ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2015; 11(6):664-671. doi:10.7150/ijbs.10783 This issue Cite
Research Paper
A Novel Role of OS-9 in the Maintenance of Intestinal Barrier Function from Hypoxia-induced Injury via p38-dependent Pathway
Department of General Surgery, Xinqiao Hospital, Third Military Medical University, Chongqing, China.
Abstract
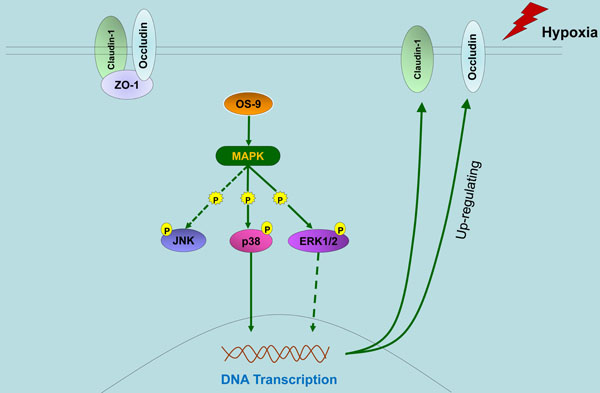
OS-9 is a lectin required for efficient ubquitination of glycosylated substrates of endoplasmic reticulum-associated degradation (ERAD). OS-9 has previously been implicated in ER-to-Golgi transport and transcription factor turnover. However, we know very little about other functions of OS-9 under endoplasmic reticulum stress. Here, we used gene knockdown and overexpression approaches to study the protective effect of OS-9 on intestinal barrier function of intestinal epithelial cell Caco-2 monolayer. We found that OS-9 attenuated intestinal epithelial barrier dysfunction under hypoxia through up-regulating occludin and claudin-1 protein expression. Furthermore, we showed that the up-regulation of occludin and claudin-1 induced by OS-9 was mediated by p38 and ERK1/2 phosphorylation and did not involve HIF-1α. In summary, our results demonstrate that OS-9 up-regulates occludin and claudin-1 by activating the MAP kinase (MAPK) pathway, and thus protects the epithelial barrier function of Caco-2 monolayer under hypoxia condition.
Keywords: OS-9, intestinal epithelial barrier, p38, tight junction
Introduction
Intestinal mucosa serves as a major functional barrier separating potentially harmful intraluminal elements such as bacteria and endotoxins from extra-intestinal tissues and the circulation system [1,2]. The integrity of the intestinal mucosa barrier is an essential preliminary defensive mechanism in the pathophysiology of systemic complications [3]. In mesenteric ischemia-reperfusion (I/R), the intestinal barrier function is compromised, resulting in destruction of tight junctions (TJs) and increase of intestinal permeability to macromolecules [4,5]. Intestinal injury associated with ischemia is integral to the pathogenesis in multiple diseases. One major consequence of intestinal ischemia is hypoxia, which leads to endoplasmic reticulum stress [6].
As a master regulator of the hypoxic response, hypoxia-inducible factor-1α (HIF-1α) plays an important role in the regulation of intestinal barrier. Some studies have showed that HIF-1α activation is deleterious to intestinal barrier function associated with hypoxia, I/R, and inflammation [7-10]. In our previous research, HIF-1α was also demonstrated to induce the loss of epithelial barrier and disruption of tight junction proteins [11]. However, the precise molecule mechanisms by which HIF-1α regulates intestinal epithelial barrier (IEB) are still elusive.
Interestingly, accumulating evidence revealed the potential relationship between endoplasmic reticulum (ER) stress and HIF-1α activation under various pathological stimuli [12,13]. Of note, a recent study showed that a novel regulator, osteosarcoma-9 (OS-9), was identified as HIF-1α binding partners [14]. Usually, OS-9 is proposed as a lectin which played a key role during the ER stress response associated with hypoxia [15-17]. Functionally, it has been implicated in ER-to-Golgi transport of the membrane protease and helps to select misfolded glycoproteins for degradation [18,19]. Recently, Carvalho and colleagues showed that OS-9 is involved in cell survival and resistance to apoptosis [20]. Should be note, the current studies suggested that OS-9 may affects hypoxic signaling via interaction with either the transcription factor HIF-1α or an HIF-1α-independent pathway [14,21,22]. Nonetheless, little data exist for the function of OS-9 in intestinal epithelial cells, especially its role in the regulation of TJs and IEB. Thus, it would be important to clarify the exact role of OS-9 in the mechanisms of IEB modulation in a HIF-1α-dependent or independent way under hypoxia conditions.
According to the above, we hypothesized that OS-9 could regulate the IEB through HIF-1α dependent pathway. In this study, for the first time we used genetic and biochemical approaches to show that OS-9 plays an important role in maintaining the IEB function under hypoxia conditions. However, this barrier-inducing effect of OS-9 is mediated by p38 and ERK1/2 phosphorylation and did not involve HIF-1α.
