ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2018; 14(11):1457-1465. doi:10.7150/ijbs.26686 This issue Cite
Research Paper
MiR-490-3p inhibits osteogenic differentiation in thoracic ligamentum flavum cells by targeting FOXO1
1. Department of Orthopedics, Peking University Third Hospital, Beijing, China
2. Department of Orthopedics, The First Affiliated Hospital of Dalian Medical University, Dalian, China
3. Central Laboratory, Peking University International Hospital, Beijing, China
4. Bone Research Laboratory, University of Texas Southwestern Medical Center, Dallas, Texas, USA
5. Department of Orthopedics, Peking University International Hospital, Beijing, China
Abstract
Thoracic ossification of the ligamentum flavum (TOLF) is a rare heterotopic ossification of spinal ligaments, which is the major cause of thoracic spinal canal stenosis and myelopathy. In this study, the roles of miR-490-3p and forkhead box O1 (FOXO1) in osteogenesis of human thoracic ligamentum flavum cells were investigated. MiR-490-3p was found to be down-regulated during osteogenic differentiation of thoracic ligamentum flavum cells, while their overexpression inhibited osteogenic differentiation. In addition, the analysis of target prediction and dual luciferase reporter assays supported that miR-490-3p directly targeted FOXO1 and suppressed the expression of FOXO1. Moreover, FOXO1 knockdown was displayed to attenuate the effect of miR-490-3p inhibition. ChIP assays showed that miR-490-3p negatively regulated the interaction of FOXO1 and RUNX2. These findings suggest that miR-490-3p performs an inhibitory role in osteogenic differentiation of thoracic ligamentum flavum cells by potentially targeting FOXO1.
Keywords: thoracic ossification of the ligamentum flavum, miR-490-3p, osteogenic differentiation, FOXO1
Introduction
Ossification of the ligamentum flavum (OLF) is a rare heterotopic ossification of spinal ligaments, and it is more likely to affect the East Asian populations. It has been reported that OLF primarily occurs in the lower thoracic spine. Thoracic ossification of the ligamentum flavum (TOLF) has been considered to be the main cause of thoracic spinal canal stenosis and myelopathy [1-4]. Many investigations have been conducted on the potential etiology of TOLF at the genetic and cellular levels. While these studies suggested that some potential contributing factors are associated with TOLF, such as mechanical effects [5-7], basic metabolic elements [8, 9], degenerative effects [4, 10], inflammatory factors [11, 12] and genetic factors [13, 14], the underlying development and progression of TOLF is inadequately understood.
MicroRNAs (miRNAs), which comprise a substantial family of small, single-stranded non-coding RNAs, have been reported to play important roles in a variety of physiological and pathological processes. The potential of miRNAs in positive or negative regulation of osteogenesis or osteoclastogenesis has been identified [15, 16]. A recent integrated study on miRNA-mRNA suggested that the regulatory networks of a series of miRNAs may play a vital role in the onset and progression of ossification of posterior longitudinal ligament (OPLL) [17]. The data suggested that the expression of miR-490-3p was down-regulated in OPLL ligamentum cells. MiR-490-3p has been previously discovered to promote cell cycle arrest and apoptosis, and was identified as a suppressor in several types of tumors [18-20]. It was also found that miR-490-3p regulates cell proliferation and apoptosis by targeting high mobility group a isoform 2 (HMGA2) in osteosarcoma, which is an osteogenic tumor with the capability of osteoblast differentiation [21]. Even its sibling miR-490-5p was reported to down-regulate the chondrogenic differentiation in human adipose-derived stem cells by targeting bone morphogenetic protein receptor type 2 (BMPR2) [22]. However, the function of miR-490-3p in osteogenesis, as well as the underlying mechanisms by which miR-490-3p modulates the thoracic ligamentum flavum cells, still need to be elucidated.
In recent years, there has been growing interest in the study of the association between the effect of inflammatory factors and new bone formation. In the previous studies, the involvement of inflammatory factors in TOLF were revealed and have been reported to play an important role in the osteogenesis of ligament flavum cells [11, 12]. Other investigations also suggested the close correlation between inflammation and oxidative stress in bone formation [23, 24]. Evidences have been provided to indicate that as a member of the FOXO family, FOXO1 involves in the TOLF progression through the induction of osteogenic differentiation [25, 26].
In our previous research, miR-132-3p was confirmed to regulate the osteogenic differentiation of thoracic ligamentum flavum cells via targeting FOXO1, GDF5 and SOX6 [25]. In addition, a high-throughput sequencing has demonstrated that miR-615-3p negatively regulated the osteogenic differentiation of human lumbar ligamentum flavum cells through suppressing osteogenic regulators GDF5 and FOXO1 [26]. In this study, the roles of miR-490-3p and FOXO1were further analyzed during the osteogenic differentiation of thoracic ligamentum flavum cells. Our results implied that FOXO1, which is negatively regulated by miR-490-3p, promotes the osteogenic differentiation of thoracic ligamentum flavum cells.
