ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2016; 12(5):594-606. doi:10.7150/ijbs.14151 This issue Cite
Research Paper
Revealing the Effects of the Herbal Pair of Euphorbia kansui and Glycyrrhiza on Hepatocellular Carcinoma Ascites with Integrating Network Target Analysis and Experimental Validation
1. Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing 100700, China.
2. College of Pharmacy, Fujian University of Traditional Chinese Medicine, Fuzhou 350122, China.
*These authors contribute equally to this study.
Abstract
Although the herbal pair of Euphorbia kansui (GS) and Glycyrrhiza (GC) is one of the so-called "eighteen antagonistic medicaments" in Chinese medicinal literature, it is prescribed in a classic Traditional Chinese Medicine (TCM) formula Gansui-Banxia-Tang for cancerous ascites, suggesting that GS and GC may exhibit synergistic or antagonistic effects in different combination designs. Here, we modeled the effects of GS/GC combination with a target interaction network and clarified the associations between the network topologies involving the drug targets and the drug combination effects. Moreover, the "edge-betweenness" values, which is defined as the frequency with which edges are placed on the shortest paths between all pairs of modules in network, were calculated, and the ADRB1-PIK3CG interaction exhibited the greatest edge-betweenness value, suggesting its crucial role in connecting the other edges in the network. Because ADRB1 and PIK3CG were putative targets of GS and GC, respectively, and both had functional interactions with AVPR2 approved as known therapeutic target for ascites, we proposed that the ADRB1-PIK3CG-AVPR2 signal axis might be involved in the effects of the GS-GC combination on ascites. This proposal was further experimentally validated in a H22 hepatocellular carcinoma (HCC) ascites model. Collectively, this systems-level investigation integrated drug target prediction and network analysis to reveal the combination principles of the herbal pair of GS and GC. Experimental validation in an in vivo system provided convincing evidence that different combination designs of GS and GC might result in synergistic or antagonistic effects on HCC ascites that might be partially related to their regulation of the ADRB1-PIK3CG-AVPR2 signal axis.
Keywords: herb-herb combination, Euphorbia kansui, Glycyrrhiza, hepatocellular carcinoma ascites, network pharmacology.
Introduction
Hepatocellular carcinoma (HCC) represents one of the most frequent malignancies, and the incidence of HCC is increasing worldwide [1]. Although great progress has been made in medical and surgical treatments for this disease, its prognosis has not conspicuously improved in recent years [2]. As one of the severe complications of HCC, malignant ascites, which is an abnormal accumulation of fluid in the peritoneal cavity caused by tumor infiltration or secretion [3], is a recognized indication of end-stage events in this malignancy and often deteriorates the patient's quality of life [4]. Currently, several treatments, such as salt restriction, peritoneal catheter drainage, intraperitoneal chemotherapy and paracentesis, have been performed for the remission of ascites [5]. However, the therapeutic efficacies of these treatments are unsatisfactory, and the treatment of malignant ascites remains a difficult problem to solve. Therefore, it is extremely necessary to develop novel and effective therapeutic strategies for the management of HCC-induced malignant ascites to improve patient quality of life.
Traditional Chinese medicine (TCM) is one of the most popular complementary and alternative medicine modalities worldwide and has been practiced in China for more than 2,000 years. TCM is increasingly used for the treatment of malignant ascites. For example, the roots of Euphorbia kansui T. N. Liou ex T. P. Wang (Gansui, GS), which is recorded in the Shennong-Bencao, has been used for centuries in China for the treatment of ascites, edema and asthma [6]. Recent studies have demonstrated that GS also has a variety of pharmacological actions including anti-fertility, anti-viral, and immune system-regulating effects. Moreover, GS is effective in the treatment of cancer, pancreatitis, and intestinal obstruction [7]. However, GS is strongly toxic to the liver and kidneys and can induce symptoms such as diarrhea, stomachache, dehydration and respiratory failure [8], which have seriously restricted the clinical application of GS.
Glycyrrhiza (Licorice, Gancao, GC) is another Chinese herb. GC is the root of Glycyrrhiza uralensis Fisch. or Glycyrrhiza glabra L., Leguminosae and has been prescribed in many TCM formulas for its medical activities, which include anti-inflammatory, immunoregulatory, and anti-allergic effects [9]. In TCM, GC is commonly combined with other herbs into a single prescription and functions as a unique “guide drug” to enhance the effectiveness of other ingredients or to reduce toxicity and improve flavor in almost half of all Chinese herbal formulas [10, 11]. In the Chinese medicinal literature, GS and GC compose one herbal pair of the so-called "eighteen antagonistic medicaments", in which two herbs are mutually incompatible and thus theoretically should not be applied simultaneously. However, the GS/GC combination is prescribed in a classic TCM formula, Gansui-Banxia-Tang, which has been used for the treatment of cancerous ascites [12, 13], which implies that the two herbs may exhibit synergistic or antagonistic effects in different combination designs.
TCM emphasizes the integrity of the human body and the interaction between human individuals and their environment. TCM herbs are multi-component and multi-target agents that essentially achieve therapeutic effectiveness by collectively modulating the molecular network of the body system using its active components [14]. System biology is a useful tool for explaining and predicting various events related to the efficacy of drugs [15] and is valuable for evaluating the rationality and compatibility of herbs or prescriptions. Because herbs manifest their actions via their targets, the effects of herb-herb combinations should depend on the interactions of their targets in a network manner. To the best of our knowledge, the rationality of the herbaceous compatibility of GS and GC has not been fully elucidated. In the current study, we modeled the effects of the GS/GC combination with a target interaction network and clarified the associations between the network topologies of the drug targets and the drug combination effects.
Materials and Methods
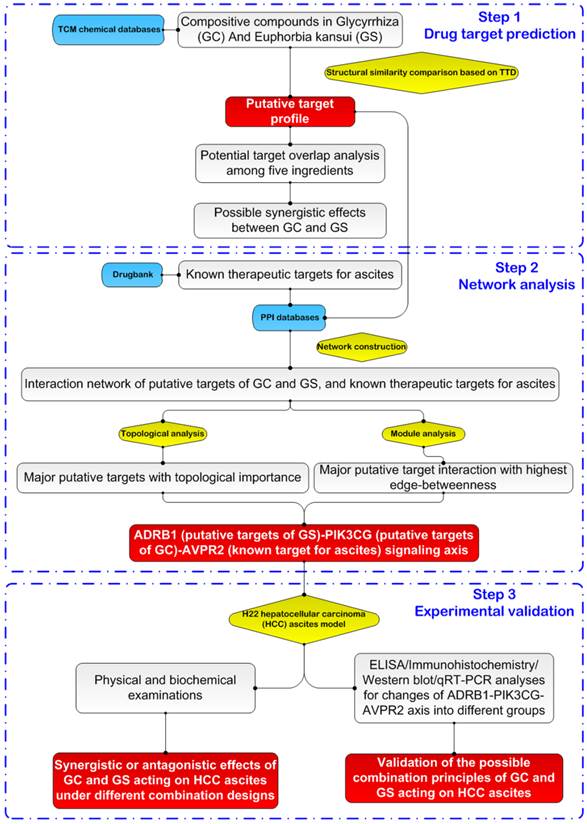
The technical strategy of this study is presented in Figure 1.