Materials and Methods
Cell culture and hypoxic treatment
HEK 293T and Caco-2 (human colon carcinoma) cells were purchased from Cell Resource Center, IBMS, CAMS/PUMC (Beijing). 293T cells were cultured in high glucose DMEM supplemented with 10% fetal bovine serum (FBS). Caco-2 cells were grown in Eagle's Minimum Essential Medium (MEM) with 20% FBS, 1% non-essential amino acids, 100 U/100μg/ml penicillin and streptomycin. Caco-2 cells were subcultured by digestion with 0.25% trypsin and 0.53 mM EDTA in Hank's balanced saline solution (HBSS). Cells were incubated in a 5% CO2 humidified incubator at 37℃. For hypoxic exposure, Caco-2 cells were placed in a modulator incubator (Thermo) flushed with 1% O2/5% CO2/balance N2 and sealed, and incubated at 37℃.
Regents
Rabbit anti-OS-9 (NB100-520) antibody was purchased from Novus Biologicals. Rabbit anti-ERK1/2 (66192-1), anti-JNK (24164-1), anti-ZO-1 (21773-1) antibodies were purchased from Proteintech (China). Rabbit anti-p38 (1544-1), anti-phospho-ERK1/2 (1481-1), anti-Occludin (6973-1) antibodies were purchased from Epitomics and R&D (MAB7074). Rabbit anti-phosphor-JNK (4668) and mouse anti-phosphor-p38 (9216) antibodies were purchased from Cell Signaling. Rabbit anti-Claudin-1 (Ab15098) antibody was purchased from Abcam. Mouse anti-HIF-1α (NB100-105) was purchased from Novus Biologicals. Anti-GAPDH antibody was purchased from Goodhere Biotechnology (China).
Western blot analysis
The cells were washed twice with ice-cold PBS, and lysed in cold RIPA buffer (PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1ug/ml APMSF, 1.0 mM sodium orthovandate) containing mammalian protease inhibitor cocktail (Sigma-Aldrich). Protein concentration was determined according to the Bradford method using BCA assay reagent (Beyotime). Samples (25 μg protein) were loaded onto SDS-PAGE gels and the gels were transferred to PVDF membranes (Millipore) after electrophoresis. Membranes were blocked by 5% bovine serum albumin (BSA) in TBST (50 mM Tris-HCl PH 7.5, 140 mM NaCl, 0.1% Tween) and then incubated with the following primary antibodies at 4℃ overnight: mouse monoclonal anti-ZO-1 (1:1000), rabbit monoclonal anti-Occludin (1:1000), rabbit polyclonal anti-Claudin-1 (1:1000), rabbit polyclonal anti-OS-9 (1:1000), rabbit polyclonal anti-GAPDH (1:1000). The membranes were then washed three times and incubated with HRP-conjugated goat anti-rabbit or goat anti-mouse secondary antibody. Membrane imaging was performed using an ECL kit (Thermo) according to the manufacturer's instructions.
Immunocytochemistry analysis
Cells were fixed in 4% paraformaldehyde for 15 min, washed 3 times with PBS, and blocked in PBS containing 5% BSA. Cells were then incubated with various primary antibodies overnight as recommended by the suppliers. The next day, cells were incubated with fluorescence-conjugated secondary antibodies for 1 hour. Cells were also stained with DAPI (Invitrogen) for nucleus visualization. All images were obtained with a TCS-SP5 confocal microscope (Leica, Germany).
siRNA transfection
Short-interfering RNA (siRNA) duplexes targeting the OS-9 mRNA were synthesized by Ribobio (Guangzhou, China) as follows: GGAAACACCUGCUUACCAAdTdT and dTdTCCUUUGUGGACGAAUGGUU. A nonspecific siRNA was used in parallel as control. Briefly, cells were incubated with siRNA for 6 hours at 37℃ according to the manufacturer's instructions. At 48 hours after transfection, cells were subjected to transepithelial electrical resistance (TER) measurement and lysed to generate lysates for immunoblot analyses.
Lentiviral transduction and stable cell line establishment
For production of recombinant lentivirus, 1×106 293T cells were co-transfected for 4 hours with 6 μg of the empty vector or target vector encoding V5-tagged plenti6.3-OS-9 (Invitrogen), 4.5 μg of psPAX2 (Addgene) and 1.5 μg pMD2G-VSVG (Addgene). After removal of the transfection mixture, cells were incubated in culture medium for 72 hours. The medium containing recombinant lentivirus was centrifuged at 3000 rpm for 15 minutes, filtered through a 0.45 μm filter (Millipore) and stored at -80℃. Virus titer was determined by infecting 293T cells with a recombinant lentivirus carrying the Plenti6.3-IRES2-EGFP vector (Invitrogen) and counting EGFP positive cells under fluorescence microscope. On average, the viral titer was 1×106 transduction units/ml.