Materials and Methods
Patient specimen
We obtained 16 patients' specimen from the BioBank of Department of Orthopedics, Peking University Third Hospital. This study was approved by the Ethics Committee for Human Subjects of Peking University Third Hospital with the Declaration of Helsinki (PUTH-REC-SOP-06-3.0-A27, #2014003). TOLF patients who visited the orthopedic clinic and signed written informed consent for the study were enrolled. The diagnosis of TOLF was made on the basis of clinical symptoms and radiological examination as previously described [25].
Cell culture and osteogenic differentiation
Ligaments were derived from patients during surgery and rinsed with phosphate-bufferedsaline (PBS). The ligaments collected were minced and digested using 0.25% trypsin (Gibco, Grand Island, NY, USA), followed by 250U/mL type I collagenase (Sigma-Aldrich, St.Louis, MO, USA). The specimen was then placed in 100mm culturing dishes containing Dulbecco's Modified Eagle's medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco), 100U/mL penicillin G sodium and 100mg/mL streptomycin sulfate in a humidified atmosphere with 5% CO2 at 37°C. For passaging, passages 2 and 3 were used for subsequent experimentation. To induce osteogenic differentiation, cells were cultured in osteogenic medium consisting of DMEM supplemented with 50 µM ascorbic acid (Sigma-Aldrich), 10 mM β-glycerophosphate (Sigma-Aldrich) and 10 nM dexamethasone (Sigma-Aldrich).
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA). Reverse transcription and qRT-PCR for miR-490-3p were performed using amiDETECTA Track™ miRNA qRT-PCR Starter Kit (RiboBio, Guangzhou, China) on a BioRad IQ5 system. Each value was normalized to that of RnU6. Reverse transcription and qPCR for the mRNA level of FOXO1 and osteogenic markers was carried out as described previously [25]. Expression levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and relative gene expression levels were calculated using the 2-ΔΔCt method. All experiments were performed in triplicate. The following primer pairs were used: miR-490-3p-RT: 5'-CAGCATGGCAGCATAGGTCACGCTTATGGAGCCTGGGACGTGACCTATGCTG-3'; miR-490-3p-Fw: 5'-TCAGGAGGTCCAACCGCG-3'; miR-490-3p-Rv: 5'-CCTGGGACGTGACCTATGCTG-3'; U6-RT: 5'-GTTAAGCACTTCGCAAGGTATAAAA-3'; U6-Fw: 5'-TCATATACACGACGGCTTCGCTC-3'; U6-Rv: 5'-TGTGCGTTTAAGCACTTCGC-3'; FOXO1-Fw: 5'-GTAGGTTCAGTGAACCCTCGAA-3'; FOXO1-Rv: 5'-AGTCCGACTCCCAATCACTCGTC-3'; GAPDH-Fw: 5'-CGGGTTATGCTGGTTTAGGC-3'; GAPDH-Rv: 5'-GTGGGTACCGTTTAAGGTAC-3'.
Western blot analysis
Total protein (50 μg) was separated in a Bis-Tris polyacrylamide gel and transferred onto a nitrocellulose membrane. The membrane was then incubated in 5% bovine serumal bumin (BSA) containing primary rabbit-anti-human polyclonal antibodies at 4°C overnight. Next, samples were incubated with IRDye®800CW goat-anti-rabbit antibody (1:5000; ab216773, Abcam) at room temperature for 1 hour and visualized via chemiluminescence with an infrared laser scanning system (OdysseyLicor, Lincoln, NE, USA). The following primary rabbit-anti-human antibodies were used: anti-FOXO1 (1:1000; ab39670, Abcam); anti-Runx2 (1:1000; ab23981, Abcam); anti-Sp7/Osterix (1:2000; ab22552, Abcam); anti-ALP (1:2000; ab95462, Abcam); anti-OCN (1:500; ab93876, Abcam); anti-OPN (1:1000; ab8448, Abcam) and anti-GAPDH (1:2500; ab9485, Abcam).
Alkaline phosphatase (ALP) activity assay and Alizarin red staining
Cells were seeded in 6-well plates at the density of 1×105/well and cultured in osteogenic medium for 18 days. ALP activity was determined using an ALP activity staining kit (GMS80033.1; GENMED Scientifics, Shanghai, China) and mineralization was assessed using an Alizarin Red S kit (GMS80046.3; GENMED Scientifics, Shanghai, China).
Luciferase reporter assay
The DNA sequences of FOXO1 containing binding site of miR-490-3p was amplified by PCR using HEK293T cells genomic DNA. The amplified DNA sequences were inserted into pmiR-RB-REPORT™ vectors (RiboBio) to generate wild type (WT) FOXO1 3'-untranslated region (UTR) (containing the binding site of miR-490-3p), with mutated (MUT) FOXO1 3'-UTR luciferase vectors generated using site-directed mutagenesis (FAST Multigenesis System, TransGen Biotech, Beijing, China), respectively. For the reporter assay, HEK293T cells were cultured in a 96-well plate with 1.5×104 cells/well in 100μL of culture medium/well for 24 h. Cells were then co-transfected with 50 nM miR-490-3p mimic and 100 ng of vector per well and cultured in fresh medium for an additional 48 h. The luciferase reporter assay was carried out using the Dual-Glo® Luciferase Assay System (Promega, Madison, WI, USA) according to the manufacturer's instructions, Firefly and Renilla luciferase activities were measured in cell lysates. The relative fluorescence intensity for each sample was calculated by normalizing the signal value of Renilla luciferase to Firefly luciferase, and then compared with the control. Luminescence was quantified using a Veritas™ 9100-002 luminometer (Promega).