Data preparation
Structural information about the chemical components of GS and GC
The TCM Database@Taiwan [16] (http://tcm.cmu.edu.tw/, Updated in Jun 28, 2012), which is currently the largest non-commercial TCM database in the world, was used to collect the structural information (*.mol or *.sdf files) of 4 GS compounds and 82 GC compounds.
Protein-protein interaction (PPI) data
PPI data were collected from the following eight existing PPI databases [17-24]. The interactions used in this study include direct (physical) and indirect (functional) associations derived from high-throughput experiments, genomic context, co-expression correlation and manually extraction from the published data. Detailed information about these PPI databases is provided in Supplementary Table S1.
Prediction of putative targets for GS and GC
Putative targets of GS and GC were predicted based on structural similarity comparisons as described in our previous study [25]. Detailed information about the prediction method of putative targets for GS and GC is provided in Supplementary Material.
Network construction and analysis
The PPI data about the putative targets of GS and GC, known therapeutic targets of ascites and other human proteins were used to construct the drug target PPI network which was visualized by Navigator software (version 2.2.1).
A schematic diagram of the systems biology-based strategies for revealing the combination principles of the herbal pair of Euphorbia kansui (GS) and Glycyrrhiza (GC) used in the therapy of hepatocellular carcinoma ascites based on target network analysis and experimental validation.
According to the previous study of Li et al. [26], we defined a node as a hub if its degree is greater than twice the median degree of all nodes in the network. Subsequently, the PPIs among the hubs were used to construct the hub network. Moreover, four topological features, 'Degree', 'Node betweenness', 'Closeness' and 'K value' (definitions are provided in Supplementary Material), were calculated to identify the major putative targets of GS and GC. These targets were defined as those with values of the four features that were higher than the corresponding median values.
Molecular docking simulation
Molecular docking simulation was performed to evaluate the direct binding efficiencies of the main chemical components of GS and GC (Supplementary Figure S1 and Table S2) with ADRB1 and PIK3CG, respectively, using the electronic high-throughput screening (eHiTS) docking module, which is a flexible ligand docking system [27]. The structures of ADRB1 and PIK3CG were obtained from the previous studies of Cushing et al. [28] and Warne et al [29]. The docking score represents the direct binding energy of the ligand to the receptor in units of log Kd. The higher the absolute value of a docking score is, the stronger the direct binding efficiency of the ligand to the receptor. According to the scoring system of the eHiTS algorithm, the direct binding efficiencies of compound-target pairs were classified into three levels: strong, i.e., the absolute value of the docking score is higher than 5.66 for GS or 5.70 for GC; middle, i.e., the absolute value of the docking score ranges from 4.66 to 5.66 for GS or 4.70 to 5.70 for GC; and weak, i.e., the absolute value of the docking score is lower than 4.66 for GS or 4.70 for GC.
Experimental validation
H22 mouse HCC ascites models were used to examine the synergistic or antagonistic effects of the actions of GS and GC on HCC ascites in different combination designs and to validate their possible combination principles (ADRB1-PIK3CG-AVPR2 signal axis).
Drug preparation
GC (Lot: 120713) and GS (Lot: 121106) were both purchased from Anhui Fengyuan Tongling Herbal Pieces Co. Ltd. High performance liquid chromatography-mass spectrometry (HPLC-MS) was used to determine the main chemical components of GS and GC. As shown in Supplementary Figure S1 and Table S2, 11 and 36 chemical components were identified in GS and GC, respectively.
GC was decocted to a mother liquor with a dosage of 1.34 g/kg. In brief, 20.1 g GC was soaked in a ten-fold volume of water for 1 h, heated with an electric heater and decocted for 1.5 h. After standing and filtrating, the residue was decocted again with the same amount of water for 1 h. After standing and filtrating, the solution was combined, concentrated and distillated at 60 ℃ to a designated volume (300 mL) and kept at 4 ℃. GS was crushed with a grinder and filtered with an 80-mesh sifter. The powder was retained for further use. The GS powder and GC decoctions (mother liquors) were mixed at different ratios and heated before intragastric administration to the different groups.
Cell culture and animal model
The experimental protocol was approved by Medical Experimental Animal Care Committee of Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences.
The murine H22 HCC ascitic cell line was purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). H22 cells were cultured in RPMI-1640 medium supplemented with 2 mM L-glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10% FCS at 37 ℃ under a humidified atmosphere of 5% CO2, and the cultures were passaged every 2 or 3 days.
Male BALB/C mice (6 weeks old) weighing 18-22 g were obtained from the Laboratory Animal Center of China Academy of Chinese Medical Sciences (Beijing, China) and were acclimatized for 1 week prior to use in the experiment. All mice were bred in laminar flow cabinets under specific pathogen-free conditions.
The H22 HCC ascites model was prepared according to previous studies [35-38]. In brief, the needle was inserted into the left lower abdomen, and H22 cells were inoculated intraperitoneally. Each mouse was inoculated with 1×10 7 H22 cells. The procedure was not associated with mortality or morbidity.
Grouping and treatment
In our previous study, we investigated the synergistic and antagonistic effects of GS/GC combinations with different ratios in the treatment of HCC ascites based on uniform design (the data have not been published; please see details in Supplementary Tables S3 and S4). In this study, all H22 HCC ascites models were divided randomly into 4 groups (10 mice in each group): (1) the model control group; (2) the GS alone group (0.93 g/kg; the diuretic effect of GS on HCC ascites was strongest); (3) the GS/GC_synergy group (GS/GC ratio was 1/0.39, i.e., 0.69 g/kg to 0.27 g/kg this combination had the strongest effects in treating HCC ascites) (see Group 5 in Supplementary Tables S3 and S4); and (4) the GS/GC_antagonism group (GS/GC was 1/1.11, i.e., 0.93 g/kg to 1.03 g/kg, the strongest antagonistic effects in treating HCC ascites) (see Group 6 in Supplementary Tables S3 and S4).