For stable cell line establishment, 2×105 Caco-2 cells were infected with 1×106 transduction units of the OS-9 expressing recombinant lentivirus for 24 hours in the presence of polybrene (1:2000). OS-9-expressing cells were selected and maintained in MEM containing 10 μg/ml blasticidin (Sigma).
Measurement of transepithelial electrical resistance (TER)
TER was measured with an electric resistance system ERS-2 (Millipore) following the manufacturer's instructions. Briefly, Caco-2 cells were grown on Millicell® filter (0.33 cm2 area, 0.4 μm pore diameter, and 6.5 mm diameter) and the culture medium was replaced before TER measurement. To calculate the actual resistance of the cell monolayer, the mean resistance of filters without cell was subtracted from the monolayer measurement, and the difference between the filter and monolayer areas was corrected.
Statistical analysis
Experimental data were shown as the Mean ± SD. Statistical analysis was performed using the SPSS software (version 13.0 for windows) and significant difference was defined as P < 0.05.
Results
OS-9 knockdown aggravates the injury of IEB function in hypoxia
To ensure that OS-9 was effectively and specifically blocked, cells were transfected with either a control scrambled siRNA or the OS-9-specific siRNA. Whole-cell lysate (WCL) from transfected cells was subjected to SDS-PAGE followed by immunoblotting. Compared to cells treated with the control siRNA, the OS-9 level markedly decreased in WCL derived from cells transfected with the OS-9-specific siRNA (Fig. 1A).
OS-9 knockdown aggravates the injury of IEB function in hypoxia. (A) Western blot analysis of OS-9 protein level in cultured Caco-2 cells transfected with OS-9 (SiOS-9) or scrambled (SiCon) siRNA for 48 hours. GAPDH was used as a standard for cellular protein input. (B) Western blot analysis of ZO-1, claudin-1 and occludin protein levels in Caco-2 cells transfected with SiOS-9 or SiCon under nomoxia or hypoxia condition. GAPDH was used as a standard for cellular protein input. (C) Immunofluorescence staining of claudin-1 (red) and occludin (green) in Caco-2 cells transfected with SiOS-9 and SiCon under nomoxia or hypoxia condition. (D) % decrease of transepithelial electrical resistance (TER) in Caco-2 cells transfected with SiOS-9 or SiCon under hypoxia condition. Results are expressed as mean±SD. (*P<0.05 between SiOS-9 and Control).
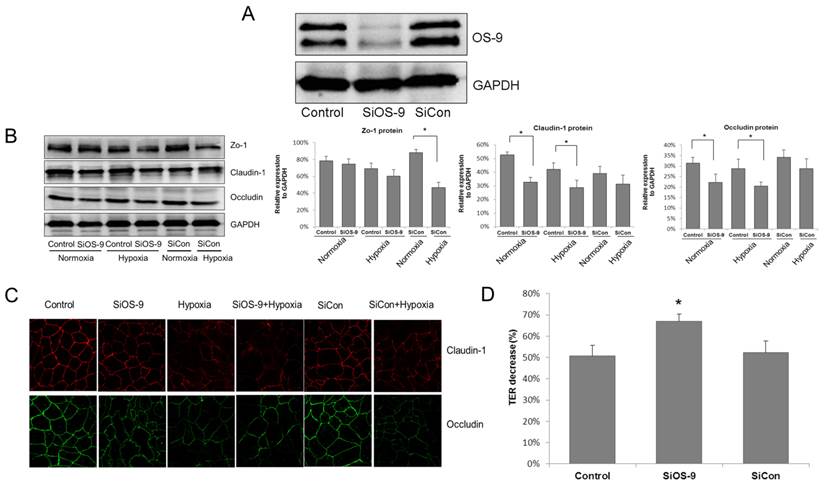
OS-9 overexpression protects intestinal epithelium barrier in hypoxia. (A) Western blot analysis of OS-9 protein level in cultured Caco-2 cells infected with a lentiviral vector expressing OS-9 (OS-9-V5) or a control empty vector (Vector). GAPDH was used as a standard for cellular protein input. (B) Western blot analysis of ZO-1, claudin-1 and occludin protein levels in Caco-2 cells infected with OS-9-V5 or Vector under nomoxia or hypoxia condition. GAPDH was used as a standard for cellular protein input. (C) Immunofluorescence staining of claudin-1 (red) and occludin (green) in Caco-2 cells infected with OS-9-V5 or Vector under nomoxia or hypoxia condition. (D) % decrease of transepithelial electrical resistance (TER) in Caco-2 cells infected with OS-9-V5 or Vector under hypoxia condition. Results are expressed as mean±SD. (*P<0.05 between OS-9-V5 and Vector).
