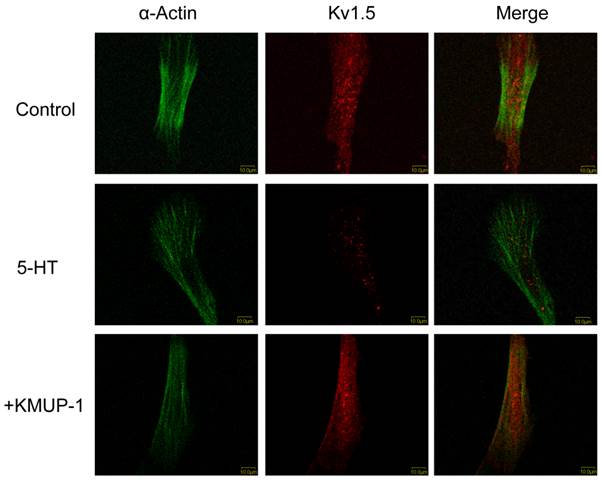
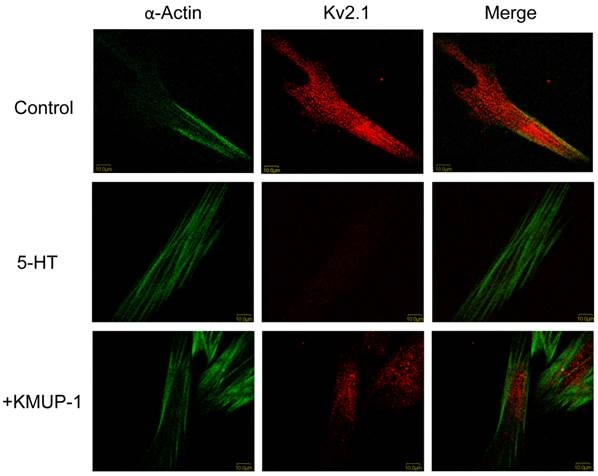
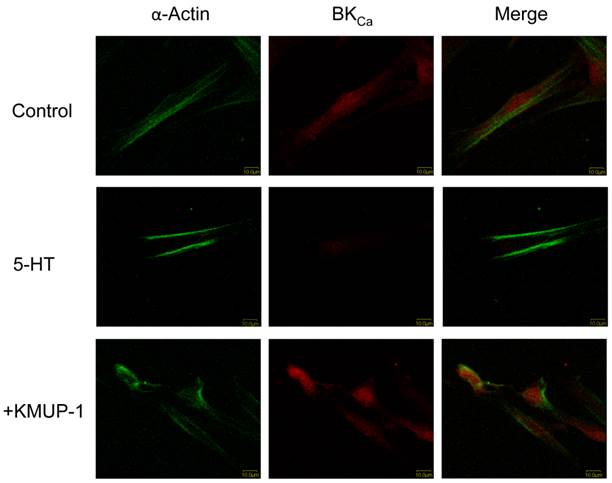
In primary cultured PASMCs, 5-HT (10 μM) significantly decreased the immunoreactivity of Kv1.5 (Fig 2), Kv2.1 (Fig 3) and BKCa (Fig 4) channel proteins as measured by confocal microscopy. α-Smooth muscle actin was used as a marker in PASMCs. Surprisingly, 5-HT-induced decreases in the immunoreactivity of these channel proteins were restored by co-incubation with KMUP-1 (1 μM) (Figs 2, 3 and 4).