The H22 HCC ascites mice in the GS, GS/GC_synergy and GS/GC_antagonism groups were intragastrically administered the appropriate doses of GS and GC mentioned above. The mice in the model control group received an equal volume of normal saline. The treated mice were cared for under standard laboratory conditions and used in different experiments. On the 10th day, the surviving mice were sacrificed by cervical dislocation. The mice were weighed, and abdominal circumferences were measured. Their ascites and blood were collected for physical examinations and enzyme-linked immunosorbent assay (ELISA). Next, the liver and kidney tissues were immediately dissected and fixed in paraformaldehyde overnight for hematoxylin-eosin (H&E) staining to detect the toxic effects of GS and GC combinations. Specifically, the kidney tissues were used for western blot and quantificational real-time polymerase chain reaction (qRT-PCR) analyses.
Physical examinations
To measure the ascites volume, the ascites fluid was collected from the opened abdominal wall following cervical dislocation and measured via syringe.
Serum biochemical analysis
The serum biochemical analyses included alanine aminotransferase (ALT, related to the liver), aspartate amino transferase (AST, related to the liver), creatinine (CREA, related to the kidney), and blood urea nitrogen (BUN, related to the kidney) and were determined by routine kinetic and fixed-rate colorimetric methods using common hospital laboratory equipment. All assays were conducted in triplicate using fresh serum.
ELISA
The ascites and sera were diluted to different concentrations and analyzed using mouse ADRB1, PIK3CG, AVPR2 ELISA kits obtained from Beijing Xinfangcheng Biotechnology (Beijing, China) following the manufacturer's instructions.
Immunohistochemical staining
Expression patterns and subcellular localizations of ADRB1, PIK3CG and AVPR2 proteins in kidney tissues obtained from different groups were examined by immunohistochemical staining according to our previous studies [34]. The following antibodies were used in this study: anti-ADRB1 (lot: GR174027-3, Abcam; Cambridge, MA), anti-PIK3CG (lot: GR104307-9, Abcam; Cambridge, MA) and anti-AVPR2 (lot: 9A08V2, Bioss; Beijing, China).
Western blot analysis
The western blot procedures are described in our previous studies [29, 39]. The primary antibodies included anti-ADRB1 (lot: GR174027-3, Abcam; Cambridge, MA), anti-PIK3CG (lot: GR104307-9, Abcam; Cambridge, MA), anti-AVPR2 (lot: GR146393-1, Abcam; Cambridge, MA), anti-pPIK3CG (Phospho-Ser1101, lot: AD 071823, Biorbyt; Cambridge, MA), anti-pAKT (Phospho-S473, lot: GR-139335-14, Abcam; Cambridge, MA), and anti-β-actin (lot: I1o813, Beijing TransGen Biotech Co. Ltd., Beijing, China). All experiments were performed in triplicate. The mean normalized protein expressions ± SEs were calculated from independent experiments.
QRT-PCR
The qRT-PCR procedures are described in our previous studies [40-41]. GAPDH was used as the internal control for the normalization and quantification of the target gene expression. The primer sequences for the ADRB1, PIK3CG and AVPR2 mRNAs are listed in Supplementary Table S5. The relative quantification of the target gene expression was evaluated using the comparative cycle threshold (CT) method as in a previous study [42]. All experiments were performed in triplicate. The mean normalized gene expressions ± the SEs were calculated from independent experiments.
Statistical analysis
The SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA) for Windows and SAS 9.1 (SAS Institute, Cary, NC) were used for the statistical analysis. The continuous variables are expressed as the means ± the S.E. For comparisons of the means of multiple groups, one-way ANOVAs followed by LSD tests were performed. Differences were considered statistically significant at P <0.05.
Results & Discussion
Putative targets for GS and GC
Based on our previously developed target prediction system [25], the numbers of putative targets for GS and GC were 13 and 102, respectively. Eleven of the putative targets of the two herbs overlapped, which was suggestive of potential interactions between GS and GC. Detailed information about putative targets is provided in Supplementary Table S6.
Combination principles of the herbal pair GS and GC in its action on HCC ascites
To shed light on the combination principles of the actions of herbal pair GS and GC on the HCC ascites, we first constructed a drug target network using the PPI information of the putative targets of GS and GC, known therapeutic targets for ascites and other human proteins. The network consisted of 3955 nodes and 7735 edges. For detailed information about this network, see Supplementary Table S7.
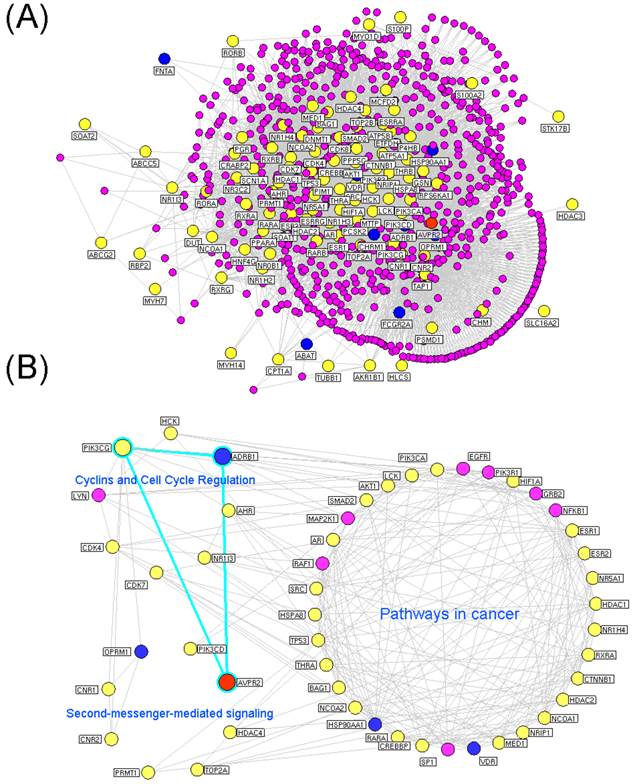
The hubs in a drug target network have extremely high levels of degree and tend to encode essential genes. According to the previous studies of Li et al [26] and our research group [38-40], we identified a node as a hub if its degree was more than twice the median degree of all nodes in the network. Consequently, 936 nodes were identified as hubs. Next, we constructed the interaction network of these hubs, which consisted of 934 nodes and 3976 edges as shown in Figure 2A. Please see the detailed information about this network provided in Supplementary Table S8.