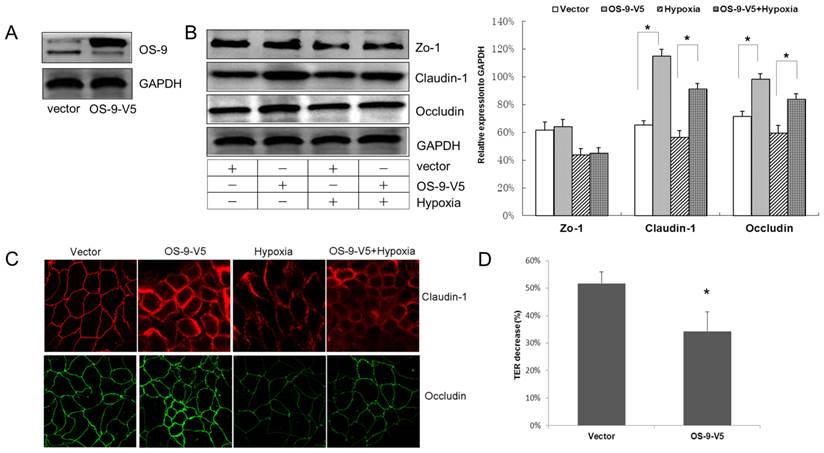
To investigate the role of OS-9 in the modulation of IEB function, western blot analysis was used to detect the expressions of tight junction (TJ) proteins in Caco-2 monolayer cells, including ZO-1, claudin-1 and occludin (Fig. 1B). In cells transfected with the control siRNA, a hypoxia treatment of 8 hours did not significantly alter the expression level of the three TJ proteins. However, OS-9 siRNA caused significant decrease of claudin-1 and occludin proteins in normoxia, and even more reduction in hypoxia (P<0.05). Western blot analysis revealed the results for the alteration of TJ proteins expression: a nearly 20 % drop on claudin-1 and occludin expression respectively in OS-9 siRNA group; while the expression of ZO-1 was unaffected by OS-9 knockdown.
Further study with immunocytochemistry (ICC) confirmed our finding that OS-9 knockdown reduced claudin-1 and occludin expression in hypoxia. As illustrated in Fig. 1C, claudin-1 and occludin were typically expressed at the cell surface as a tidy network when cells reached confluence. The TJ protein network was obviously disrupted in hypoxia; while OS-9 knockdown further reduced claudin-1 and occludin expression in Caco-2 cells.
The TJ functional impact of OS-9 knockdown on Caco-2 cell monolayer was further examined by measuring the transepithelial electrical resistance (TER) value. As shown in Fig. 1D, hypoxia resulted in a 50.77%±5.02% reduction of TER in control cells whereas OS-9 knockdown further decreased TER to 66.93%±3.39% in cells expressing the OS-9 siRNA (P< 0.05). These results indicate that OS-9 plays a protective role in hypoxia by up-regulating the expression of TJ proteins.
OS-9 overexpression protects intestinal epithelium barrier in hypoxia
To directly address the barrier-protective role of OS-9 in hypoxia-induced intestinal epithelial injury, we used an OS-9 expressing lentiviral vector to deliver OS-9 into Caco-2 cell monolayer and monitored TJ protein expression and TER as above. Western blot analysis confirmed efficient delivery of OS-9 into Caco-2 cells at protein level (Fig. 2A).
Overexpression of OS-9 significantly up-regulated claudin-1 nearly 1-fold and occludin nearly 30% under both normoxia and hypoxia conditions (P<0.05) (Fig. 2B). Consistent with the knockdown result, OS-9 overexpression did not affect the protein level of ZO-1. Again, ICC data confirmed the western blot results; OS-9 overexpression resulted in thicker TJ network of claudin-1 and occludin (Fig. 2C). Importantly, OS-9 overexpression significantly decreased the reduction of TER (Fig. 2D), indicating less damage to the Caco-2 monolayer against hypoxia threaten. Together, these results demonstrated a protective role of OS-9 on IEB function during hypoxia exposure, likely via up-regulating claudin-1 and occludin protein level.
OS-9 does not regulate HIF-1α protein level in Caco-2 cell
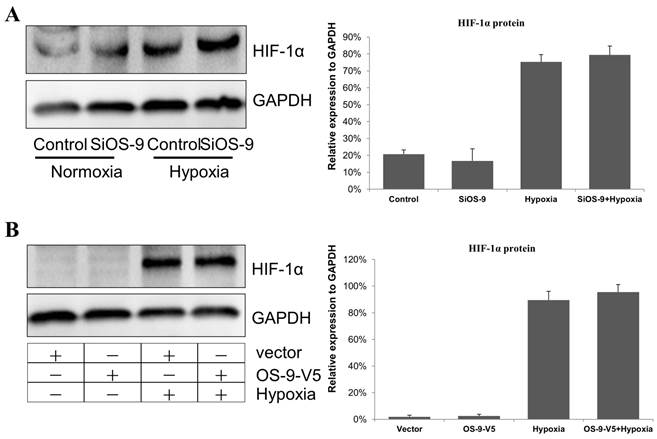
Previous studies reported that OS-9 downregulatd HIF-1α mRNA and protein levels [14]. In our previous researches, HIF-1α pathways aggravate IEB dysfunction caused by proinflammatory cytokine IFN-γ [11]. So, we tested the hypothesis that OS-9 protects Caco-2 cell monolayer by downregulating HIF-1α. Unexpectedly, we found that HIF-1α protein level was unaffected by OS-9 knockdown (Fig. 3A) or overexpression (Fig. 3B) under either normoxia or hypoxia condition. These results suggested that OS-9 does not regulate HIF-1α protein level, at least in Caco-2 cells.
