ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2021; 17(1):204-219. doi:10.7150/ijbs.51362 This issue Cite
Review
Biological characteristics of IL-6 and related intestinal diseases
1. Department of Oral Medicine, Basic Medical College, Capital Medical University, Beijing 100069, China.
2. Undergraduate Student of 2018 Eight Program of Clinical Medicine, Peking University Health Science Center, Beijing, 100081, China.
3. Department of Physiology and Pathophysiology, Basic Medical College, Capital Medical University, Beijing 100069, China.
4. Department of Bioinformatics, College of Bioengineering, Capital Medical University, Beijing 100069, China.
5. Department of Clinical Medicine, Basic Medical College, Capital Medical University, Beijing 100069, China.
6. Experimental Center for Morphological Research Platform, Capital Medical University, Beijing 100069, China.
*These authors contributed equally to this work.
Abstract
The intestine serves as an important digestive and the largest immune organ in the body. Interleukin-6(IL-6), an important mediator of various pathways, participates in the interactions between different kinds of cells and closely correlates with intestinal physiological and pathological condition. In this review we summarize the signaling pathways of IL-6 and its functions in maintaining intestinal homeostasis. We also explored its relation with nervous system and highlight its potential role in Parkinson's disease. Based on its specialty of the double-side influences on intestinal tumors and inflammation, we summarize how they are done through distinctive process.
Keywords: Interleukin-6(IL-6), Intestinal diseases, Inflammation, Tumor, Nerve Immune barrier
Introduction
Nearly all of the nutrient digestion and absorption are carried out in the intestine tract and abundant microbes parasitic in the gastrointestinal (GI) tract may be involved in the process. Through this complex process microbial metabolites, together with remains in the digestive tract, interact with host cells widely and even a seemingly small dysregulation may lead to the breakdown of intestinal homeostasis just like the 'butterfly effect' [1]. The maintenance of this precise balance requires the control of epithelial cells via different immune mechanisms constituting the intestinal immune barrier. Though there is no common definition of the intestine immune barrier, here we put its contents as the mucous layer, immune cells and tissues and immunoactive substance [2]. Immune cells are further distinguished different immune cell types or cell type-specific subsets based on the expression of cell surface and intracellular markers. With a wide variety of biological functions, IL-6 is the hub in mediating the communications among these cells and the cross-talk between intestinal epithelium and the gut microbiota. Not limited to the gastrointestinal tract, its receptor, IL-6R, is widely expressed, which causes inflammation in response to a wide variety of stimuli including infection, stress and trauma [3], for example, in the liver leading to the acute phase protein response, in the hypothalamus together with IL-1β leading to systemic fever [4], and in the gut leading to Th17 activation [5]. Within the scope of this review, we just limit it to the functions related to the intestine.
As a polypeptide, IL-6 consists of α and β chains whose structure has been fully elucidated and used for targeted therapy widely [6, 7]. It can be secreted by several kinds of cells, from intestinal epithelial cells to lymphocytes, and a large amount of studies has offered promising targets. However, as it is difficult to control the multiple sources, there is still a long way to go from theoretical feasibility of pharmacology to clinical application. In this review, we focused on the role of IL-6 in the function and overlapping relation with intestinal dysfunction.
Discovery and structure of IL-6
When Toshio Hirano et al. first reported the molecular cloning, structural analysis and functional expression of the cDNA encoding B-cell stimulatory factor 2(BSF-2), they deduced that it is a novel interleukin containing 184 amino acids [8]. At that time, the dideoxy method and gas-phase protein sequencer were used to obtain the complete neucleotide sequence of the insert cDNA as well as the partial amino-acid sequence of each fragment of the BSF-2. With those methods researchers further compared the protein sequence of BSF-2 with all proteins in the National Biomedical Research Foundation Database (NBRF) and Genetic Sequence Data Bank (GSDB) but no strong sequence similarity was revealed in the comparison. Then its many other functions such as promoting the proliferation of hepatocytes were verified and they are not limited to plasma cells. As most of them are related to the acute phase protein and interactions among lymphocytes, it was finally termed as lnterleukin-6 (IL-6). With technology advancing, its structure has been fully illuminated [6] and the history of its discovery has also been specially reviewed [9], which may have strong implications for future investigations.
Maintaining homeostasis in physiological state (Function of IL-6)
In normal conditions, serum IL-6 concentration is about 1.6pg/ml, narrowly mediating mild immune responses in defense of invasive pathogens [10]. Based on its interaction with vagus [11], it may also impacts the smooth muscle cells or secretory cells and then consequently the intestinal motility or secretion.