Effects of KMUP-1 on 5-HT-inhibited Kv and BKCa channel proteins
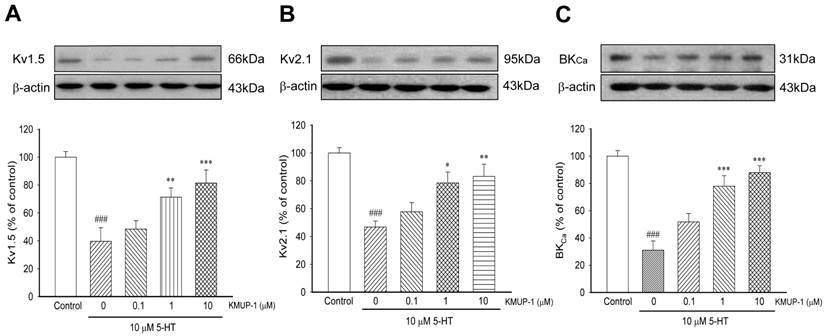
In primary PASMCs, 5-HT (10 μM) significantly reduced the expression of Kv1.5 (Fig 5A), Kv2.1 (Fig 5B) and BKCa (Fig 5C) channel proteins. After incubation with KMUP-1 (0.1, 1, 10 μM), the expression of these channel proteins was gradually reversed in a dose-dependent manner (Fig 5).
KMUP-1 modulation of 5-HT-inhibited Kv1.5 proteins via the PKC pathway
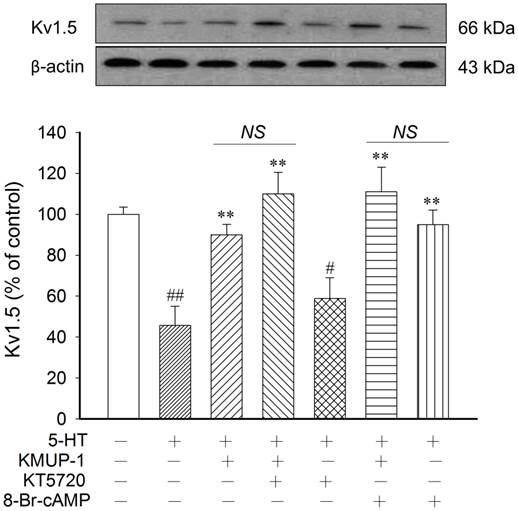
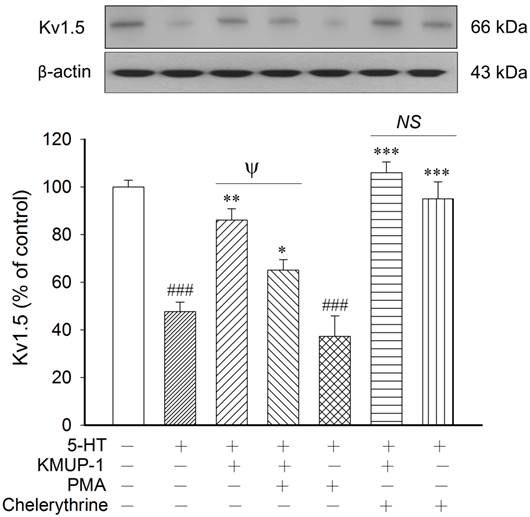
In this study, we found that the protein expression and immunereactivity of Kv1.5, Kv2.1 and BKCa channels were parallel in PASMCs. Therefore, we chose the Kv1.5 protein to explore the intracellular response to 5-HT in Kv channels and to observe the possible biochemical mechanisms of KMUP-1. To determine whether PKA is involved in 5-HT inhibition of K+ channels, the PKA activator 8-Br-cAMP and inhibitor KT5720 were used in primary PASMCs. KMUP-1 (1 μM) alone increased Kv1.5 expression from 100 ± 3.4% to 123.1 ± 4.3% (p<0.05), whereas 5-HT (10 μM) markedly inhibited this protein. Additionally, this channel protein was little affected by the PKA inhibitor KT5720 alone. 5-HT inhibition of Kv1.5 expression was reversed by KMUP-1 (1 μM), 8-Br-cAMP (100 μM), KMUP-1+KT5720 (1 μM) or KMUP-1+8-Br-cAMP (Fig 6). The PKC activator PMA and inhibitor chelerythrine were used to ascertain the role of PKC in 5-HT-inhibited K+ channels. Kv1.5 protein was little influenced by the PKC inhibitor chelerythrine. 5-HT inhibition of Kv1.5 protein was reversed by KMUP-1 (1 μM), chelerythrine (1 μM), KMUP-1+PMA (1 μM) or KMUP-1+chelerythrine, but not affected by the PKC activator PMA (1 μM). Notably, KMUP-1 reversed 5-HT-inhibited Kv1.5 protein and this effect was attenuated significantly by adding the PKC activator PMA, suggesting that KMUP-1 did modulate the 5-HT-mediated PKC pathway (Fig 7). Taken together, KMUP-1 alone increased Kv1.5 and it also reversed 5-HT-inhibited Kv1.5 protein, suggesting that KMUP-1 is not simply an inhibitor of 5-HT-mediated PKC signaling, but might have the direct actions on AC/PKA pathways.