SiRNA/miRNA mimic/miRNA inhibitor transfection
Synthetic miRNA mimic/inhibitor and siRNA were purchased from RiboBio. The sequence of miR-490-3p mimic is as follows: 5'-CAACCUGGAGGACUCCAUGCUG-3'/3'-GCAUGGAGUCCUCCAGGUUGUU-5'. The sequence of miR-490-3p inhibitor is as follows: 5'-CAGCAUGGAGUCCUCCAGGUUG-3'. Ligamentum flavum cells were transfected with siRNA/miRNA mimic/miRNA inhibitor using Lipofectamine® 2000 Transfection Reagent (Life Technologies, NY, USA), according to the manufacturer's instructions.
MiR-490-3p mimic or inhibitor was transfected into cells at a concentration of 20 nM with nonspecific microRNA or nonspecific microRNA inhibitor used as negative control (NC). SiRNA targeting FOXO1was transfected at a concentration of 50 nM with non-targeting siRNA used as NC.
Chromatin immunoprecipitation (ChIP)
The ChIP analysis was carried out using ChIP Assay Kit (P2078, Beyotime). Briefly, 1% formaldehyde was added to culture medium to cross-link DNA and bound protein. Cell lysates after being diluted with ChIP dilution buffer were immunoprecipitated with anti-FOXO1 antibody or normal mouse IgG (A7028, Beyotime) overnight, mixed with Protein A+G Agarose/Salmon Sperm DNA and collected by centrifugation. Protein-DNA complexes were washed and eluted followed by a cross-link reversal step. DNA from each immunoprecipitation reaction was examined by qPCR. The FOXO1 binding sites in RUNX2 gene promoter were searched from SABiosciences. The primer for the FOXO1-responsive region of Runx2 promoters was purchased from QIAGEN (EpiTect ChIP qPCR Primer Assay for Human RUNX2: GPH1011389 (-) 02A, QIAGEN, Hilden, Germany).
Statistical analysis
Data were presented as the mean ± SD. The two-tailed unpaired Student's t-tests were used for comparisons of two groups. The ANOVA multiple comparison test (SPSS 17.0) followed by Turkey post hoc test were used for comparisons of more than two groups. Each experiment was repeated at least three times. P<0.05 was considered to be statistically significant.
Results
MiR-490-3p inhibits osteogenic differentiation in thoracic ligamentum flavum cells
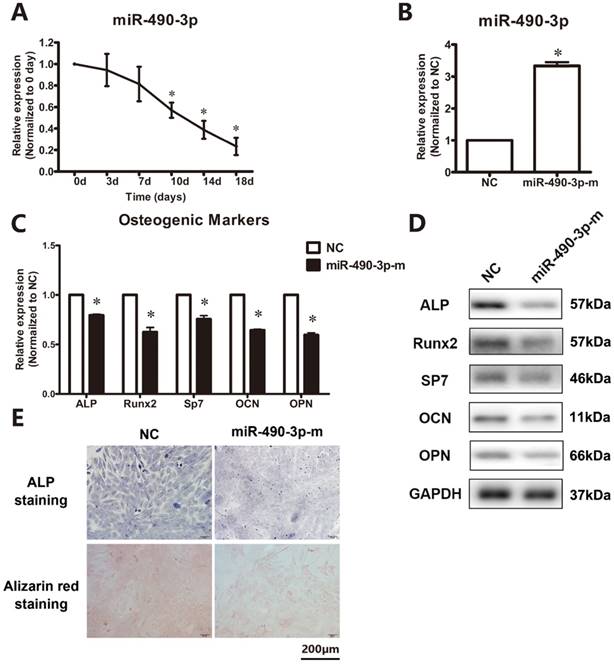
The temporospatial expression of miR-490-3p in thoracic ligamentum flavum cells was determined. As showed in Figure 1A, the expression level was decreased by day 7 compared with day 0 and continued to decline until day 18. To further clarify the role of miR-490-3p in the regulation of osteogenesis in thoracic ligamentum flavum cells, the osteogenic capacity was examined after being transfected with miR-490-3p mimic for 48 h. The miRNA level of miR-490-3p was increased (Figure 1B), and the osteogenic differentiation of ligamentum flavum cells was significantly inhibited, as indicated by a reduced expression of osteoblastic markers (ALP, Runx2, SP7, OPN and OCN) and reduced ALP and Alizarin red staining (Figure 1C-E).