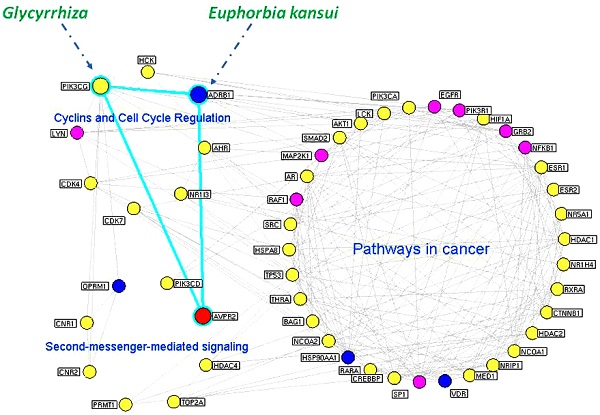
Putative targets of the Euphorbia kansui (GS) and Glycyrrhiza (GC) interaction networks. (A) Interaction network of hub nodes in the interaction network of the putative targets of GS and GC. (B) Three functional modules of the interaction network of the major putative targets of GS and GC. The yellow and blue nodes denote the putative targets of GS and GC, respectively. The red node denotes the known therapeutic target for the treatment of ascites. The purple nodes denote other human proteins that directly interact with the putative targets of GS and GC. The blue edges denote the ADRB1-PIK3CG-AVPR2 signal axis.
Based on the hub interaction network, four topological features, i.e., 'Degree,' 'Node betweenness', 'Closeness' and 'K value' were calculated to screen the major putative targets of GS and GC. In total, we identified 3 and 39 major putative targets for GS and GC, respectively (Supplementary Table S9).
Modularity has been reported to be another important aspect of a PPI network. Nodes that are highly interconnected within the network are usually involved in the same biological modules or pathways. Using a Markov clustering algorithm, we divided the interaction network of major putative targets into 3 functional modules containing 36, 8 and 8 nodes (Figure 2B). According to an enrichment analysis based on the GO annotation system and the KEGG pathway, the biggest functional module was significantly associated with pathways in cancer. The other modules were involved in cyclins and cell cycle regulation and second-messenger-mediated signaling.
Because an interaction with a high 'edge-betweenness' has been defined as a bottleneck with many 'shortest paths' going through it that controls the rate of information flow, we further calculated the 'edge-betweenness' of each interaction in the network of major putative targets. As shown in Supplementary Table S10, the interaction between ADRB1 and PIK3CG exhibited the greatest edge-betweenness value (52.08), which suggested its importance in connecting the different modules in the network.
As illustrated in Figure 2B, ADRB1 and PIK3CG were major putative targets of GS and GC, respectively, and they both had functional interactions with AVPR2 according to the information of existing interaction databases (ADRB1-AVPR2: reaction from String/HAPPI; ADRB1-PIK3CG: direct interaction from HPRD; PIK3CG-AVPR2: reaction from I2D). AVPR2 has been recognized as a known therapeutic target for ascites; therefore, we propose that the effects of the GS/GC combination on ascites might be associated with the ADRB1-PIK3CG-AVPR2 signal axis.
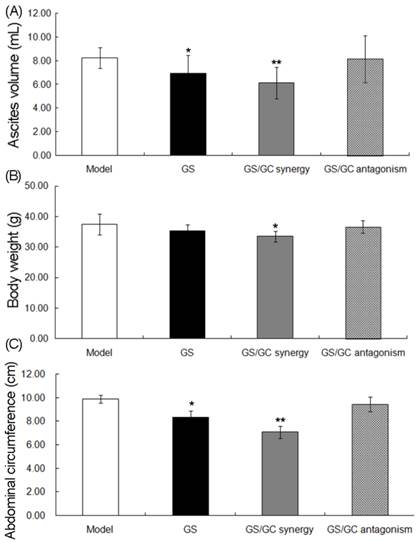
Changes in ascites volumes. (A) body weights (B) and abdominal circumferences (C) in the H22 HCC ascites model group (n=9), Euphorbia kansui (GS) alone treatment group (n=8), GS/Glycyrrhiza (GC) combination_synergistic effect group (GS/GC_synergy, n=8) and GS/GC combination_antagonistic effect group (GS/GC_antagonism, n=9). The data are represented as the means ± the S.E. '*' P<0.05 compared with the model group, '**' P<0.01 compared with the model group.
Prior to the experimental validation, a molecular docking simulation was performed using the eHiTS software to investigate whether the main chemical components of GS and GC could bind directly to ADRB1 and PIK3CG, respectively. This structure-based method has been reported to play important roles in the discovery of ligand-protein interactions and the elucidation of binding mechanisms. Consequently, seven compound-target pairs were deleted either because their structural information was unavailable or negative results were output from eHiTS. The positive docking results obtained from the eHiTS software are summarized in Supplementary Table S11. Among the 12 chemical components of GS, 8 (66.67%) exhibited middle-strong binding efficiencies with ADRB1. Regarding to 30 chemical components of GC, 20 (66.67%) exhibited middle-strong binding efficiencies with PIK3CG. These findings imply that two-thirds of the chemical components of GS and GC might have strong potential to directly bind with ADRB1 and PIK3CG, respectively; this supposition should be validated experimentally in further studies because molecular docking remains a computer simulation procedure that is used to predict the conformation of a receptor-ligand complex.
Experimental Validation
The synergistic and antagonistic effects of GS and GC acting on HCC ascites under different combination designs
Compared to the model control group, the ascites volumes (Figure 3A), body weights (Figure 3B) and abdominal circumferences (Figure 3C) in the GS, GS/GC_synergy and GS/GC_antagonism groups were all reduced. Notably, the differences in these three physical features between the GS/GC_synergy and model control groups were greater than those between the GS and model control groups (Figure 3). However, there were no significant differences between the GS/GC_antagonism and model control groups in terms of ascites volume, body weight or abdominal circumference (all P>0.05, Figure 3).