Participating in the immune-epithelial-bacteria function regulation cross-talk
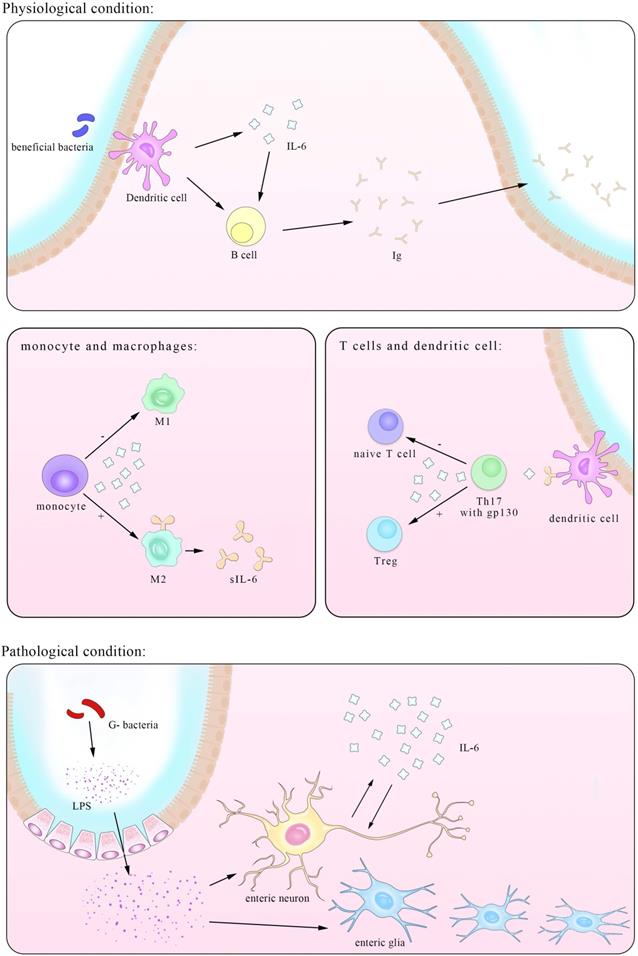
Gastrointestinal tract, an essential part of digestive system, is mainly responsible for absorbing nutrients. A total number of about 1013-1014 CFU/mL bacteria residing in the intestinal tract are involved in this process, whose metabolites together with food residues make up most of contents in the intestine directly or indirectly in contact with the mucus layer isolated from epithelial cells. IL-6 plays an essential role during the immune-epithelial-bacteria cross-talk in both the beneficial and harmful processes carried out by different types of microbiota. Pediococcus acidilactici K15, selected as the most effective strain of lactic acid bacteria for stimulating IgA production, induce the secretion of IL-6 in BDCA1+ DCs (mDC1s) via its double-stranded RNA and enhance the production of protective IgA by B plasma cells [12]. As excessive IL-6 may initiate inflammation, another experiment by Vinderola et al. showed increasing level of iIgA-producing cells without corresponding enhancement in the CD4+ T-cell population in mice after orally administered strains of lactobacillus (LAB), indicating that the effect was limited to plasma cells secreting IgA. Further comparation between the influence by LAB and E.coli found that higher level of IL-6 secreted in a TLR2-dependent manner is responsible for the enhancement of IgA and this could also be seen in other probiotics such as L. casei CRL 431 and L. helveticus R389 [13]. IL-6 level induced by these probiotics variety is precisely enough for B cell differentiation but not adequate for T cells, consequently maintaining the immune response to a proper extent. However, with IL-6 being able to promote inflammation progress most of the time; many probiotics alleviate intestinal pathology via reducing its level. Forit was verified that the mixture of Lactobacillus mucosae NK41 and Bifidobacterium longum NK46 from human feces significantly mitigated IL-6 level and immobilization stress (IS)-induced anxiety-like/depressive behaviors [14]. And short chain fatty acids (SCFAs) generated by bacteria such as L. acidophilus KLDS1.0738 [15] act as stimuli for macrophage and reduce IL-6 secretion in vivo which is studied using mice. In turn, injection SCFAs has achieved promising results that allow for further clinical trials [16]. Despite those beneficial microflora, some metabolisms or its own metabolisms serve as pathogens, cause inflammation and put the intestine in danger, many of which involve the increase of IL-6 level [13, 17, 18], But exceptions also exist, such as that in Lycium barbarum polysaccharides (LBPS) treatment of cyclophosphamide (CTX)-induced mice, the increasing level of IL-6 is concomitant with abundances of Bacteroidaceae, Lactobacillaceae, Prevotellaceae and Verrucomicrobiaceae positively associated with immune traits [19].
The imbalance of those beneficial and harmful species is regarded as the origin for many diseases and clinical trials found mitigatory symptoms in various intestinal diseases such as UC and colorectal cancer after probiotic treatment, always accompanied by lower IL-6 level [20, 21]. But this kind of treatment remains conflicting, suggesting more multifaceted interactions between bacteria and intestinal immune system in which IL-6 serve as an important mediator [22]. Further, much detailed work remains to be done due to unique characteristics of microflora as even the same bacteria family can exert opposite influence on the same cells for its complex compositions and interactions, such that in the Bifidobacterium adolescentis strain IF1-11 induced significantly higher IL-6 and lower IL-10, contrary to that in IF1-03 [23].