OS-9 overexpression induces p38 and ERK1/2 phosphorylation in Caco-2 cell
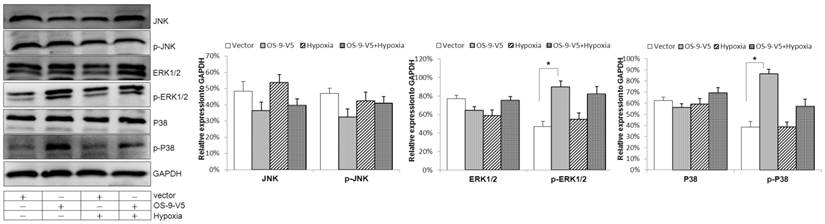
In an attempt to identify the signaling pathway downstream of OS-9, we tested the effect of OS-9 overexpression on three distinct MAPKs: JNK, ERK1/2 and p38. As shown in Fig. 4, the levels of both total and phosphorylated/activated JNK were not significantly affected by OS-9 overexpression in either normoxia or hypoxia. In addition, the total protein levels of ERK1/2 and p38 were also not affected by OS-9 overexpression. In contrast, the phosphorylated ERK1/2 and p38 were significantly increased upon OS-9 overexpression (P<0.05), and under both nomoxia and hypoxia conditions. These results suggested that OS-9 is able to induce the MAPK signaling pathway via ERK1/2 and p38 phosphorylation.
P38 activity is required for the protective function of OS-9 on IEB
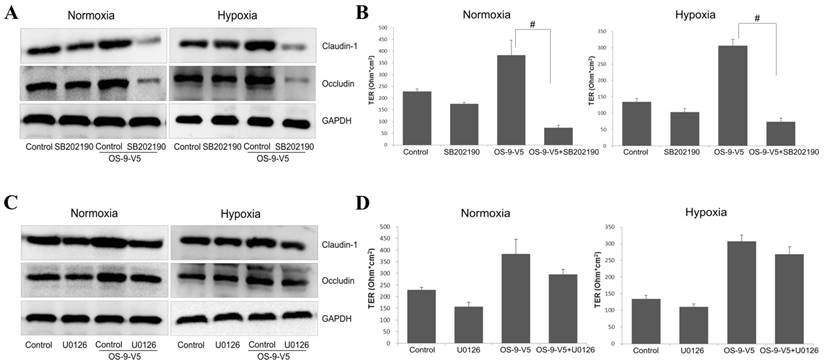
We next tested whether p38 or ERK1/2 phosphorylation/activation was required for OS-9-mediated protection of Caco-2 epithelial barrier function. We treated normal or OS-9 overexpressing cells with SB202190 or U0126, phosphorylation inhibitor of p38 or ERK1/2, respectively, and measured claudin-1 and occludin expression and TER. SB202190 dramatically reduced both claudin-1 and occludin protein levels (P<0.05) in OS-9 overexpressing cells but not in normal cells either normoxia or hypoxia (Fig. 5A). Consistent with this result, although OS-9 overexpression significantly increased TER, as expected, this protection effect of OS-9 is dramatically negated upon SB202190 treatment; the inhibitor only slightly reduced TER in normal cells (Fig. 5B). In contrast, U0126 did not alter claudin-1 or occludin protein level, nor did it significantly affect TER in control or OS-9 overexpressing cells (Fig. 5C and 5D). These results suggested that OS-9 exerts its protective effects on Caco-2 epithelial barrier function via the p38 signaling pathway.
OS-9 does not regulate HIF-1α protein level in Caco-2 cell. (A) Western blot analysis of HIF-1α protein level in cultured Caco-2 cells transfected with OS-9 siRNA (SiOS-9) or control cells (Control) under nomoxia or hypoxia condition. GAPDH was used as a standard for cellular protein input. (B) Western blot analysis of HIF-1α protein level in cultured Caco-2 cells infected with a lentiviral vector expressing OS-9 (OS-9-V5) or a control empty vector (Vector) under nomoxia or hypoxia condition. GAPDH was used as a standard for cellular protein input.
OS-9 overexpression induces p38 and ERK1/2 phosphorylation in Caco-2 cell. Western blot analysis of total and phosphorylated/activated JNK, ERK1/2 and p38 protein levels in cultured Caco-2 cells infected with a lentiviral vector expressing OS-9 (OS-9-V5) or a control empty vector (Vector) under nomoxia or hypoxia condition. GAPDH was used as a standard for cellular protein input. (*P<0.05 between OS-9-V5 and Vector).