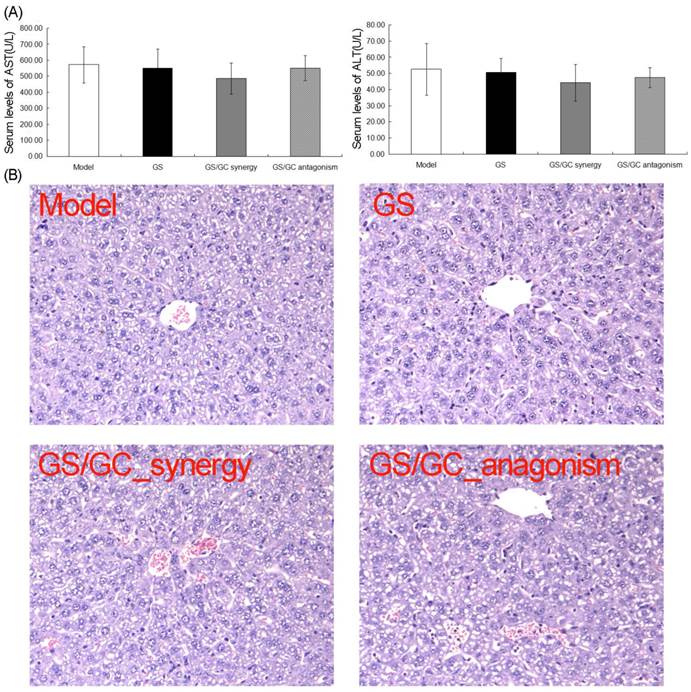
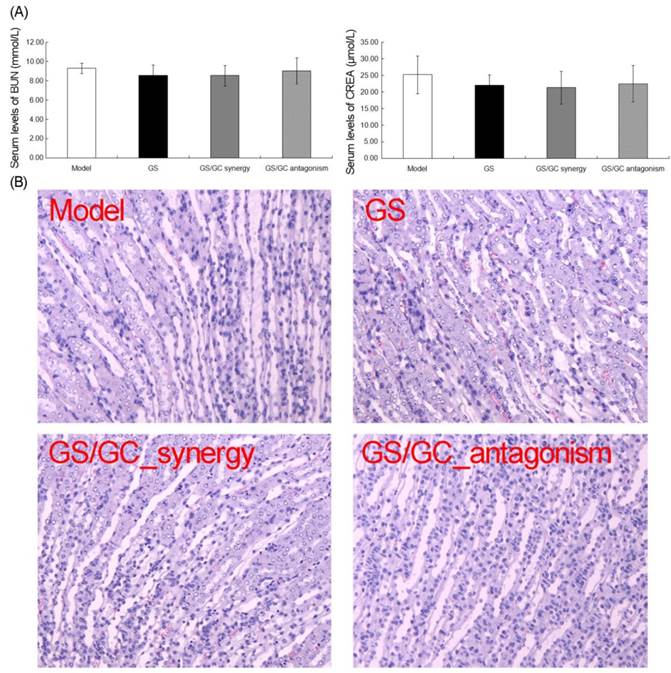
Because GS is strong toxic to the liver and kidney (which has seriously restricted its clinical application), and GC is extensively used to enhance the effectiveness and reduce the toxicity of other drugs in TCM, we sought to observe the changes in toxicity of the GS/GC combinations of different ratios on the HCC ascites mice. Figure 4A illustrates the serum levels of two enzymes related to liver metabolism. No increases in AST or ALT were observed in response to any of the combinations of GS/GC compared to the ascites model group, although these enzymes did decrease slightly in the GS/GC_synergy group (Figure 4A). Additionally, evaluation of the liver sections stained with H&E revealed that GS and GS/GC combination treatment did not aggravate the damage of ascites to the liver tissues, which was shown in the model control group, such as inflammation or necrosis (Figure 4B). Moreover, the kidney toxicities of the different drug treatments were evaluated in H22 HCC ascites mice with serum biochemical and histological analyses. We found that the serum levels of BUN and CREA were not altered significantly in any of the treatment groups compared to the model control group (Figure 5A). Furthermore, no specific pathological symptoms were observed in any of the groups. Nearly all the kidney cells maintained normal structures, the degeneration and necrosis of the glomerulus and renal tubes induced by toxicants and immunological factors were not observed in any region of the kidney, no edema or swelling were observed in the renal tubes of the kidney tissue (Figure 5B). These results suggest that the GS/GC combinations at different ratios did not aggravate the pathological changes in either the livers or kidneys of the H22 HCC ascites mice.
The GS/ GC combination attenuates HCC ascites partially by regulating the ADRB1-PIK3CG-AVPR2 signal axis
We further detected the regulatory effects of the GS/GC combinations of different ratios on the expressions of ADRB1, PIK3CG and AVPR2 at both the protein and mRNA levels in the ascites, sera and kidney tissues of the different groups by ELISA, immunohistochemistry, western blot and qRT-PCR analyses.
Liver toxicity in the H22 HCC ascites mice. (A) No increases in AST or ALT levels were observed in response to any of combinations of GS/GC, although these levels did decrease slightly in the GS/GC_synergy group. (B) Evaluation of the liver sections stained with Hematoxylin-eosin (H&E) revealed that GS and GS/GC combination treatment did not aggravate the damage of ascites to the liver tissues, which was shown in the model control group, such as inflammation or necrosis. H&E staining, 200×magnification.
Kidney toxicity in the H22 HCC ascites mice. (A) The serum levels of BUN and CREA were not significantly altered in any of the treatment groups. (B) No specific pathological symptoms were detected in any of the groups. Almost all the kidney cells maintained their normal structures, degeneration and necrosis of the glomeruli and renal tubes induced by toxicants and immunological factors were not observed in any region of the kidney, and no edema or swelling were observed in the renal tubes of the kidney. Hematoxylin-eosin (H&E) staining, 200×magnification.
ADRB1 (official full name: adrenoceptor beta 1, ADRβ1) is an adrenergic receptor that belongs to the prototypic family of guanine nucleotide-binding regulatory protein-coupled receptors that mediate the physiological effects of the hormone epinephrine and the neurotransmitter norepinephrine [41]. Previous animal studies have revealed direct relationships of the lymph flow rate and liquid clearance with adrenergic receptors activity in the lymphatic system [42]. It also has been reported that beta-adrenergic receptors may play a role in ascites susceptibility [43]. In this study, ADRB1 was predicted to be a major putative target of GS. As shown in Figure 6A, the positive staining of ADRB1 protein was localized in cellular cytoplasm of kidney tissues. Statistically, immunohistochemistry, western blot and qRT-PCR all showed that the ADRB1 protein and mRNA levels in the ascites, sera and kidney tissues of the GS group were significantly decreased compared to the model control group (all P<0.01, Figure 6B and Figure 7A), which implies a regulatory effect of GS on ADRB1. Notably, the ADRB1 protein and mRNA levels in the GS/GC_antagonism group were higher than those in the GS group (all P<0.01, Figure 6B and Figure 7A), suggesting that GC reverse the downregulation of ADRB1 caused by GS treatment when the ratio of GS and GC was 1:1.11. Moreover, we found that the GS/GC combination ratio of 1:0.39 (GS/GC_synergy group) also dramatically reduced the expression of ADRB1 in the ascites, sera and kidney tissues (all P<0.01, Figure 6B and Figure 7A), but no significant differences compared to the GS/GC_antagonism group, were noted, which suggests that GC might not have affected the regulation of ADRB1 expression by of GS in this combination design.