Also considering that the bacteria-associated treatment mainly modulates the long-term intestinal functions while the drugs targeted IL-6 are generally used to alter the acute symptoms, combined treatment of them may offer the patients better therapeutic efficacy.
Maintaining mucosal integrity
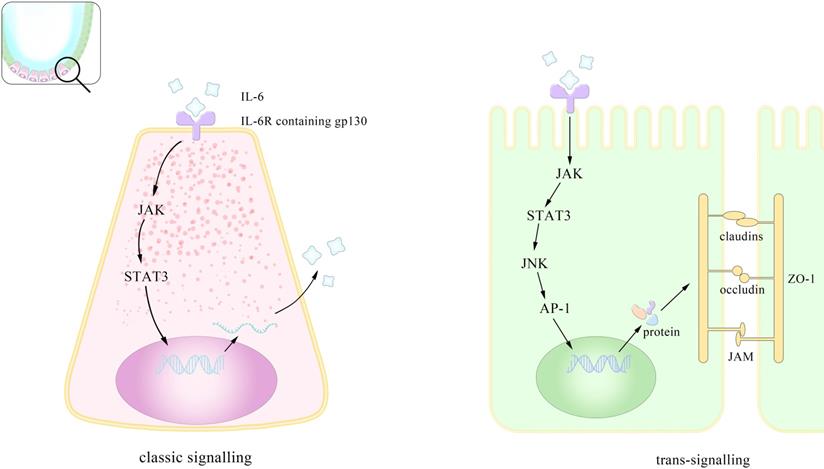
The mucous layer serves as the out-most colonic barrier exposed to pathogens and contains mainly mucin2 (MUC2) secreted largely by the goblet cells with toll-like receptors (TLRs). Studies have demonstrated that the IL-6 mediated Jak/STAT3 pathway may drive goblet cells differentiation via its downstream PI3-kinase/Akt signal peptide corroborating the previous finding of visible damage of mucosa in IL-6-/- mice [24]. The second defensive barrier are the junctions between epithelial cells including tight junction (TJ), adheion junction, desmosomes connection and gap junction from top to basement. TJ, deemed to be cardinal, is composed of occludin, claudins, junctional adhesion molecule (JAM), zonula occludens (ZO) and limits the passage of macromolecules [25] and microorganism [26, 27]. TJs are important intestinal barrier against exogenous pathogens and largely determine the intestinal permeability modulated by IL-6. To investigate the underlying mechanism between them, researchers carried out experiments both in vivo and in vitro (consisting of filter-grown Caco-2 monolayer), corroborating that IL-6 enhance the intestinal TJ barrier in a size dependent manner relying on the increasing expression of claudin-2. IL-6 caused about a 3- to 4-fold increase in trans-epithelial flux of smaller-sized paracellular marker urea without affecting the flux of larger-sized molecules including mannitol, coinciding with the factor that claudin-2 dependent pore pathways allowing paracellular flux of ions and smaller sized molecules <4.0 Å in molecular radius also indicating that the increasing permeability is limited to smaller-sized molecules. They further verified that IL-6 activated JNK (c-Jun N-terminal kinase) signaling pathway, leading to the activation of transcription factor AP-1 and consequently a sequential activation of claudin-2 gene. Despite the same finding that IL-6 increase TJ permeability via enhancing claudin-2, Takuya Suzuki et al. attested the up-grading level of claudin-2 gene in a MEK/ERK and PI3K-dependent manner with the involvement of transcriptional factor Cdx2 (caudal-related homeobox-2) [28]. Considering that Cdx2 is only expressed in the epithelial cells at the luminal surface in normal colons but is found in the colonic crypt in patients with Crohn disease (CD) and ulcerative colitis (UC), and that its expression in mice intestine also correlates with claudin-22, the different models they used may account for the distinctive outcomes in two studies [29]. In fact, besides the role in physiological conditions, IL-6 also pathologically promotes the mucosa preservation and facilitates mucosal repairing, which will be discussed in part 3.3. Composing both phospholipid (PL) and protein, integrity of membrane in intestinal epithelial cells can also be influenced by IL-6 mediated alteration of secretory phospholipase A2 (sPLA2). Researchers believed that IL-6 leads to changing in the composition of (PL), more exactly, an increase in phosphatidylethanolamine (PE) and sphingomyelin (SM) and a decrease in phosphatidylcholine (PC) and lysophosphatidylcholine (LPC) [30]. PE as well as SM serves as inflammatory signaling factors in IBD suggesting that they may function as mediators of the ascending level of sPLA2 induced by IL-6. Previous studies also found this enhancement in IL-6 level was achieved via the regulation of C/EBP-β dependent on epithelial detachment rather than certain proteases [31].