Discussion
This study investigated whether the xanthine derivative KMUP-1 inhibits 5-HT-induced vasoconstriction and K+-channel inhibition in PAs, to explore the applicability of KMUP-1 for reversing the development of PAH during 5-HT exposure. In this study, we observed that 5-HT-mediated PA vasoconstriction was blunted by pretreatment with KMUP-1, and these effects were reversed by co-incubation with nonselective K+, selective Kv and BKCa channel blockers (TEA, 4-AP and paxilline). Additionally, 5-HT inhibition of Kv1.5, Kv2.1 and BKCa channel proteins in PASMCs was reversed by KMUP-1 in a dose-dependent manner. Those data were further confirmed by immunofluorescent localization. Our findings suggest that the underlying mechanism by which KMUP-1 reverses 5-HT-mediated vasoconstriction is partially attributable to its prevention of the K+ channel inhibition triggered by 5-HT.
5-HT exerts vasoconstrictor and mitogenic effects, both phenomena being dependent on an increase of cytosolic [Ca2+]i in PASMCs [20, 21]. PAH is characterized by high circulating 5-HT concentration, 5-HT-induced hyperreactivity and SMC proliferation, suggesting a major role for 5-HT in both vascular wall remodeling and elevated vascular resistance [9]. Sustained Ca2+ elevation contributes to PASMCs contraction and proliferation [22]. In our previous reports, we have shown that the PA relaxation and anti-proliferation effects of KMUP-1 involve the inhibition of Ca2+-influx and RhoA/Rho kinase signaling [5, 6]. On the other hand, one report suggested that 5-HT-induced vasoconstriction was mediated by at least two types of receptors, 5-HT2A and 5-HT1B, but only the 5-HT2A receptor involves Kv channel inhibition in rat PASMCs [13]. KMUP-1 has been used to attenuate monocrotaline-induced PA proliferation by targeting 5-HT2A, 5-HT2B and 5-HT2C receptors [6]. In the present isolated PA experiments, we observed that KMUP-1 attenuation of 5-HT-mediated contractile response was reversed by adding K+ channel inhibitors. Taken together, we suggest that KMUP-1 caused PA relaxation in addition to the blockade of Ca2+-influx and the RhoA/Rho kinase cascade by binding to 5-HT2 receptor subtypes [5, 6], and this could be due, at least in part, to the modulation of K+ channel activation and/or opening.
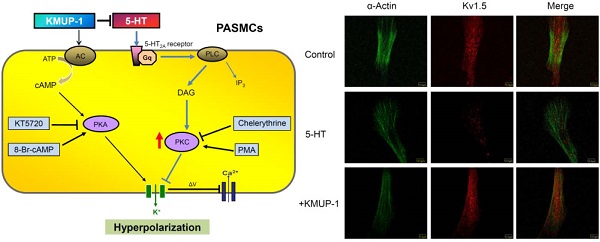
KMUP-1 restores 5-HT inhibited Kv1.5 expression in PASMCs. Confocal immunofluorescence images of PASMCs stained with Kv1.5 proteins. PASMCs were incubated with 5-HT (10 μM) or 5-HT (10 μM) + KMUP-1 (1 μM). α-Actin was indicated by FITC-conjugated antibody (green) and Alexa Fluor® 555-conjugated antibody was used to detect Kv1.5 (red). Images were obtained using the same laser intensity.