P38 activity is required for the protective function of OS-9 on IEB. (A) Western blot analysis of claudin-1 and occludin protein levels in Caco-2 cells infected with a lentiviral vector expressing OS-9 (OS-9-V5) or an empty vector in nomoxia and hypoxia, in the presence (SB202190) or absence (Control) of 10 μM SB202190, a specific inhibitor of p38 phosphorylation. GAPDH was used as a standard for cellular protein input. (B) The transepithelial electrical resistance (TER) value in Caco-2 cells infected with OS-9-V5 (OS-9-V5) or uninfected cells (Control), in the presence or absence of 10 μM SB202190. Results are expressed as mean±SD. (C) Western blot analysis of claudin-1 and occludin protein levels in Caco-2 cells infected with a lentiviral vector expressing OS-9 (OS-9-V5) or an empty vector, in the presence (U0126) or absence (Control) of 10 μM U0126, a specific inhibitor of ERK1/2 phosphorylation. GAPDH was used as a standard for cellular protein input. (D) The TER value in Caco-2 cells infected with OS-9-V5 (OS-9-V5) or uninfected cells (Control), in the presence or absence of 10 μM U0126. Results are expressed as mean±SD. (# P<0.01 between OS-9-V5+SB202190 and OS-9-V5).
Discussion
In this study, we show that: (1) OS-9 significantly improves the epithelial barrier function of Caco-2 monolayer via up-regulation of the TJ protein level of occludin and claudin-1 but not ZO-1; (2) OS-9 does not regulate HIF-1α protein level in Caco-2 cells under either normoxia or hypoxia condition; (3) OS-9 markedly stimulates the phosphorylation of p38; (4) SB202190, a specific inhibitor of p38 phosphorylation, impairs the barrier protective function of OS-9 by reducing claudin-1 and occludin protein levels. Together, our results display a novel barrier-protective role of OS-9 on intestinal epithelial cells against hypoxia injury via a p38- but not HIF-1α-dependent pathway.
Mesenteric hypoxia/ischemia causes mucosal barrier injury with increased mucosal permeability [23]. Impaired intestinal barrier is often associated with changes in TJ protein expression after hypoxia/ischemia [24]. Consistently, our result showed decrease in claudin-1 and occludin of intestinal epithelial cells after hypoxia. Furthermore, the TER value fell significantly, indicating increased epithelial permeability and damage of barrier function.
OS-9 is a lectin required for efficient ubquitination of glycosylated endoplasmic reticulum-associated degradation (ERAD) substrates [16,25]. It interacts with meprin-B, N-copine, DC-STAMP and TRPV4, proteins associated with the endoplasmic reticulum, and plays a role in ER-to-Golgi transport of membrane proteins in mammalian and yeast cells [18,19,26-28]. It was not known whether OS-9 regulates other membrane proteins such as TJ proteins, a group of proteins tightly connected around cells [29,30]. In this study, we used loss-of-function and gain-of-function approaches to demonstrate that OS-9 protects the Caco-2 monolayer barrier function by up-regulating occludin and claudin-1 protein levels. It did not regulate ZO-1, which is a cytoplasmic plaque protein [29]. Our future studies will address how OS-9 modulates the expression as well as TJ functions of claudin-1 and occludin protein, including a possible role in protein transport to cell surface.
The HIF-1α signaling pathway has emerged as a major regulator of intestinal homeostasis and appears to manifest a dichotomous role in some gut disease [31]. It was reported that HIF-1α is injurious to intestinal barrier function, possibly via inhibiting the expression of TJ proteins [7-11,32]. Since previous studies identified OS-9 as a negative regulator of HIF-1α [14,33], we investigated the potential role of HIF-1α in the barrier-modulation function of OS-9. However, HIF-1α protein level was not affected by OS-9 knockdown or overexpression, suggesting that the barrier protective function of OS-9 in Caco-2 cells is mediated by a HIF-1α independent pathway. These results are consistent with the results of some other studies [22].
In an effort to identify the pathway utilized by OS-9 to regulate TJ proteins and exert its barrier protective function, we found that p38 and ERK1/2 phosphorylation both were stimulated by OS-9 overexpression. Using a specific inhibitor of p38 phosphorylation, we went on to show that activation of the p38 MAPK pathway is required for OS-9 induced up-regulation of claudin-1 and occludin protein levels and TER value. However, we did not obtain the similar results with p38 from the U0126, which is a specific inhibitor of ERK1/2 phosphorylation. This finding provided insight into the mechanism underlying the intestinal barrier protective functions of OS-9. Previously, activation of p38 MAPK pathway has been reported to mediate IEB dysfunction [34,35]. Furthermore, the p38 MAPK was originally identified as proteins activated in response to cellular stresses such as hyperosmolarity, arsenite or taxol and pro-inflammatory cytokines [36-38]. These studies provide further support for our results that OS-9 protect IEB by a p38 dependent pathway. Future studies will focus on the identification of molecules downstream of p38 that carry out the protective functions of OS-9 in intestine epithelial cells.