Apart from the induction of proteins as junctions, IL-6 up-regulate the expression of several genes responsible for proliferation and anti-apoptosis after treatment with C. rodentium, including the Bcl family members Bcl-xL and Mcl-1 (but not Bcl-2), the IAP family member cIAP-2 (but not survivin), and the NF-κB family member, Bcl-3 [32]. Also a meta-analysis showed no difference in IL-6 level whether in the appearance of probiotics or not in the colorectal cancer after operation, so considering the various types of probiotics more human experiments with the control of single strain are demanded [33].
Despite the fact that most pathogens are resisted by integrated mucous layer, the remaining ones still cause danger to epithelium and their removal require the participation of other epithelial cells by direct phagocytose or initiating immune responses. During the process, IL-6, an indispensable important factor, involves in most of the immune response and a heavy load of researches have given detailed descriptions [34]. In fact, apart from the well-known role in inflammation and tumor which we will discuss in part 3 and 4, IL-6 is required for tissue repairing after injury for its induction of intestinal epithelial proliferation. Here we just highlight its relationship with Paneth cells, the ones exclusively exist in the intestine crypt [35]. Recently Jeffery et al. verified its role in the maintenance of the crypt stem cell niche using mice in vitro crypt organoid and in vivo models [36, 37] This is in the control of autocrine IL-6 secretion through the Wnt signaling pathway, supporting previous hypothesis that besides the role in promoting intestinal regeneration after injury, IL-6 also play a part in modulating crypt homeostasis [38]. To determine the impact of IL-6R signaling on intestinal proliferation and repair under exogenous stress, it was exposed IL-6Rfl and IL-6RΔIEC mice to DSS, the results indicated that the mice of both genotypes started to lose weight, but IL-6RΔIEC mice seemed to cope better with DSS-induced colitis than WT as they significantly lost less weight than IL-6Rfl mice.
Pattern of mechanisms of IL-6 promoting the integrity of the intestinal epithelium. IL-6 assists maintain intestinal homeostasis through both classical and cross-signaling pathway. This is the enlarged view of the role IL-6 plays in the intestinal epithelium. IL-6 promotes the proliferation of paneth cells in the crypt through (JAK/STAT3 classic signaling). This preserves the intestinal integrity by inducing gene transcription via trans-signaling (sIL-6R: soluble IL-6 receptor; ZO: zonula occludins).
Originally termed as B-cell stimulating factor-2 (BSF-2), IL-6 first came to be known as irritation for B cells to secrete immunoglobulin (Ig) [39]. Then it gradually won the name of cytotoxic T-cell differentiation factor for its role in inducing T cells maturation [40]. Now IgA, M, D, E and G have been recognized for their roles in polymerizing and adhering pathogens though alterations of different kinds of Ig varied among experiments in UC [41]. Also IL-6 is generally acknowledged to be the transmitting factor which induces the proliferation and differentiation of plasma cells from immune cells like macrophages [42]. Most of the subsets and their respective functions have been clarified, and what is deserved to be mention is a recent investigation on the connection among IL-6, T helper17 cells (Th17) and T regular cells (Tregs) for Th17's unique characteristic as secreting IL-17. The break of this precise balance has a close correlation with inflammation and we will discuss it in part 3.2.
Based on its close connections to multiple cells and functions, IL-6 related targeted treatments have received long-term attention. However, several trails have shown that its blockade may lead to unveiled by-effects unless more specific antibodies or drugs are found or synthesized. In brief, Figure 1 showed that IL-6 help, with the maintenance of intestinal homeostasis through both classic signaling and trans-signaling pathways.