Expression patterns and subcellular localizations of ADRβ1. (A), PI3Kγ (B) and AVPR2 (C) protein in kidney tissues of the different groups as detected by immunohistochemistry. The data are represented as the means ± the S.E. '*' and '**' P<0.05 and P<0.01, respectively, compared with the model group; '#' and '##' P<0.05 and P<0.01, respectively, compared with the GS group.
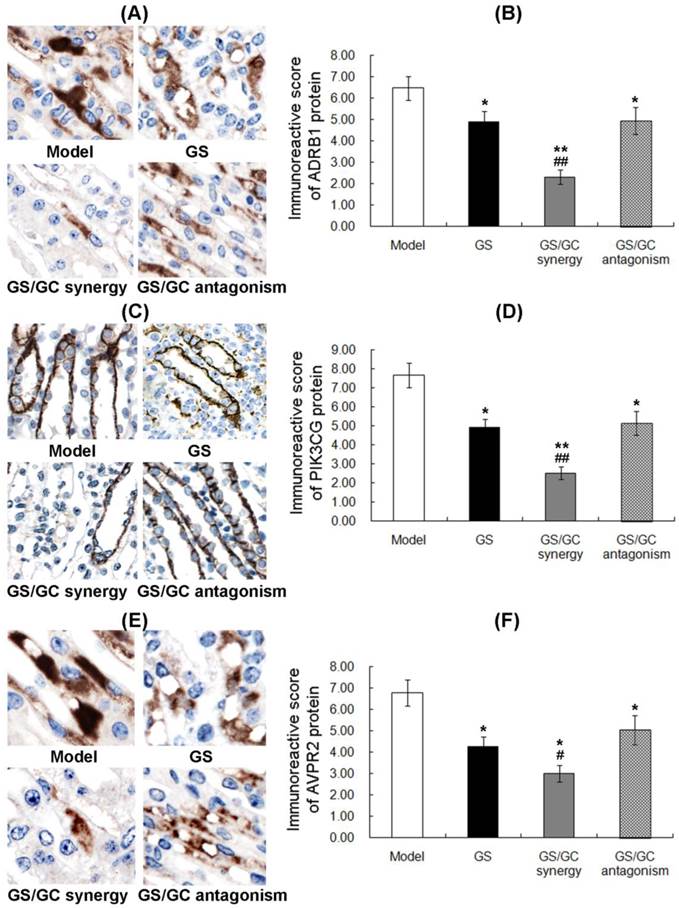
Phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit gamma (PIK3CG) is an enzyme that phosphorylates phosphoinositides on the 3-hydroxyl group of the inositol ring. PIK3CG is an important modulator of extracellular signals, including those elicited by E-cadherin-mediated cell-cell adhesion, which plays an important role in the maintenance of the structural and functional integrity of the epithelia. Functionally, PIK3CG is responsible for coordinating a diverse range of cellular processes that includes proliferation, cell survival, degranulation, vesicular trafficking and cell migration [44]. As a main component of PI3K/AKT pathway that has been reported to be involved in carcinogenesis and the formation of malignant ascites [45, 46], PIK3CG may also contribute to these pathogeneses. Here, we identified PIK3CG as a major putative target of GC. The immunohistochemistry showed the cytoplasmic staining of PIK3CG protein in kidney tissues (Figure 6C). Our data also indicated that the expression levels of PIK3CG protein and mRNA in the ascites, sera and kidney tissues of the GS/GC_synergy group were significantly lower than those in the model control and GS groups, which suggests a regulatory effect of GC on PIK3CG. However, the levels of PIK3CG in the ascites, sera and kidney tissues of the GS/GC_antagonism group exhibited no significant changes compared to the model control or GS groups possibly because the dosage of GC in this group was too low to downregulate its putative target (Figure 6D and Figure 7B). More interestingly, similar results regarding the changes in phosphorylated PIK3CG (p-PIK3CG) and p-AKT in the different groups are presented in Supplementary Figure S2 and imply a role of GC in the activation of the PI3K/AKT pathway.
Arginine vasopressin receptor 2 (AVPR2) belongs to the seven-transmembrane domain G protein-coupled receptor (GPCR) superfamily and couples to Gs to stimulate adenylate cyclase. AVPR2 expressed in the kidney tubule, predominantly in the distal convoluted tubule and collecting ducts, where its primary function is to respond to the pituitary hormone arginine vasopressin (AVP) by stimulating mechanisms that concentrate the urine and maintain water homeostasis in the organism [47]. Notably, AVPR2 has been recognized as a therapeutic target for the treatment of malignant ascites. Increasing evidence indicates that several AVPR2 antagonists, such as satavaptan, tolvaptan and lixivaptan, can improve the control of ascites in cirrhosis [48-50]. More interestingly, our network analysis identified interactions between ADRB1 and AVPR2 and between PIK3CG and AVPR2, which suggest a role of the ADRB1-PIK3CG-AVPR2 signal axis in attenuating ascites. Similar to ADRB1 and PIK3CG protein, the positive staining of AVPR2 protein was localized in cellular cytoplasm of kidney tissues.
Expressions of ADRβ1. (A) PI3Kγ (B) and AVPR2 (C) at the protein and mRNA levels in the ascites, sera and kidney tissues of the different groups as detected by ELISA, western blot and qRT-PCR analyses. The data are represented as the means ± the S.E. '*' and '**' P<0.05 and P<0.01, respectively, compared with the model group; '#' and '##' P<0.05 and P<0.01, respectively, compared with the GS group. Lanes 1-4 in the western blot denote the Model, GS, GS/GC_synergy and GS/GC_antagonism groups, respectively.