MiR-490-3p inhibits osteogenic differentiation in thoracic ligamentum flavum cells. (A) Endogenous miR-490-3p expression levels were measured via qRT-PCR at different time points during osteogenic differentiation in ligamentum flavum cells for 18 days; *P<0.05 compared with day 0. (B) miR-490-3p expression assessed via qRT-PCR in ligamentum flavum cells transfected with miRNA mimic for 48h; *P<0.05 compared with NC group. (C, D) Osteogenic marker mRNA and protein expression examined via qRT-PCR and Western blot after miR-490-3p overexpression for 48h; *P<0.05 compared with NC group. (E) ALP staining and Alizarin red staining at day 18 following miR-490-3p mimic transfection for 48h; scale bar represents 200µm. N=5 in each group.
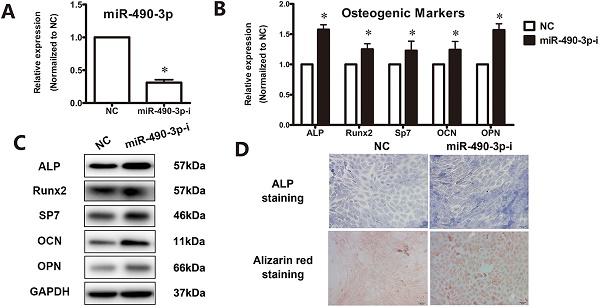
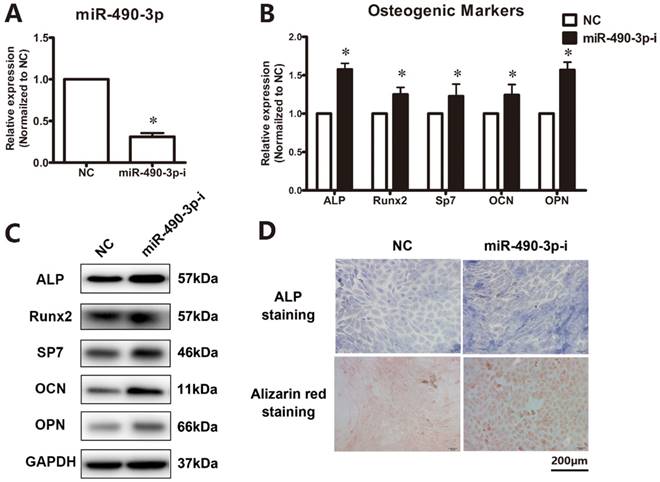
Inhibition of miR-490-3p promotes osteogenesis in thoracic ligamentum flavum cells
Next, to further clarify the effect of miR-490-3p on the regulation of osteogenesis in thoracic ligamentum flavum cells, the osteogenic ability was determined after transfecting miR-490-3p inhibitor for 48 h. The inhibition rate of miR-490-3p was shown in Figure 2A, and the osteogenic differentiation of ligamentum flavum cells was remarkably up-regulated, as indicated by an increased expression of osteoblastic markers (Figure 2B-D).
MiR-490-3p directly targets FOXO1
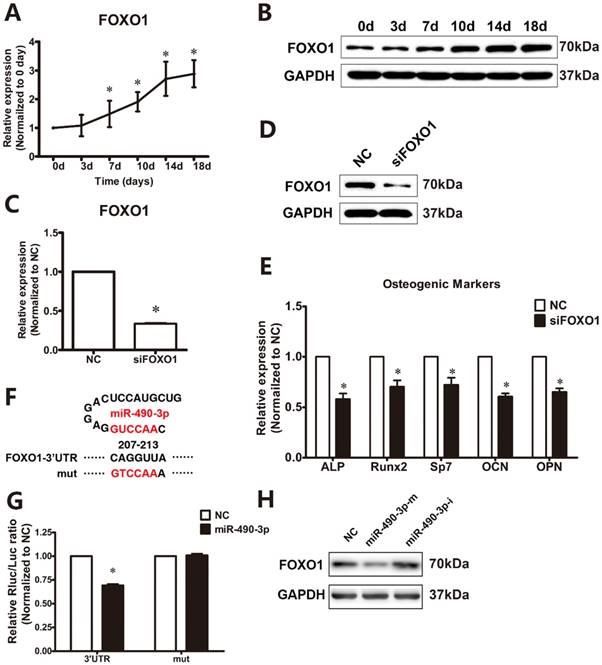
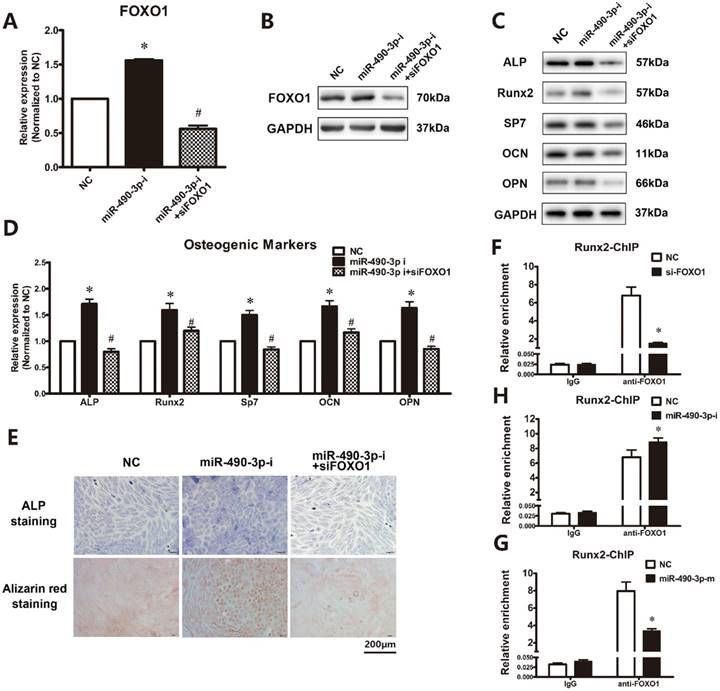
As showed in Figure 3A and 3B, osteogenic induction resulted in a significant increased mRNA and protein expression of FOXO1 from day 7 to day 18. To examine the functional effects of FOXO1 on osteogenic differentiation of ligamentum flavum cells, siFOXO1 was employed and significantly reduced both FOXO1 mRNA and protein expression (Figure 3C and 3D). Following FOXO1 knockdown, osteogenic differentiation of ligamentum flavum cells was significantly inhibited, as indicated by reduced RUNX2, SP7, ALP, OPN and OCN expression (Figure 3E) at day 18.