Receptor and signaling pathways of IL-6
Researchers have corroborated IL-6 signaling in three pathways, classic-signaling, trans-signaling and trans-presentation. All of the three ways above demand the combination of IL-6, monopeptide glycoprotein IL-6 receptor (IL-6R), and glycoprotein130 (gp130) (mainly site III) and the differences lie in the existing forms of the substances as well as their differentiated functions as a result. The classic signaling requires membrane IL-6R and is normally seen in leukocytes and liver cells while the trans signaling utilizes soluble IL-6R (sIL-6R) and plays important roles in times of tumors. The dose response curve for the classic one is bell-shaped coinciding with the fact that high concentration of IL-6 induces the cleavage of sIL-6R and consequently the trans-signaling pathway [43]. Both the two ways involve the formation of a complex hexameric formed by double sequentially combination of IL-6, IL-6R and gp130 and further activate different downstream pathways related to gene transcription [44]. The downstream pathways include JAK/signal transducer and activator of transcription (STAT3), Src homology 2 domain-containing protein tyrosine phosphatase-2 (SHP-2)/extracellular-signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK), phosphoinositol-3 kinase (PI3K)/protein kinase B (PkB)/Akt and SRC/YAP [45, 46]. In JAK/STAT pathway the major activation is performed on STAT3 and one of its downstream molecules, the suppressor of cytokine signaling (SOCS3), can inhibits the catalytic activity of JAK. Controlled by gene DNMT1 [47], the expression of SOCS3 serves as a natural inhibitor of STAT3, with the same effect happening in the protein inhibitors of activated STAT (PIAS) [24]. Intriguingly a clinical investigation showed that SOCS3 level increased in UC but dropped in UC-CRC. SOCS3 gene methylation may account for its decrease in UC-CRC and its increase in UC likely explains the lesions but this contrary alteration overall suggests a more complex association between IL-6 and SOCS3 [48]. Besides SOCS3, there exists another kind of natural inhibitor of IL-6 signaling. By binding to the complex of IL-6/sIL-6R, it selectively block the trans-signaling with high affinity and studies have shown its concentration highly related to the tumor progress [49]. Although recombinant sgp130 has been used in the treatment of inflammation, illuminating the modulating factors of it in vivo may benefit the immunotherapy against tumor [50].
The third pathway recognized recently involves similar molecules but in different cells. As figure 2 shown that A subset of CD11b+ DCs that are Sirpα+ express IL-6Rα which bound to IL-6 and then presented to the gp130 on myelin peptide specific encephalitogenic T cells. Although classic IL-6 signaling is sufficient to suppress the transforming growth factor β (TGF-β)-induced expression of Foxp3, this process is proved indispensable for the priming of pathogenic TH17 cells [51].
Many blockers in this process have been synthesized or discovered and experiments aimed at testing their efficacy and side effects are also operated on a large scale. Not only the antibody targeted directly on IL-6 but also the one on its membrane receptors and the components of its upstream activators, such as JNK, extracellular signal-regulated kinases (ERK) and p38 are intensively researched. Although blockers of critical molecules in the downstream pathways mentioned above are meticulously studied with reviews for comprehensive summary [6]. Now several approaches such as genetic knockout and antibody incubation have been put into use clinically and could probably offer patients a bright prospect.
Relevant to inflammation associated diseases
The gut wall is protected by a well-developed immune system and an intestinal mucosal barrier composed of epithelial cells and mucus layers secreted by goblet cells, and this contains commensal bacteria that regulate the passage of fluids, macromolecules and antigens. It may help in limiting bacterial colonization by releasing mucus, antimicrobial peptides and immunoglobulins [52]. Normally it assists with the elimination of pathogens but long-term inflammation such as the CD and UC may lead to a serious of pathological manifestation seriously affect the patients quality of life. IL-6 is normally known to aggravate inflammation by both directly driving lymphocytes proliferation as well as differentiation and directly/indirectly through nervous system. Nevertheless, the anti-inflammatory functions like fresh for many people, was gradually unveiled. Like a key hub in the busy transporting net, IL-6 acts as a mediate in many functions in the gastrointestinal immune barrier [53-55].
IL-6 as a vital mediator between the neuro-immune axis
Apart from the well-known regulatory role in immune system, IL-6 is an important mediator in the interactions between nervous system and immune system in both direct and indirect ways. Overwhelming studies have demonstrated highly bi-directional relationship between intestinal diseases and mental diseases accompanied with neuron lesions [56]. It is reported that increasing IL-6 level in adult mice intestine is secreted by enteric glia with the stimuli of IL-1β or LPS [57, 58]. Intriguingly, exogenous IL-6 suppresses the expression of IL-6 mRNA in a dose-dependent way. Another experiment by Burgueño et al. further demonstrated that upon treated with LPS, enteric neurons expressing TLR2/4/9 secrete IL-6 in NF-kB dependent pathway. But the way IL-6 secretion is not limited to this for pretreated with Bay 11-7082 largely reduced the production of IL-6 but not completely abrogated it [58]. Besides the direct secretion of IL-6, nerves inhibit IL-6 expression in lymphocytes, based on the fact that Ach receptors are found on them and that IL-6 level in vagotomized mice were higher than that in sham-operated mice [59]. Activation of β-adrenoceptors protects the intestine from the detrimental effects by inhibition of IL-6 elevation as well as other cytokines [60] and acetylcholine is found to have the same impact in a post-transcription manner depending on MФ nicotinic acetylcholine receptor (alpha7nAChR) [61].