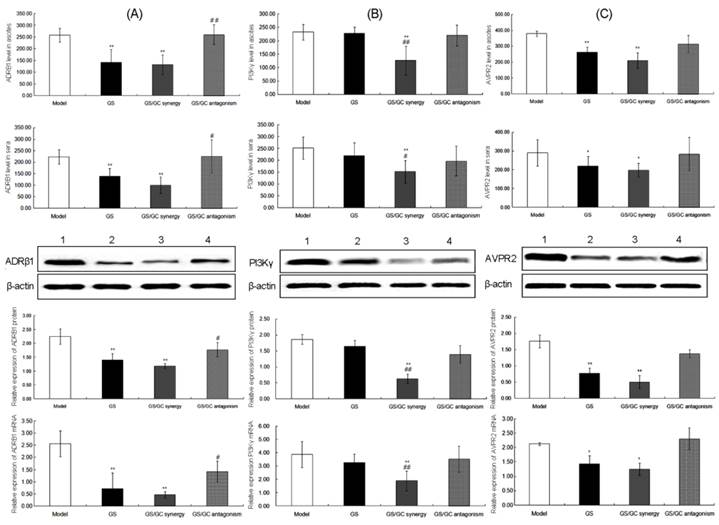
As shown in Figure 6F and Figure 7C, the expression levels of AVPR2 in the ascites, sera, and kidney tissues of the GS and GS/GC_synergy groups were significantly lower than those of the model control group (all P<0.05), which implies a diuretic function of the two-drug combination. However, the levels of AVPR2 in the ascites, sera, and kidney tissues of the GS/GC_antagonism group were not significantly different from those of the model control group, which suggests that the GS/GC combination at the ratio 1:0.39 might exhibit antagonistic effects on malignant ascites.
Conclusion
This systems-level investigation that integrated drug target prediction, network analysis and experimental validation provided the convincing evidence that GS and GC under different combination designs might exert synergistic or antagonistic effects on HCC ascites, which might be partially related to their regulations of the ADRB1-PIK3CG-AVPR2 signal axis. These findings also indicated that the herbal pair of the 'eighteen antagonistic medicaments' could exhibit synergistic effects with rational drug combination design. Therefore, additional studies should explore promising drug combinations of other herbal pairs.
Supplementary Material
Additional File 1Supplementary information, Figures S1-S2.
Table S1-Table S5.
Table S6-Table S7.
Table S8-Table S9.
Table S10-Table S11.
Acknowledgements
This study was supported by the National Basic Research Program of China (973 Program) (2011CB505300, 2011CB505305), the National Natural Science Foundation of China (81303153), Beijing Nova program (Z1511000003150126), Beijing Joint Project Specific Funds and the Fundamental Research Funds for the Central public welfare research institutes (ZZ2014008) and the Prospective Study Platform Project of Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences (QZPT002). All authors have read the journal's authorship agreement and policy on disclosure of potential conflicts of interest.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255
2. Sherman M. Recurrence of hepatocellular carcinoma. N Engl J Med. 2008;359:2045-2047
3. Cavazzoni E, Bugiantella W, Graziosi L. et al. Malignant ascites: pathophysiology and treatment. Int J Clin Oncol. 2013;18:1-9
4. Chung M, Kozuch P. Treatment of malignant ascites. Curr Treat Options Oncol. 2008;9:215-233
5. Tamsma J. The pathogenesis of malignant ascites. Cancer Treat Res. 2007;134:109-118
6. Yu FR, Lian XZ, Guo HY. et al. Isolation and characterization of methyl esters and derivatives from Euphorbia kansui (Euphorbiaceae) and their inhibitory effects on the human SGC-7901 cells. J Pharm Pharm Sci. 2005;8:528-535
7. Nie S, Li Z, Liang A. et al. Effects of processing on specific toxicity and pharmacodynamics of radix Kansui, radix Achyranthis bidentatae and semen Armeniacae amarum. Zhongguo Zhong Yao Za Zhi. 1996;21:153-6 190
8. Yan X, Zhang L, Guo J. et al. Processing of kansui roots stir-baked with vinegar reduces kansui-induced hepatocyte cytotoxicity by decreasing the contents of toxic terpenoids and regulating the cell apoptosis pathway. Molecules. 2014;19:7237-54
9. Hou YC, Lin SP, Chao PD. Liquorice reduced cyclosporine bioavailability by activating P-glycoprotein and CYP 3A. Food Chem. 2012;135:2307-12
10. Wang X, Zhang H, Chen L. et al. Liquorice, a unique "guide drug" of traditional Chinese medicine: a review of its role in drug interactions. J Ethnopharmacol. 2013;150:781-90
11. Sheela ML, Ramakrishna MK, Salimath BP. Angiogenic and proliferative effects of the cytokine VEGF in Ehrlich ascites tumor cells is inhibited by Glycyrrhiza glabra. Int Immunopharmacol. 2006;6:494-8
12. Song WQ. Utilization pattern of Gansui-Banxia-Tang in clinics. J Tradit Chin Med. 1993;34:492 In Chinese
13. Zhang Y, Guo X, Wang D. et al. A systems biology-based investigation into the therapeutic effects of Gansui Banxia Tang on reversing the imbalanced network of hepatocellular carcinoma. Sci Rep. 2014;4:4154
14. Li M, Qiao C, Qin L. et al. Application of traditional Chinese medicine injection in treatment of primary liver cancer: a review. J Tradit Chin Med. 2012;32:299-307
15. van der Greef J, van Wietmarschen H, Schroën J. et al. Systems biology-based diagnostic principles as pillars of the bridge between Chinese and Western medicine. Planta Med. 2010;76:2036-47
16. Chen CY-C. TCM Database@Taiwan: The World's Largest Traditional Chinese Medicine Database for Drug Screening In Silico. PLoS ONE. 2011;6:e15939
17. Chen JY, Mamidipalli S, Huan T. HAPPI: an online database of comprehensive human annotated and predicted protein interactions. BMC Genomics. 2009;10(Suppl 1):S16
18. Matthews L, Gopinath G, Gillespie M. et al. Reactome knowledgebase of human biological pathways and processes. Nucleic Acids Res. 2009;37:D619-D622
19. Brown KR, Jurisica I. Online predicted human interaction database. Bioinformatics. 2005;21:2076-2082
20. Aranda B, Achuthan P, Alam-Faruque Y. et al. The IntAct molecular interaction database in 2010. Nucleic Acids Res. 2010;38:D525-D531
21. Keshava Prasad TS, Goel R. et al. Human Protein Reference Database - 2009 update. Nucleic Acids Res. 2009;37:D767-D772
22. Ceol A, Chatr Aryamontri A, Licata L. et al. MINT, the molecular interaction database: 2009 update. Nucleic Acids Res. 2010;38:D532-D539