Inhibition of miR-490-3p promotes osteogenesis in thoracic ligamentum flavum cells. (A) miR-490-3p expression assessed via qRT-PCR in ligamentum flavum cells transfected with miR-490-3p inhibitor for 48h; *P<0.05 compared with NC group. (B, C) Osteogenic marker mRNA and protein expression examined via qRT-PCR and Western blot after miR-490-3pinhibitior transfection for 48h; *P<0.05 compared with NC group. (D) ALP staining and Alizarin red staining at day 18 following miR-490-3p inhibitor transfection for 48h; scale bar represents 200µm. N=5 in each group.
To further examine whether miR-490-3p directly targets FOXO1, TargetScan was utilized to forecast potential miRNAs targets in the FOXO1 3'-UTR. FOXO1 was found to have a miR-490-3p binding site in the 3'-UTR, and luciferase reporters were constructed based on this finding (Figure 3F). Dual luciferase reporter gene assay indicated that miRNA-490-3p targets the 3'-UTR of FOXO1 in the binding site and repress the luciferase activity (Figure 3G). Furthermore, FOXO1 expression was decreased by the overexpression of miR-490-3p, while miR-490-3p inhibitor up-regulates the protein expression of FOXO1 (Figure 3H).
FOXO1 knockdown could block the effect of miR-490-3p inhibition
Then we analyzed the effect of miR-490-3p inhibitor on the osteogenesis of ligamentum flavum cells with or without siFOXO1, respectively. Furthermore, as shown in Figure 4A and 4B, miR-490-3p inhibitor promotes the expression of FOXO1. As expected, the effect of miR-490-3p inhibitor on osteogenic differentiation was attenuated after transfection of siFOXO1 for 48h (Figure 4C-4E). Moreover, ChIP assay showed that miR-490-3p negatively regulates the interaction of FOXO1 and RUNX2 (Figure 4F-4H).
Discussion
TOLF is characterized by pathological heterotopic ossification in ligamentum flavum. The pathological process of TOLF involves the chondrogenic and osteogenic differentiation of fibroblasts into osteoblasts. Thus, investigating osteogenic differentiation of thoracic ligamentum flavum cells would likely lead to better understanding of the pathogenesis of TOLF. In a recent integrated study on microRNA-mRNA, miR-490-3p was found to be one of the specific down-regulated miRNAs in OPLL cells compared with normal posterior longitudinal ligament cells [17], suggesting that miR-490-3p may be involved in the process of TOLF.
MiR-490-3p, which is located on chromosome 7q33, has been previously indicated to play an important role in the carcinogenesis of multiple types of cancer. It has been reported that miR-490-3p exerted suppressive effects on gastric cancer cells through directly targeting SMARCD1 [19], and modulates cell growth and epithelial to mesenchymal transition of hepatocellular carcinoma cells by targeting endoplasmic reticulum-Golgi intermediate compartment protein 3 (ERGIC3) [20]. Similar evidence is found that miR-490-3p regulates cell proliferation and apoptosis by directly down-regulating HMGA2 expression in osteosarcoma. In the present study, we showed that miR-490-3p was down-regulated during osteogenic differentiation of thoracic ligamentum flavum cells, while the in vitro experiments suggested that miR-490-3p acts as the negative regulator of osteogenic differentiation in ligamentum flavum cells.
MiR-490-3p directly targets FOXO1. (A, B) FOXO1 mRNA and protein expression levels examined via qRT-PCR and Western blot at different time points during osteogenic differentiation of ligamentum flavum cells for 18 days; *P<0.05 compared with day 0. (C, D) FOXO1 mRNA and protein expression level examined via qRT-PCR and Western blot following siFOXO1 transfection in ligamentum flavum cells for 48h; *P<0.05 compared with NC group. (E) Osteogenic marker mRNA expression examined via qRT-PCR at day 18 after FOXO1 knockdown for 48h; *P<0.05 compared with NC group. (F) Schematic diagrams indicating the wild-type and mutated type miR-490-3p binding sites in the FOXO1 3'-UTR. (G) Wild-type (WT) FOXO1 3'-UTR or mutant (MUT) FOXO1 3'-UTR reporter plasmids were co-transfected into HEK293T cells with miR-490-3p for 48h and fluorescence was quantified; *P<0.05 compared with NC group. (H) FOXO1 protein expression level examined via Western blot with either miR-490-3p mimic or inhibitor transfection for 48h in ligamentum flavum cells. N=5 in each group.