Besides the impacts ENS exert on IL-6 secretion, IL-6 in turn affects ENS via several ways. In dissected and dissociated myenteric plexus of newborn rats, the highest average neurite outgrowth per neuron was observed in GDNF-treated and Hyper-IL-6-treated cultures, indicating an essential role it plays in ENS survival and differentiation [62]. And IL-6 is capable of reducing the expression of vasoactive intestinal peptide (VIP) in biopsies of CD patients and healthy subjects, although the precise source remains unconfirmed [63]. VIP is an important peptide in the brain-gut axis and its function in repressing the activation of tumor-associated macrophages is also reported [64]. IL-6 can also exert direct excitatory effect on a subset of myenteric neurons and reversibly promote the presynaptic inhibition of acetylcholine released from cholinergic nerve terminals [65]. It also suppresses norepinephrine release by myenteric nerves in a dose-dependent way and with IL-1β synergizing, subthreshold level of IL-6 can also exert similar influences as in higher concentration [66]. Taken together, these findings suggest that IL-6 is an important neuromodulator of gastrointestinal motility which correlates closely with many diseases within and outside the gastrointestinal tract (refer to Box2 for more details).
Moreover, higher level of IL-6 is witnessed in irritable bowel syndrome (IBS) patients but its correlation with gastrointestinal dysfunction remains unknown. Researchers using maternal separation (MS) mice found increase activity of submucosal neurons is partly dependent on IL-6 in a Ca2+-mediated manner. This process requires membrane-bound IL-6R which is expressed predominantly in neuronal fibers and further initiates different pathway for gene transcription of various functions [67, 68]. Noticing that the calcium response could be potentiated by the corticotropin-releasing factor (CRF) [69], further studies investigated the cross-talk between IL-6 and CRF in colonic submucosa neurons and found that IL-6 also potentiate CRF-induced calcium response and CRF stimulates the colonic secretion of IL-6 by submucosal neurons and T-helper lymphocytes with CRFR1. It also enhances IL-6R on neurons and activates IL-6 induced MAPK but not STAT3 pathway [70]. Studies using LPS or directly administration of IL-6 showed its role in hypothalamic-pituitary-adrenal axis(HPA) activation but as those IL-6 could hardly been restricted to intestine, we will not give more descriptions of it due to the scope of this review [71]. Similar studies have convinced more and more places where nerve physiological conditions are related to IL-6 levels, but unfortunately many cytokine concentration alterations emerge simultaneously so it is hard to make variety control [63]. The use of monoclonal antibodies or gene knockout technology is likely to solve the problem. Also now a large proportion of articles concerning the impact of IL-6 on neurons are confined to the CNS, so more attention to peripheral nerves probably shed light on novel therapies.
Box 2: IL-6 and gastrointestinal motility
Gastrointestinal motility is essential for food digestion and daily detoxification and its stasis is frequently concomitant with multiple organ dysfunction syndrome (MODS), which show marked up-regulation of IL-6 correlated with reduced colonic motility and probably result in gastrointestinal smooth muscle dysfunction [72]. Although IL-6 has no impact on the spontaneous contraction of colon in normal rats, it has conflicting influences in different disease models [73]. Studies showed that higher IL-6 level could be induced by damaged muscle fibers and neutralizing IL-6 receptor antibodies (xIL-6R) improved the symptoms in Dystrophin-deficient C57BL/10ScSn-Dmdmdx/J (mdx, dystrophic) mice [74]. Another type of intestinal dyskinesia is postoperative ileus (POI) accompanied with higher IL-6 level, and IL-6 mRNA from muscularis extracts demonstrated a significant induction after intestinal manipulation [75, 76]. The negative correlation between IL-6 level and muscle contractility was also verified in an experiment using repeated LPS injection to induce muscularis cross-tolerance to POI. And this relation, mainly via the NF-kB, was not shown in mucosa, indicating the exclusive effects IL-6 exert on the muscularis [77], this phenomenonis also elucidated in UC [78] as well as diseases in other organs[79] and various kinds of colonic damages [80, 81].
Many systemic diseases also show alterations in gut motility. Colonic dysmotility accompanied with significantly elevated blood plasma IL-6 levels occurs in type 1 diabetes and studies demonstrated the increased contraction of distal colon mediated by IL-6 in the process [73]. Similar to IBS, exogenous IL-6 in myenteric plexus also facilitates contraction of proximal colon in chronic unpredictable mild stress (CUMS) induced depression model, coinciding with the higher level of IL-6 in depressed patients [82]. Considering the Ca2+ response IL-6 could induce and findings that increased Ca2+ by ERK and p38MAPK pathways lead to colonic smooth muscle hypercontractility, it may be reasonable provide an explanation and even a treatment for those findings mentioned above [83].