KMUP-1 restores 5-HT inhibited Kv2.1 expression in PASMCs. Confocal immunofluorescence images of PASMCs stained with Kv2.1 proteins. PASMCs were incubated with 5-HT (10 μM) or 5-HT (10 μM) + KMUP-1 (1 μM). α-Actin was indicated by FITC-conjugated antibody (green) and Alexa Fluor® 555-conjugated antibody was used to detect Kv2.1 (red). Images were obtained using the same laser intensity.
KMUP-1 restores 5-HT inhibited BKCa expression in PASMCs. Confocal immunofluorescence images of PASMCs stained with BKCa proteins. PASMCs were incubated with 5-HT (10 μM) or 5-HT (10 μM) + KMUP-1 (1 μM). α-Actin was indicated by FITC-conjugated antibody (green) and Alexa Fluor® 555-conjugated antibody was used to detect BKCa (red). Images were obtained using the same laser intensity.
KMUP-1 (01, 1, 10 μM) reversed 5-HT (10 μM) inhibited (A) Kv1.5, (B) Kv2.1 and (C) BKCa channel proteins in a dose-dependent manner in PASMCs. Protein quantitation is shown in the lower panel. Data are means ± SE, n=6-8. ###P< 0.001 compared with control; *P< 0.05, **P< 0.01, ***P< 0.001 compared with 5-HT alone.
KMUP-1 activates AC/PKA-increased Kv1.5 proteins directly but does not modulate the 5-HT-mediated PKA pathway. 5-HT (10 μM) inhibition of Kv1.5 protein was not affected by the PKA inhibitor KT5720 (1 μM). 5-HT inhibition of Kv1.5 expression was reversed by KMUP-1 (1 μM), 8-Br-cAMP (100 μM), KMUP-1+KT5720 (1 μM) or KMUP-1+8-Br-cAMP in PASMCs. Protein quantitation is shown in the lower panel. Data are means ± SE, n=6-8. #P< 0.05, ##P< 0.01 compared with control; **P< 0.01 compared with 5-HT alone. AC: Adenylate cyclase; NS: Not significant.
KMUP-1 modulates 5-HT-inhibited Kv1.5 proteins via the PKC pathway. 5-HT inhibition of Kv1.5 protein was reversed by KMUP-1 (1 μM), chelerythrine (1 μM), KMUP-1+PMA (1 μM) or KMUP-1+chelerythrine, but not affected by the PKC activator PMA (1 μM). KMUP-1 reversed 5-HT-inhibited Kv1.5 protein and this effect was attenuated significantly by co-incubation with the PKC activator PMA, suggesting that KMUP-1 modulates the 5-HT-mediated PKC pathway. Protein quantitation is shown in the lower panel. Data are means ± SE, n=6-8. ###P< 0.001 compared with control; *P< 0.05, **P< 0.01, ***P< 0.001 compared with 5-HT alone; ψP< 0.05 5-HT+KMUP-1 compared with 5-HT+KMUP-1+PMA. PMA: Phorbol 12-myristate 13-acetate; NS: Not significant.