MiRNAs regulate the target mRNA by binding the 3'-UTR and subsequently mediating its degradation via the RNA-induced silencing complex (RISC) [27]. In this study, we first demonstrated that miR-490-3p down-regulated the osteogenic differentiation by directly targeting the 3'-UTR of FOXO1 mRNA in the thoracic ligamentum flavum cells. Members of FOXO transcription factors were up-regulated in several types of cells and were involved in a series of pathologic and physiologic processes, which impacted a number of clinical conditions. Among the FOXO family, FOXO1 is the main member expressed in bone, involving in a variety of pathologic and physiologic processes [28-30]. Animal studies have demonstrated that mouse model after FOXO1 knockdown was decreased in bone mineral density and osteogenic differentiation [31, 32]. Another study of Bcl2-deficient mouse model suggested that Bcl2 deficiency induces and activates FOXO1 and accelerates osteoblast differentiation [33]. Moreover, in vitro and in vivo investigation showed that FOXO1 directly interacts with the promoter of Runx2 and regulates its expression to affect mesenchymal cell differentiation into osteoblast [34, 35]. The location expression of FOXO1 was visually observed in both cartilage and ossified area of TOLF by immunohistochemistry. Meanwhile, FOXO1 was found to encourage osteogenic differentiation of thoracic ligamentum flavum cells [25]. The similar effect of FOXO1 has also been shown in the osteogenic differentiation of lumbar ligamentum flavum cells [26]. In the present study, FOXO1 knockdown attenuated the effect of miR-490-3p inhibition on the osteogenic differentiation of thoracic ligamentum flavum cells. ChIP results in this study suggested that miR-490-3p negatively regulated the osteogenic differentiation by targeting FOXO1 with the interaction of RUNX2.
FOXO1 knockdown could block the effect of miR-490-3p inhibition. (A, B) FOXO1 mRNA and protein expression levels examined via qRT-PCR and Western blot at day 18 following miRNA inhibitor and siFOXO1 transfection in ligamentum flavum cells for 48h; *P<0.05 compared with NC group; #P<0.05 compared with miR-490-3p inhibitor. (C, D) Osteogenic marker protein and mRNA expression examined via Western blot and qRT-PCR after miRNA inhibitor for 48h with and without siFOXO1 transfection; *P<0.05 compared with NC group; #P<0.05 compared with miR-490-3p inhibitor group. (E) ALP staining and Alizarin red staining at day 18 following miR-490-3p inhibitor transfection for 48h with and without siFOXO1 transfection; scale bar represents 200µm. (F-H) The level of FOXO1 in the promoter regions of RUNX2 with siFOXO1, miR-490-3p mimic or inhibitor for 48h was analyzed in ligamentum flavum cells by ChIP assay. *P<0.05 compared with NC group. N=5 in each group.
TOLF is a highly regulated pathological process, which involves chondrogenic and osteogenic differentiation associated with genetic, mechanical and inflammatory factors. In our study, we identified that miR-490-3p could directly target the 3'-UTR of FOXO1 mRNA and is identified as a regulator of FOXO1 in thoracic ligamentum flavum cells. This finding led us to further explore the effect of miR-490-3p and FOXO1 on osteogenic differentiation. However, we cannot rule out other potential pathways involved in the regulation of miR-490-3p. Previous studies provided evidence for the involvement and possible mechanism of transforming growth factor beta (TGF-β) in TOLF [13, 36]. It is known that the role of FOXO1 is remarkable in the regulation of TGF-β expression [37-39]. MiR-490-3p was also reported to repress the migration and invasion abilities of colorectal cancer cells partially by targeting the TGF-β signaling pathway [40]. In addition, β-catenin signaling pathway was also an important pathway in osteogenic differentiation in ligamentum flavum cells [5], while miR-490-3p seemed to have an inhibitory effect on β-catenin expression in nuclear fractions of colorectal cancer cells [19]. Therefore, further research is obligatory to better understand how miR-490-3p modulates osteogenic differentiation in ligamentum flavum cells.
In conclusion, our study demonstrated that miR-490-3p inhibits the osteogenic differentiation of ligamentum flavum cells by targeting FOXO1. Further, FOXO1 and miR-490-3p could possibly be viable therapeutic target for ossification management in thoracic ligamentum flavum and other skeletal disorders.
