ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2016; 12(2):198-209. doi:10.7150/ijbs.13716 This issue Cite
Research Paper
Cyclosporine A Suppressed Glucose Oxidase Induced P53 Mitochondrial Translocation and Hepatic Cell Apoptosis through Blocking Mitochondrial Permeability Transition
1. Department of Toxicology, the Ministry of Education Key Lab of Hazard Assessment and Control in Special Operational Environment, Shaanxi Provincial Key Lab of Free radical biology and medicine, School of Public Health, The Fourth Military Medical University, Xi'an, 710032, P. R. China
2. Department of Cardiology, Xijing Hospital, the Fourth Military Medical University, Xi'an, Shaanxi, 710032, China
Abstract
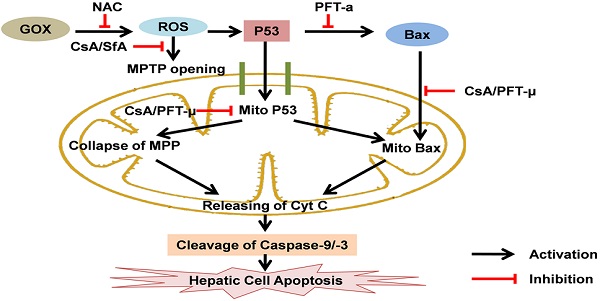
P53 is known as a transcription factor to control apoptotic cell death through regulating a series of target genes in nucleus. There is accumulating evidences show that p53 can directly induce cell apoptosis through transcription independent way at mitochondria. However, the mechanism by which p53 translocation into mitochondria in response to oxidative stress remains unclear. Here, glucose oxidase (GOX) was used to induce ROS generation in HepG2 cells and liver tissues of mice. The results showed that p53 was stabilized and translocated to mitochondria in a time and dose dependent manner after GOX exposure. Interestingly, as an inhibitor of mitochondrial permeability transition, cyclosporine A (CsA) was able to effectively reduce GOX mediated mitochondrial p53 distribution without influencing on the expression of p53 target genes including Bcl-2 and Bax. These indicated that CsA could just block p53 entering into mitochondria, but not affect p53-dependent transcription. Meanwhile, CsA failed to inhibit the ROS generation induced by GOX, which indicated that CsA had no antioxidant function. Moreover, GOX induced typical apoptosis characteristics including, mitochondrial dysfunction, accumulation of Bax and release of cytochrome C in mitochondria, accompanied with activation of caspase-9 and caspase-3. These processions were suppressed after pretreatment with CsA and pifithrin-μ (PFT-μ, a specific inhibitor of p53 mitochondrial translocation). In vivo, CsA was able to attenuate p53 mitochondrial distribution and protect mice liver against from GOX mediated apoptotic cell death. Taken together, these suggested that CsA could suppress ROS-mediated p53 mitochondrial distribution and cell apoptosis depended on its inhibition effect to mitochondrial permeability transition. It might be used to rescue the hepatic cell apoptosis in the patients with acute liver injury.
Keywords: Cyclosporine A, Reactive oxygen species, p53, mitochondrial permeability transition, liver disease, cell apoptosis