In conclusion, our results demonstrate that OS-9 maintains IEB function by up-regulating occludin and claudin-1 expressions. The signal molecule p38 is closely involved in the barrier protective mechanisms of OS-9 under hypoxia. It will be important to determine the precise mechanism of how OS-9 maintains the epithelial barrier function from hypoxia-induced injury.
Acknowledgements
This research was supported by grants from the National Natural Science Foundation of China (NSFC 81330013 and NSFC 81272078 to H.Y., NSFC 81300275 to L.H.S, NSFC 81270451 and 81470803 to W.D.X), and the Program of Changjiang Scholars and Innovative Research (IRT 13050 to HY).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Assimakopoulos SF, Scopa CD, Charonis A, Spiliopoulou I, Georgiou C. et al. Experimental obstructive jaundice disrupts intestinal mucosal barrier by altering occludin expression: beneficial effect of bombesin and neurotensin. J Am Coll Surg. 2004;198:748-757
2. Schenk M, Mueller C. The mucosal immune system at the gastrointestinal barrier. Best Pract Res Clin Gastroenterol. 2008;22:391-409
3. Colak T, Ozturk C, Polat A, Bagdatoglu O, Kanik A. et al. Effects of trapidil on intestinal mucosal barrier function and bacterial translocation after intestinal ischemia and reperfusion in an experimental rat model. Curr Ther Res Clin Exp. 2003;64:355-366
4. Arda-Pirincci P, Bolkent S. The role of epidermal growth factor in prevention of oxidative injury and apoptosis induced by intestinal ischemia/reperfusion in rats. Acta Histochem. 2014;116:167-175
5. Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med (Berl). 2007;85:1295-1300
6. Grenz A, Clambey E, Eltzschig HK. Hypoxia signaling during intestinal ischemia and inflammation. Curr Opin Crit Care. 2012;18:178-185
7. Rosenberger P, Khoury J, Kong T, Weissmuller T, Robinson AM. et al. Identification of vasodilator-stimulated phosphoprotein (VASP) as an HIF-regulated tissue permeability factor during hypoxia. FASEB J. 2007;21:2613-2621
8. Feinman R, Deitch EA, Watkins AC, Abungu B, Colorado I. et al. HIF-1 mediates pathogenic inflammatory responses to intestinal ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2010;299:G833-843
9. Kannan KB, Colorado I, Reino D, Palange D, Lu Q. et al. Hypoxia-inducible factor plays a gut-injurious role in intestinal ischemia reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2011;300:G853-861
10. Liu H, Li M, Wang P, Wang F. Blockade of hypoxia-inducible factor-1α by YC-1 attenuates interferon-γ and tumor necrosis factor-α-induced intestinal epithelial barrier dysfunction. Cytokine. 2011;56:581-588
11. Yang S, Yu M, Sun L, Xiao W, Yang X. et al. Interferon-gamma-induced intestinal epithelial barrier dysfunction by NF-kappaB/HIF-1alpha pathway. J Interferon Cytokine Res. 2014;34:195-203
12. Mahfoudh-Boussaid A, Zaouali MA, Hadj-Ayed K, Miled AH, Saidane-Mosbahi D. et al. Ischemic preconditioning reduces endoplasmic reticulum stress and upregulates hypoxia inducible factor-1alpha in ischemic kidney: the role of nitric oxide. J Biomed Sci. 2012;19:7
13. Kim HJ, Jeong JS, Kim SR, Park SY, Chae HJ. et al. Inhibition of endoplasmic reticulum stress alleviates lipopolysaccharide-induced lung inflammation through modulation of NF-kappaB/HIF-1alpha signaling pathway. Sci Rep. 2013;3:1142
14. Baek JH, Mahon PC, Oh J, Kelly B, Krishnamachary B. et al. OS-9 Interacts with Hypoxia-Inducible Factor 1α and Prolyl Hydroxylases to Promote Oxygen-Dependent Degradation of HIF-1α. Molecular Cell. 2005;17:503-512
15. Alcock F, Swanton E. Mammalian OS-9 is upregulated in response to endoplasmic reticulum stress and facilitates ubiquitination of misfolded glycoproteins. J Mol Biol. 2009;385:1032-1042
16. Bernasconi R, Pertel T, Luban J, Molinari M. A Dual Task for the Xbp1-responsive OS-9 Variants in the Mammalian Endoplasmic Reticulum: INHIBITING SECRETION OF MISFOLDED PROTEIN CONFORMERS AND ENHANCING THEIR DISPOSAL. Journal of Biological Chemistry. 2008;283:16446-16454
17. Satoh T, Chen Y, Hu D, Hanashima S, Yamamoto K. et al. Structural basis for oligosaccharide recognition of misfolded glycoproteins by OS-9 in ER-associated degradation. Mol Cell. 2010;40:905-916