Acknowledgements
We would like to thank the Central Laboratory of Peking University Third Hospital for the technical support. This work was supported by the National Natural Science Foundation of China (Grant Number: 81772381 and 81572101). This work was also supported by Beijing Municipal Science & Technology Commission (Grant Number: Z181100001818006).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Feng FB, Sun CG, Chen ZQ. Progress on clinical characteristics and identification of location of thoracic ossification of the ligamentum flavum. Orthopaedic surgery. 2015;7:87-96
2. Guo JJ, Luk KD, Karppinen J, Yang H, Cheung KM. Prevalence, distribution, and morphology of ossification of the ligamentum flavum: a population study of one thousand seven hundred thirty-six magnetic resonance imaging scans. Spine (Phila Pa 1976). 2010;35:51-6
3. Hou X, Sun C, Liu X, Liu Z, Qi Q, Guo Z. et al. Clinical Features of Thoracic Spinal Stenosis-associated Myelopathy: A Retrospective Analysis of 427 Cases. Clin Spine Surg. 2016;29:86-9
4. Lang N, Yuan HS, Wang HL, Liao J, Li M, Guo FX. et al. Epidemiological survey of ossification of the ligamentum flavum in thoracic spine: CT imaging observation of 993 cases. Eur Spine J. 2013;22:857-62
5. Cai HX, Yayama T, Uchida K, Nakajima H, Sugita D, Guerrero AR. et al. Cyclic tensile strain facilitates the ossification of ligamentum flavum through beta-catenin signaling pathway: in vitro analysis. Spine (Phila Pa 1976). 2012;37:E639-46
6. Fan D, Chen Z, Wang D, Guo Z, Qiang Q, Shang Y. Osterix is a key target for mechanical signals in human thoracic ligament flavum cells. Journal of cellular physiology. 2007;211:577-84
7. Ning S, Chen Z, Fan D, Sun C, Zhang C, Zeng Y. et al. Genetic differences in osteogenic differentiation potency in the thoracic ossification of the ligamentum flavum under cyclic mechanical stress. International journal of molecular medicine. 2017;39:135-43
8. Fan D, Chen Z, Chen Y, Shang Y. Mechanistic roles of leptin in osteogenic stimulation in thoracic ligament flavum cells. The Journal of biological chemistry. 2007;282:29958-66
9. Sohn S, Yoon JW, Chung CK. Increased bone mineral density in patients with ossification of the ligamentum flavum: a case-control study. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2014;17:195-9
10. Hou XF, Fan DW, Sun CG, Chen ZQ. Recombinant human bone morphogenetic protein-2-induced ossification of the ligamentum flavum in rats and the associated global modification of histone H3. Journal of neurosurgery Spine. 2014;21:334-41
11. Wang B, Chen Z, Meng X, Li M, Yang X, Zhang C. iTRAQ quantitative proteomic study in patients with thoracic ossification of the ligamentum flavum. Biochemical and biophysical research communications. 2017;487:834-9
12. Zhang C, Chen Z, Meng X, Li M, Zhang L, Huang A. The involvement and possible mechanism of pro-inflammatory tumor necrosis factor alpha (TNF-alpha) in thoracic ossification of the ligamentum flavum. PloS one. 2017;12:e0178986
13. Qu X, Chen Z, Fan D, Xiang S, Sun C, Zeng Y. et al. Two novel BMP-2 variants identified in patients with thoracic ossification of the ligamentum flavum. European journal of human genetics: EJHG. 2017;25:565-71
14. Liu Y, Zhao Y, Chen Y, Shi G, Yuan W. RUNX2 polymorphisms associated with OPLL and OLF in the Han population. Clinical orthopaedics and related research. 2010;468:3333-41
15. Huang C, Geng J, Jiang S. MicroRNAs in regulation of osteogenic differentiation of mesenchymal stem cells. Cell and tissue research. 2017;368:229-38
16. Peng S, Gao D, Gao C, Wei P, Niu M, Shuai C. MicroRNAs regulate signaling pathways in osteogenic differentiation of mesenchymal stem cells (Review). Molecular medicine reports. 2016;14:623-9
17. Xu C, Chen Y, Zhang H, Chen Y, Shen X, Shi C. et al. Integrated microRNA-mRNA analyses reveal OPLL specific microRNA regulatory network using high-throughput sequencing. Scientific reports. 2016;6:21580
18. Chen S, Chen X, Xiu YL, Sun KX, Zhao Y. MicroRNA-490-3P targets CDK1 and inhibits ovarian epithelial carcinoma tumorigenesis and progression. Cancer letters. 2015;362:122-30
19. Shen J, Xiao Z, Wu WK, Wang MH, To KF, Chen Y. et al. Epigenetic silencing of miR-490-3p reactivates the chromatin remodeler SMARCD1 to promote Helicobacter pylori-induced gastric carcinogenesis. Cancer research. 2015;75:754-65
20. Zhang LY, Liu M, Li X, Tang H. miR-490-3p modulates cell growth and epithelial to mesenchymal transition of hepatocellular carcinoma cells by targeting endoplasmic reticulum-Golgi intermediate compartment protein 3 (ERGIC3). The Journal of biological chemistry. 2013;288:4035-47