Several types of K+ channels are present in PAs, and activation or inhibition of these channels plays an important role in pulmonary circulation [23]. Activation of K+ channels in PASMCs hyperpolarizes the cell membrane, and subsequently closes voltage-dependent calcium channels, resulting in a decrease in intracellular calcium and vascular relaxation [24]. In contrast, inhibition of K+ channels results in pulmonary vasoconstriction. Previous studies have shown that the primary targets of 5-HT in the vasculature and neurons are the Kv channels [18, 25, 26]. Kv1.5 channels appear to be functionally linked to 5-HT signaling in PA and contribute to the pathogenesis of PAH. Moreover, Kv1.5 channels were internalized when PASMCs were stimulated with 5-HT [13, 18]. Kv2.1 channels play an important role in establishing the resting membrane potential of PASMCs from resistance arteries [27]. Kv channel inhibition accounts, at least in part, for 5-HT-induced pulmonary vasoconstriction and might play a role in PAH. Both 5-HT and Kv channels have been implicated in the pathogenesis of PAH [13]. Additionally, Kv channel inhibition might also be involved in the PA wall thickness and medial hypertrophy induced by 5-HT [10]. Activation of BKCa channels in PASMCs results in K+ efflux and membrane hyperpolarization, which leads to decreases in cytosolic calcium and vascular tone [16, 27]. Based on data from Western blotting and immunofluorescent staining, the expression and immunoreactivity of K+ channels in 5-HT-treated PASMCs are downregulated, especially for the Kv1.5, Kv2.1 and BKCa channel proteins. These results appear to be consistent with the study by Cogolludo et al. (2006) who found that activation of the 5-HT2A receptor inhibits Kv currents in rat PASMCs and human Kv1.5 channels. However, the findings are in contrast to our previous findings in vivo in monocrotaline-treated rats, where the Kv1.5, Kv2.1 and BKCa channel proteins are upregulated [5]. We suggest that monocrotaline-induced chronic PAH rats upregulate such functional K+ channel proteins, which could be a protective mechanism to compensate for increases of right ventricular systolic pressure, a marker of systolic pulmonary pressure [5].
To elucidate the role of PKA and/or PKC in 5-HT inhibition of K+ channels and in KMUP-1's effects on this channel, PKA and PKC activators (8-Br-cAMP, PMA) and inhibitors (KT5720, chelerythrine) were used in rat PASMCs. 5-HT-inhibited Kv1.5 protein was unaffected by the PKA inhibitor KT5720 but significantly affected by the PKC inhibitor chelerythrine, suggesting that 5-HT is not involved in cross-talk with AC/PKA cascades [19, 28] but exclusively involved in the PKC pathway in rat PASMCs. PKC plays a central role inhibiting K+ efflux and depolarizing membrane potential, and accordingly results in PA vasoconstriction. This finding is consistent with previous reports [13, 15, 29]. Surprisingly, in addition to increases in Kv1.5 channel protein with KMUP-1, KMUP-1 also reversed 5-HT inhibition of Kv1.5 and this effect was markedly blunted in combination with the PKC activator PMA. Thus, we suggest that KMUP-1 not only inhibits 5-HT-mediated PKC signaling, but also could activate AC/PKA cascades (Fig 8) as previously described [16].
Though the K+ channel inhibitory effects of 5-HT can be significant in the contractile response of PAs or PASMCs, we still need to keep in mind that other mechanisms are also important actors, such as [Ca2+]I and RhoA/ROCK among others. In summary, this study presents evidence that PKC plays a key role in regulating 5-HT-mediated PA vasoconstriction and K+-channel inhibition via activation of 5-HT2A receptors in PASMCs. This investigation also further addresses the underlying mechanisms by which KMUP-1 prevents 5-HT-induced vasoconstriction and K+-channel inhibition through PKC signaling in PAs. Finally, we suggest that KMUP-1 could be of value as a pharmacotherapeutic agent in PAH, especially for 5-HT-induced PA vasoconstriction and PASMC proliferation.
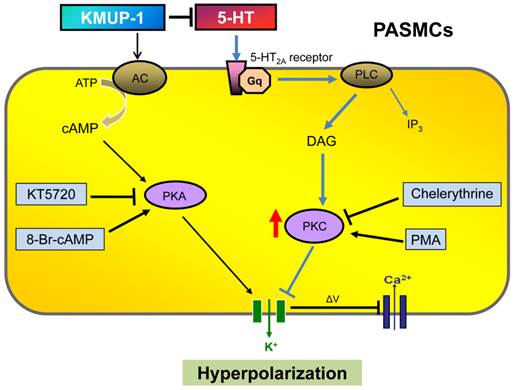
Diagram summarizing KMUP-1 amelioration of 5-HT-induced K+ channel inhibition in PASMCs. 5-HT binds to 5-HT2A receptor, which is a G protein-coupled receptor that interacts with Gq proteins. Gq activates phospholipase C (PLC) resulting in the production of diacylglycerol (DAG) and inositol triphosphate (IP3) and activation of protein kinase C (PKC), which is capable of phosphorylating tyrosine kinases and inhibiting K+ efflux. KMUP-1 does not simply inhibit 5-HT-mediated PKC signaling but can directly activate adenylate cyclase (AC)/protein kinase A (PKA) cascades, resulting in K+ channel opening. PMA: Phorbol 12-myristate 13-acetate.
