ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2019; 15(5):929-941. doi:10.7150/ijbs.32489 This issue Cite
Research Paper
Feedback Activation of SGK3 and AKT Contributes to Rapamycin Resistance by Reactivating mTORC1/4EBP1 Axis via TSC2 in Breast Cancer
1. Department of Cell Engineering, Beijing Institute of Biotechnology, Beijing 100850, China.
2. State Key Laboratory of Toxicology and Medical Countermeasures, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China.
3. State Key Laboratory of Experimental Hematology, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin 300200, China.
4. Zhuhai People's Hospital, Zhuhai Hospital Affiliated Jinan University, Zhuhai 519000, China.
*These authors contributed equally to this work.
Abstract
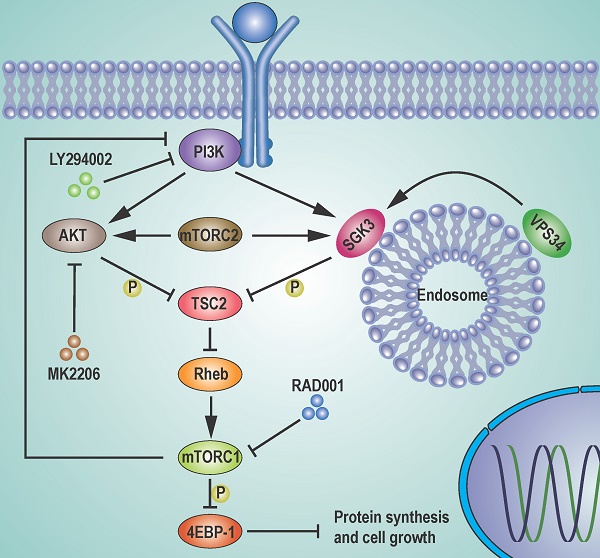
The mTORC1 inhibitors, such as rapamycin and its analogs, show limited antitumor activity in clinic, reasons for which have not been clearly elucidated. Here, we undertook an effort to uncover the mechanisms underlying the limited efficacy of rapamycin, and found that the transit suppression of 4EBP1 phosphorylation led to cap-dependent translation and cell proliferation in breast cancer cells. AKT only partially contributed to 4EBP1 re-phosphorylation. By taking advantage of mass spectrometry-based phosphoproteomic analysis, we identified SGK3 as a potent kinase involved in 4EBP1 re-phosphorylation. SGK3 deletion inhibited 4EBP1 phosphorylation and cap-dependent translation. Importantly, 4EBP1 phosphorylation was positively correlated with SGK3 activity in 67 clinical breast cancer specimens. Moreover, SGK3 deletion in combination with AKT inhibition almost blocked the 4EBP1 re-phosphorylation that was induced by rapamycin and profoundly enhanced rapamycin-induced growth inhibition in vitro and in an MCF7 breast cancer mouse xenograft model in vivo. Mechanistically, the feedback activation of SGK3 by rapamycin was dependent on hVps34 and mTORC2, and reactivated mTORC1/4EBP1 axis by phosphorylating TSC2. Collectively, our study reveals a critical role of SGK3 in mediating rapamycin resistance, and provides a rationale for targeting SGK3 to improve mTOR-targeted therapies.
Keywords: SGK3, AKT, rapamycin resistance, 4EBP1, mTOR, breast cancer