IL-6 involved in lymphocytes orchestration and promoting proliferation or differentiation
Inflammatory bowel diseases (IBD), including UC and CD, a kind of chronic disease in intestine, seriously diminish patient's life quality. They are tricky clinical problems due to ambiguous causes and lack in radical cure, indicating a complex etiology lying behind. Multiple factors such as susceptible genes [84, 85], dietary habits and psychological condition [86, 87] are shown to be relative although clear pathology remains unveiled. Both CD and UC share common symptoms like recurrent abdominal pain and constipation or diarrhea in the intestine accompanied with multiple uncertain systemic diseases that universally occurred in gastrointestinal dysfunction. However, they also distinguish from each other in many aspects, such as typically CD is profiled by more T helper1 (Th1) and Th17 compare with Th2 whereas UC is characterized by a majority of Th17 and Th2. The up-regulated level of IL-6 has been confirmed in both diseases and depletion of IL-6 alleviates both symptoms, suggesting a pivotal role it plays in the process [7]. Studies confirmed that macrophages (Mφ) and CD4+ T cells are major sources of IL-6 in IBD, with Mφ being mainly influenced by macrophage-migration inhibitory factor (MIF). IL-6 polarized Mφ into a pro-inflammatory type M1 which secret cytokines and molecules aggravating inflammation [88]. In fact, with the expression of mIL-6R decreasing in epithelial cells in chronic colitis (CC) and colitis-associated premalignant cancer (CApC) mice model, sIL-6R shed mainly from the Mφ are important for the downstream effects of IL-6 [89]. These include the enhanced expression of anti-apoptotic genes Bcl-2 and Bcl-xl in T cells via STAT3 pathway while STAT3- independent pro-apoptotic gene Bax was unaffected [90]. IL-6 also involve in the disease progress by regulating T cell differentiation, for it is demonstrated that IL-6 in combination with TGF-β triggers STAT3 pathway and induces retinoid-related orphan receptor γt (RORγt) and RORa expression in Th17, a decisive transcription factor controlling the differentiation of Th17 lineage [91, 92], which may secrete pro-inflammatory cytokines including IL-17 and IL-6 thus increase the inflammatory response. Intriguingly, in IL-6-/- mice, TGF-β sorely promote the generation of Foxp3+ and initiate Treg suppressing inflammation [5]. In fact, further studies confirmed the role of IL-6 largely caused by disturbing the intercellular FOXP3 and chromatin-modifying enzyme EZH2 interaction [93], and CD103+CD11b+ DC serve as the main IL-6 source [94]. Based on this, PF-04236921, a human monoclonal antibody against IL-6, was exploited to cure IBD in phase II RCT. Crohn's Disease Activity Index (CDAI)-70 response rates with PF-04236921 50 mg were notably improved than placebo despite later trails were forced to an end due to unreasonable abscess and perforation [95].
Besides the most typical T cells involved in IBD, IL-6 exert influence on many other cells. In CD, the secretion of IL-6 in epithelial cells leads to the reduction of intercellular cell adhesion molecule-1 (ICAM1) via NF-κB [96, 97]. Also incubation with IL-6 result in lower expression of endothelial protein C receptor (EPCR) and thrombomodulin (TM) by the vascular endothelial cell (VEC) which consequently enhances coagulation and limit the anti-inflammation effect [41]. These results hinted that treatment targeted IL-6 may bring patients a promising prospect.
Intestinal epithelial cells as well as immune cells like antigen presenting cells (for example monocytes [98] and Mφ [99]) are major sources of IL-6 whose secreting mechanisms have been widely reported (refer to Box3 for more interactions between IL-6 and immune cells in the intestine). But studies in mice found that other types of cells including intestinal smooth muscle cells could also secrete IL-6 with the stimuli of IL-1β [100]. This is conflicting since in another experiment, after infected with Trichinella spiralis, mice showed that no significant distinction between the euthymic and athymic despite the general enhanced expression level of IL-6 in the longitudinal muscle myenteric plexus (LMMP), suggesting a less essential role for its contribution in muscle growth and contraction [101].
Box 3: Interactions between IL-6 and intestinal immune cells in other diseases
Pathologically intestinal manifestations are related to various organ diseases. Immunohistochemistry in decompensated cirrhosis patients showed colocalization of IL-6 with CD68+ iNOS+ Mφ and IL-6 was predominantly present in CD11c- cells [99]. Another study in mice showed IL-6 specifically derived from DCs are crucial in defense of Giardia duodenalis. In mice infected with Giardia Lamblia, IL-6 generated by mast cells were necessary for intestinal protective immunity [102]. In cirrhotic patients with portal hypertension (PHT), hepatic venous pressure gradient (HVPG) significantly correlates with abnormal gut permeability and higher IL-6 levels [103]. And IL-6 contributed to colitis-induced platelet responses including thrombocytosis and platelet hyperreactivity via the maturation and activation of megakaryocytosis [104]. Further studies demonstrated that IL-6, secreted by bone marrow-derived blood cells, mainly leads to accelerated thrombus development within the inflamed colon [105].
And the role of IL-6 is not limited to the digestive tract but in correlation with many diseases in other systems as well as some systemic diseases [106]. For example, obesity induced IL-6 promote the polarization of Mφ into a tumor-promoting type and thus exacerbate CAC progression [107]. In brief, altering the intestinal microenvironment are closely related to body physiological functions and IL-6 is an important mediator in both interior intestine and interactions between intestine and other organs just like heart and kidney.