Acknowledgements
We thank Ms Li-Mei An for her excellent technical assistance and ex-tenure Professor Maw-Shung Liu at School of Medicine, Saint Louis University for his editorial assistance with the manuscript. The authors also thank the Center for Research Resources and Development of Kaohsiung Medical University for providing the service of Olympus Fluoview FV500 confocal microscope. This study was supported by grants NSC 101-2320-B-037-032-MY3 and NSC 101-2632-B-037-001-MY2 from the Ministry of Science and Technology, Taiwan, NSYSUKMU 102-P005 from Kaohsiung Medical University, and KMUH 100-0R29 from Kaohsiung Medical University Hospital, Kaohsiung, Taiwan.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM. et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S-24S
2. McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417-31
3. Wu BN, Lin RJ, Lin CY, Shen KP, Chiang LC, Chen IJ. A xanthine-based KMUP-1 with cyclic GMP enhancing and K+ channels opening activities in rat aortic smooth muscle. Br J Pharmacol. 2001;134:265-74
4. Liu CP, Dai ZK, Huang CH, Yeh JL, Wu BN, Wu JR. et al. Endothelial nitric oxide synthase-enhancing G-protein coupled receptor antagonist inhibits pulmonary artery hypertension by endothelin-1-dependent and endothelin-1-independent pathways in a monocrotaline model. Kaohsiung J Med Sci. 2014;30:267-78
5. Dai ZK, Cheng YJ, Chung HH, Wu JR, Chen IJ, Wu BN. KMUP-1 ameliorates monocrotaline-induced pulmonary arterial hypertension through the modulation of Ca2+ sensitization and K+-channel. Life Sci. 2010;86:747-55
6. Chung HH, Dai ZK, Wu BN, Yeh JL, Chai CY, Chu KS. et al. KMUP-1 inhibits pulmonary artery proliferation by targeting serotonin receptors/transporter and NO synthase, inactivating RhoA and suppressing AKT/ERK phosphorylation. Vascul Pharmacol. 2010;53:239-49
7. Chung HH, Dai ZK, Wu BN, Yeh JL, Chai CY, Chu KS. et al. The xanthine derivative KMUP-1 inhibits models of pulmonary artery hypertension via increased NO and cGMP-dependent inhibition of RhoA/Rho kinase. Br J Pharmacol. 2010;160:971-86
8. Guignabert C, Raffestin B, Benferhat R, Raoul W, Zadigue P, Rideau D. et al. Serotonin transporter inhibition prevents and reverses monocrotaline-induced pulmonary hypertension in rats. Circulation. 2005;111:2812-9
9. Rodat L, Savineau JP, Marthan R, Guibert C. Effect of chronic hypoxia on voltage-independent calcium influx activated by 5-HT in rat intrapulmonary arteries. Pflugers Arch. 2007;454:41-51
10. Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G. et al. Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension. Circ Res. 2004;94:1263-70
11. MacLean MR, Herve P, Eddahibi S, Adnot S. 5-hydroxytryptamine and the pulmonary circulation: receptors, transporters and relevance to pulmonary arterial hypertension. Br J Pharmacol. 2000;131:161-8
12. Dempsie Y, MacLean MR. Pulmonary hypertension: therapeutic targets within the serotonin system. Br J Pharmacol. 2008;155:455-62
13. Cogolludo A, Moreno L, Lodi F, Frazziano G, Cobeno L, Tamargo J. et al. Serotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells: role of 5-HT2A receptors, caveolin-1, and KV1.5 channel internalization. Circ Res. 2006;98:931-8
14. Hardin CD, Vallejo J. Caveolins in vascular smooth muscle: form organizing function. Cardiovasc Res. 2006;69:808-15
15. Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A. et al. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101:2319-30