23. Lehne B, Schlitt T. Protein-protein interaction databases: keeping up with growing interactomes. Hum Genomics. 2009;3:291-297
24. Beuming T, Skrabanek L, Niv MY. et al. PDZBase: a protein-protein interaction database for PDZ-domains. Bioinformatics. 2005;21:827-828
25. Zhang Y, Wang D, Tan S. et al. A systems biology-based investigation into the pharmacological mechanisms of Wu Tou Tang acting on rheumatoid arthritis by integrating network analysis. Evidence-Based Complementary and Alternative Medicine. 2013;2013:548498
26. Li S, Zhang ZQ, Wu LJ. et al. Understanding ZHENG in traditional Chinese medicine in the context of neuro-endocrine-immune network. IET Syst Biol. 2007;1:51-60
27. Zsoldos Z, Reid D, Simon A, Sadjad BS, Johnson AP. eHiTS: an innovative approach to the docking and scoring function problems. Curr Protein Pept Sci. 2006;7:421-435
28. Cushing TD, Hao X, Shin Y, Andrews K. et al. Discovery and in vivo evaluation of (S)-N-(1-(7-fluoro-2-(pyridin-2-yl)quinolin-3-yl)ethyl)-9H-purin-6-amine (AMG319) and related PI3Kδ inhibitors for inflammation and autoimmune disease. J Med Chem. 2015;58:480-511
29. Warne T, Moukhametzianov R, Baker JG. et al. The structural basis for agonist and partial agonist action on a β(1)-adrenergic receptor. Nature. 2011;469:241-4
30. Hao C, Shi Y, Yu J. et al. The therapeutic function of the chemokine RANTES on the H22 hepatoma ascites model. Mol Cell Biochem. 2012;367:93-102
31. Zhang J, Wang X, Lu H. Amifostine increases cure rate of cisplatin on ascites hepatoma 22 via selectively protecting renal thioredoxin reductase. Cancer Lett. 2008;260:127-36
32. Wang Y, Juan LV, Ma X. et al. Specific hemosiderin deposition in spleen induced by a low dose of cisplatin: altered iron metabolism and its implication as an acute hemosiderin formation model. Curr Drug Metab. 2010;11:507-15
33. Wang GP, Guan YS, Jin XR. et al. Development of novel 5-fluorouracil carrier erythrocyte with pharmacokinetics and potent antitumor activity in mice bearing malignant ascites. J Gastroenterol Hepatol. 2010;25:985-90
34. Zhang Y, Bai M, Zhang B. et al. Uncovering pharmacological mechanisms of Wu-tou decoction acting on rheumatoid arthritis through systems approaches: drug-target prediction, network analysis and experimental validation. Sci Rep. 2015;5:9463
35. Zhang Y, Guo X, Xiong L. et al. MicroRNA-101 suppresses SOX9-dependent tumorigenicity and promotes favorable prognosis of human hepatocellular carcinoma. FEBS Lett. 2012;586:4362-70
36. Lin ZY, Huang YQ, Zhang YQ. et al. MicroRNA-224 inhibits progression of human prostate cancer by downregulating TRIB1. Int J Cancer. 2014;135:541-50
37. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-8
38. Zhang Y, Li Z, Yang M. et al. Identification of GRB2 and GAB1 coexpression as an unfavorable prognostic factor for hepatocellular carcinoma by a combination of expression profile and network analysis. PLoS One. 2013;8:e85170
39. Zhang Y, Guo X, Yang M. et al. Identification of AKT kinases as unfavorable prognostic factors for hepatocellular carcinoma by a combination of expression profile, interaction network analysis and clinical validation. Mol Biosyst. 2014;10:215-22
40. Xu H, Zhang Y, Lei Y. et al. A Systems Biology-Based Approach to Uncovering the Molecular Mechanisms Underlying the Effects of Dragon's Blood Tablet in Colitis, Involving the Integration of Chemical Analysis, ADME Prediction, and Network Pharmacology. PLoS One. 2014;9:e101432
41. http://www.genecards.org/cgi-bin/carddisp.pl?gene=ADRB1
42. Kessel I, Makhoul IR, Sujov P. Congenital hypothyroidism and nonimmune hydrops fetalis: associated?. Pediatrics. 1999;103:E9
43. Ladmakhi MH, Buys N, Vanderpooten A. et al. Myocardial beta-adrenergic receptor characteristics in T(3)-induced ascites and in broiler lines differing in ascites susceptibility. Avian Pathol. 1997;26:293-303
44. http://www.genecards.org/cgi-bin/carddisp.pl?gene=PIK3CG
45. Tapia-Abellán A, Ruiz-Alcaraz AJ, Antón G. et al. Regulatory role of PI3K-Akt on the release of IL-1β in peritoneal macrophages from the ascites of cirrhotic patients. Clin Exp Immunol. In press
46. Tapia-Abellán A, Ruiz-Alcaraz AJ, Hernández-Caselles T. et al. Role of MAP kinases and PI3K-Akt on the cytokine inflammatory profile of peritoneal macrophages from the ascites of cirrhotic patients. Liver Int. 2013;33:552-60
47. http://www.genecards.org/cgi-bin/carddisp.pl?gene=AVPR2
48. Sakaida I. Tolvaptan for the treatment of liver cirrhosis oedema. Expert Rev Gastroenterol Hepatol. 2014;8:461-70
49. Watson H, Jepsen P, Wong F. et al. Satavaptan treatment for ascites in patients with cirrhosis: a meta-analysis of effect on hepatic encephalopathy development. Metab Brain Dis. 2013;28:301-5
50. Habib S, Boyer TD. Vasopressin V2-receptor antagonists in patients with cirrhosis, ascites and hyponatremia. Therap Adv Gastroenterol. 2012;5:189-97
Author contact
![]() Corresponding authors: Prof. Na Lin & Yanqiong Zhang. Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, No. 16, Nanxiaojie, Dongzhimennei, Beijing 100700, China. E-mail: nlinac.cn & yqzhangac.cn; Phone:+861064014411-2869; Fax: +861064013996.
Corresponding authors: Prof. Na Lin & Yanqiong Zhang. Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, No. 16, Nanxiaojie, Dongzhimennei, Beijing 100700, China. E-mail: nlinac.cn & yqzhangac.cn; Phone:+861064014411-2869; Fax: +861064013996.
Received 2015-10-18
Accepted 2016-2-22
Published 2016-3-25