21. Tang B, Liu C, Zhang QM, Ni M. Decreased expression of miR-490-3p in osteosarcoma and its clinical significance. European review for medical and pharmacological sciences. 2017;21:246-51
22. Yang Z, Hao J, Hu ZM. MicroRNA expression profiles in human adipose-derived stem cells during chondrogenic differentiation. International journal of molecular medicine. 2015;35:579-86
23. Micha D, Voermans E, Eekhoff MEW, van Essen HW, Zandieh-Doulabi B, Netelenbos C. et al. Inhibition of TGFbeta signaling decreases osteogenic differentiation of fibrodysplasia ossificans progressiva fibroblasts in a novel in vitro model of the disease. Bone. 2016;84:169-80
24. Kim EJ, Choi IS, Yoon JY, Park BS, Yoon JU, Kim CH. Effects of propofol-induced autophagy against oxidative stress in human osteoblasts. Journal of dental anesthesia and pain medicine. 2016;16:39-47
25. Qu X, Chen Z, Fan D, Sun C, Zeng Y. MiR-132-3p Regulates the Osteogenic Differentiation of Thoracic Ligamentum Flavum Cells by Inhibiting Multiple Osteogenesis-Related Genes. International journal of molecular sciences. 2016:17
26. Yin J, Zhuang G, Zhu Y, Hu X, Zhao H, Zhang R. et al. MiR-615-3p inhibits the osteogenic differentiation of human lumbar ligamentum flavum cells via suppression of osteogenic regulators GDF5 and FOXO1. Cell biology international. 2017;41:779-86
27. Chen L, Holmstrom K, Qiu W, Ditzel N, Shi K, Hokland L. et al. MicroRNA-34a inhibits osteoblast differentiation and in vivo bone formation of human stromal stem cells. Stem cells (Dayton, Ohio). 2014;32:902-12
28. Kousteni S. FoxO1: a molecule for all seasons. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2011;26:912-7
29. Kousteni S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone. 2012;50:437-43
30. Rached MT, Kode A, Xu L, Yoshikawa Y, Paik JH, Depinho RA. et al. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell metabolism. 2010;11:147-60
31. Iyer S, Han L, Ambrogini E, Yavropoulou M, Fowlkes J, Manolagas SC. et al. Deletion of FoxO1, 3, and 4 in Osteoblast Progenitors Attenuates the Loss of Cancellous Bone Mass in a Mouse Model of Type 1 Diabetes. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2017;32:60-9
32. Iyer S, Ambrogini E, Bartell SM, Han L, Roberson PK, de Cabo R. et al. FOXOs attenuate bone formation by suppressing Wnt signaling. The Journal of clinical investigation. 2013;123:3409-19
33. Moriishi T, Kawai Y, Komori H, Rokutanda S, Eguchi Y, Tsujimoto Y. et al. Bcl2 deficiency activates FoxO through Akt inactivation and accelerates osteoblast differentiation. PloS one. 2014;9:e86629
34. Siqueira MF, Flowers S, Bhattacharya R, Faibish D, Behl Y, Kotton DN. et al. FOXO1 modulates osteoblast differentiation. Bone. 2011;48:1043-51
35. Teixeira CC, Liu Y, Thant LM, Pang J, Palmer G, Alikhani M. Foxo1, a novel regulator of osteoblast differentiation and skeletogenesis. The Journal of biological chemistry. 2010;285:31055-65
36. Yayama T, Uchida K, Kobayashi S, Kokubo Y, Sato R, Nakajima H. et al. Thoracic ossification of the human ligamentum flavum: histopathological and immunohistochemical findings around the ossified lesion. Journal of neurosurgery Spine. 2007;7:184-93
37. Matsuzaki T, Alvarez-Garcia O, Mokuda S, Nagira K, Olmer M, Gamini R. et al. FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis. Science translational medicine. 2018:10
38. Hameedaldeen A, Liu J, Batres A, Graves GS, Graves DT. FOXO1, TGF-beta regulation and wound healing. International journal of molecular sciences. 2014;15:16257-69
39. Vivar R, Humeres C, Munoz C, Boza P, Bolivar S, Tapia F. et al. FoxO1 mediates TGF-beta1-dependent cardiac myofibroblast differentiation. Biochimica et biophysica acta. 2016;1863:128-38
40. Xu X, Chen R, Li Z, Huang N, Wu X, Li S. et al. MicroRNA-490-3p inhibits colorectal cancer metastasis by targeting TGFbetaR1. BMC cancer. 2015;15:1023
Author contact
![]() Corresponding authors: Zhongqiang Chen, E-mail: puth_czqcom, Department of Orthopedics, Peking University Third Hospital, 49 North Garden Road, Haidian District, Beijing 100191, China and Chi Zhang, E-mail: chi.zhangedu, Department of Orthopedics, Peking University International Hospital, Changping District, Beijing 102206, China; Bone Research Laboratory, University of Texas Southwestern Medical Center, Dallas, Texas 75390, USA
Corresponding authors: Zhongqiang Chen, E-mail: puth_czqcom, Department of Orthopedics, Peking University Third Hospital, 49 North Garden Road, Haidian District, Beijing 100191, China and Chi Zhang, E-mail: chi.zhangedu, Department of Orthopedics, Peking University International Hospital, Changping District, Beijing 102206, China; Bone Research Laboratory, University of Texas Southwestern Medical Center, Dallas, Texas 75390, USA
Received 2018-4-15
Accepted 2018-7-16
Published 2018-8-6