16. Wu BN, Tu HF, Welsh DG, Chen IJ. KMUP-1 activates BKCa channels in basilar artery myocytes via cyclic nucleotide-dependent protein kinases. Br J Pharmacol. 2005;146:862-71
17. Yuan XJ, Wang J, Juhaszova M, Gaine SP, Rubin LJ. Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet. 1998;351:726-7
18. Bae YM, Kim A, Kim J, Park SW, Kim TK, Lee YR. et al. Serotonin depolarizes the membrane potential in rat mesenteric artery myocytes by decreasing voltage-gated K+ currents. Biochem Biophys Res Commun. 2006;347:468-76
19. Kuo HF, Lai YJ, Wu JC, Lee KT, Chu CS, Chen IJ. et al. A xanthine-derivative K+-channel opener protects against serotonin-induced cardiomyocyte hypertrophy via the modulation of protein kinases. Int J Biol Sci. 2013;10:64-72
20. Guibert C, Marthan R, Savineau JP. Modulation of ion channels in pulmonary arterial hypertension. Curr Pharm Des. 2007;13:2443-55
21. Rodat-Despoix L, Aires V, Ducret T, Marthan R, Savineau JP, Rousseau E. et al. Signalling pathways involved in the contractile response to 5-HT in the human pulmonary artery. Eur Respir J. 2009;34:1338-47
22. Ducret T, Guibert C, Marthan R, Savineau JP. Serotonin-induced activation of TRPV4-like current in rat intrapulmonary arterial smooth muscle cells. Cell Calcium. 2008;43:315-23
23. Dospinescu C, Widmer H, Rowe I, Wainwright C, Cruickshank SF. Hypoxia sensitivity of a voltage-gated potassium current in porcine intrapulmonary vein smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2012;303:L476-86
24. Zheng YM, Park SW, Stokes L, Tang Q, Xiao JH, Wang YX. Distinct activity of BK channel beta1-subunit in cerebral and pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol. 2013;304:C780-9
25. Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR. et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S20-31
26. Jin NG, Crow T. Serotonin regulates voltage-dependent currents in type IeA and Ii interneurons of Hermissenda. J Neurophysiol. 2011;106:2557-69
27. Yan J, Chen R, Liu P, Gu Y. Docosahexaenoic acid attenuates hypoxic pulmonary vasoconstriction by activating the large conductance Ca2+-activated K+ currents in pulmonary artery smooth muscle cells. Pulm Pharmacol Ther. 2014;28:9-16
28. Tournois C, Mutel V, Manivet P, Launay JM, Kellermann O. Cross-talk between 5-hydroxytryptamine receptors in a serotonergic cell line. Involvement of arachidonic acid metabolism. J Biol Chem. 1998;273:17498-503
29. Fike CD, Kaplowitz MR, Zhang Y, Madden JA. Voltage-gated K+ channels at an early stage of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1169-76
Author contact
![]() Corresponding authors: Jiunn-Ren Wu M.D., Ph.D., Department of Pediatrics, Division of Pediatric Pulmonology and Cardiology, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung 807, Taiwan. Fax: 886-7-3213931; E-mail: jirewuedu.tw. Bin-Nan Wu Ph.D., Department of Pharmacology, Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, 100 Shih-Chuan 1st Road, Kaohsiung 807, Taiwan. Fax: 886-7-3234686; E-mail: binnanedu.tw
Corresponding authors: Jiunn-Ren Wu M.D., Ph.D., Department of Pediatrics, Division of Pediatric Pulmonology and Cardiology, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung 807, Taiwan. Fax: 886-7-3213931; E-mail: jirewuedu.tw. Bin-Nan Wu Ph.D., Department of Pharmacology, Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, 100 Shih-Chuan 1st Road, Kaohsiung 807, Taiwan. Fax: 886-7-3234686; E-mail: binnanedu.tw
Received 2014-11-20
Accepted 2015-3-23
Published 2015-4-25