18. Friedmann E, Salzberg Y, Weinberger A, Shaltiel S, Gerst JE. YOS9, the putative yeast homolog of a gene amplified in osteosarcomas, is involved in the endoplasmic reticulum (ER)-Golgi transport of GPI-anchored proteins. J Biol Chem. 2002;277:35274-35281
19. Litovchick L. A Selective Interaction between OS-9 and the Carboxyl-terminal Tail of Meprin beta. Journal of Biological Chemistry. 2002;277:34413-34423
20. Vourvouhaki E, Carvalho C, Aguiar P. Model for Osteosarcoma-9 as a potent factor in cell survival and resistance to apoptosis. Phys Rev E Stat Nonlin Soft Matter Phys. 2007;76:011926
21. Flashman E, McDonough MA, Schofield CJ. OS-9Another Piece in the HIF Complex Story. Molecular Cell. 2005;17:472-473
22. Bielinsky A-K, Brockmeier U, Platzek C, Schneider K, Patak P. et al. The Function of Hypoxia-Inducible Factor (HIF) Is Independent of the Endoplasmic Reticulum Protein OS-9. PLoS ONE. 2011;6:e19151
23. Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of mesenteric ischemia/reperfusion: a review. Acta Cir Bras. 2005;20:336-343
24. Li Q, Zhang Q, Wang C, Liu X, Qu L. et al. Altered distribution of tight junction proteins after intestinal ischaemia/reperfusion injury in rats. J Cell Mol Med. 2009;13:4061-4076
25. Alcock F, Swanton E. Mammalian OS-9 Is Upregulated in Response to Endoplasmic Reticulum Stress and Facilitates Ubiquitination of Misfolded Glycoproteins. Journal of Molecular Biology. 2009;385:1032-1042
26. Jansen BJH, Eleveld-Trancikova D, Sanecka A, van Hout-Kuijer M, Hendriks IAM. et al. OS9 interacts with DC-STAMP and modulates its intracellular localization in response to TLR ligation. Molecular Immunology. 2009;46:505-515
27. Wang Y, Fu X, Gaiser S, Kottgen M, Kramer-Zucker A. et al. OS-9 Regulates the Transit and Polyubiquitination of TRPV4 in the Endoplasmic Reticulum. Journal of Biological Chemistry. 2007;282:36561-36570
28. Nakayama T, Yaoi T, Kuwajima G, Yoshie O, Sakata T. Ca2(+)-dependent interaction of N-copine, a member of the two C2 domain protein family, with OS-9, the product of a gene frequently amplified in osteosarcoma. FEBS Lett. 1999;453:77-80
29. Yu QH, Yang Q. Diversity of tight junctions (TJs) between gastrointestinal epithelial cells and their function in maintaining the mucosal barrier. Cell Biol Int. 2009;33:78-82
30. Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213-1228
31. Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281-287
32. Shah YM, Ito S, Morimura K, Chen C, Yim SH. et al. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology. 2008;134:2036-2048 2048 e2031-2033
33. Koh MY, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends in Biochemical Sciences. 2008;33:526-534
34. Samak G, Narayanan D, Jaggar JH, Rao R. CaV1.3 channels and intracellular calcium mediate osmotic stress-induced N-terminal c-Jun kinase activation and disruption of tight junctions in Caco-2 CELL MONOLAYERS. J Biol Chem. 2011;286:30232-30243
35. Elamin E, Masclee A, Troost F, Pieters HJ, Keszthelyi D. et al. Ethanol Impairs Intestinal Barrier Function in Humans through Mitogen Activated Protein Kinase Signaling: A Combined In Vivo and In Vitro Approach. PLoS ONE. 2014;9:e107421
36. Lee MY, Jung SC, Lee JH, Han HJ. Estradiol-17beta protects against hypoxia-induced hepatocyte injury through ER-mediated upregulation of Bcl-2 as well as ER-independent antioxidant effects. Cell Res. 2008;18:491-499
37. Nguyen DT, Kebache S, Fazel A, Wong HN, Jenna S. et al. Nck-dependent activation of extracellular signal-regulated kinase-1 and regulation of cell survival during endoplasmic reticulum stress. Mol Biol Cell. 2004;15:4248-4260
38. Darling NJ, Cook SJ. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim Biophys Acta. 2014;1843:2150-2163
Author contact
![]() Corresponding authors: Hua Yang, M.D., Ph.D. or Weidong Xiao, M.D., Ph.D.. Department of General Surgery, Xinqiao Hospital, Third Military Medical University, Chongqing 400037, China, Phone: (0086)23-687-55705 (Office), E-mail: huayangedu.cn or xiaoweidongedu.cn
Corresponding authors: Hua Yang, M.D., Ph.D. or Weidong Xiao, M.D., Ph.D.. Department of General Surgery, Xinqiao Hospital, Third Military Medical University, Chongqing 400037, China, Phone: (0086)23-687-55705 (Office), E-mail: huayangedu.cn or xiaoweidongedu.cn
Received 2014-10-10
Accepted 2015-2-18
Published 2015-4-27