IL-6 as an important member of the anti-inflammatory effect
As mentioned above, apart from its physiological functions, the impact of IL-6 in mucosal repairing after injury are vital for body recovery and are an essential part of its anti-inflammatory roles. It is reported that probiotics like Bacteroidales can recruit IL-6 and enlighten TJ by promoting the secretion of claudin-1 and mucin-2 which consequently alleviate inflammation. This further convinced their former observation that the colitis mice with anti-IL-6 mAb treatment beginning at the onset of co-housing led to significantly greater weight loss and decrease in crypt number over time compared with control group [108]. Diseases in other organs can also influence the intestinal epithelial permeability during which IL-6 possess an indispensable role. During hemorrhagic shock and resuscitation (HS/R), IL-6-/- mice showed markedly attenuated tissue injury and intestinal permeability compared with the wild type (WT) group [109]. However, there lies some difference in the inflammatory state among outcomes from various laboratories. Inflammation was down-regulated in IL-6-/- mice after HS/R in the experiment by Yang et al., while this condition appeared after intravenous administration of IL-6 by Meng et al. [110]. Considering the pleiotropical nature of IL-6, this distinction may arise from the dose of IL-6 they used.
Studies have found that exogenous IL-6 inhibit acute inflammatory responses and prevents ischemia/reperfusion (I/R) injury after intestinal transplantation. Although no differences are observed in TJ, the higher level of SOCS3 compared with control group and related lower serum level of pro-inflammatory cytokines may be a reasonable explanation [111]. Other protective roles of IL-6 in I/R lies in its restoration of microvascular by oral administration and reduced apoptosis via increased bcl gene expression [112, 113]. Also in systemic bacteraemia following haemorrhagic shock oral administration IL-6 alleviate the symptom by reducing bacteria translocation from the gut and with less bacteriological cultures within the mesenteric lymph nodes [114, 115].
In active inflammation, adenosine released in the intestinal lumen could induce IL-6 secreted by intestinal epithelial cells, which activated neutrophils degranulation by an intracellular Ca2+ flux [116]. This recruiting and activation of neutrophil could exert double effects for both recovering after colitis in bacteria-depleted mice and secreting substances such as IL-6 stimulated by IL-1 [17, 117]. The relationship and underlying mechanisms between IL-6 and inflammation have received much attention and contrary opinions existed concerning whether classic-signaling promotes anti-inflammatory effects and epithelium proliferation [7, 118]. Apart from the need for clarification on mechanisms, propagating drugs into clinical trials and applications is also a pressing work that cannot be neglected.
The controversy over the essential characteristics of IL-6 has received long-time attention. Here we summarize its roles in inflammation in Figure 2 and find that many of them are in fact precise balance.
Double-edged sword effect of IL-6 in tumorigenesis
Tumor-promoting effects
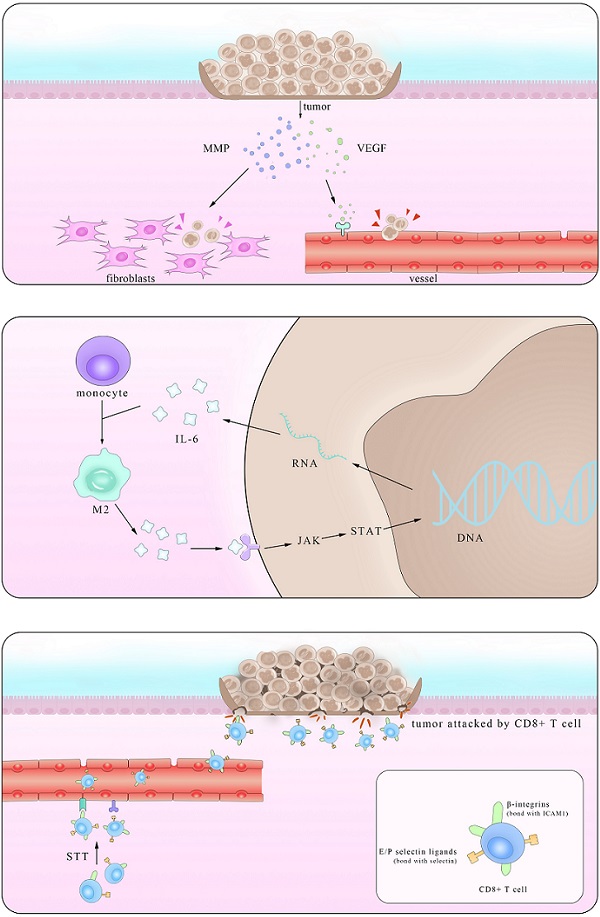
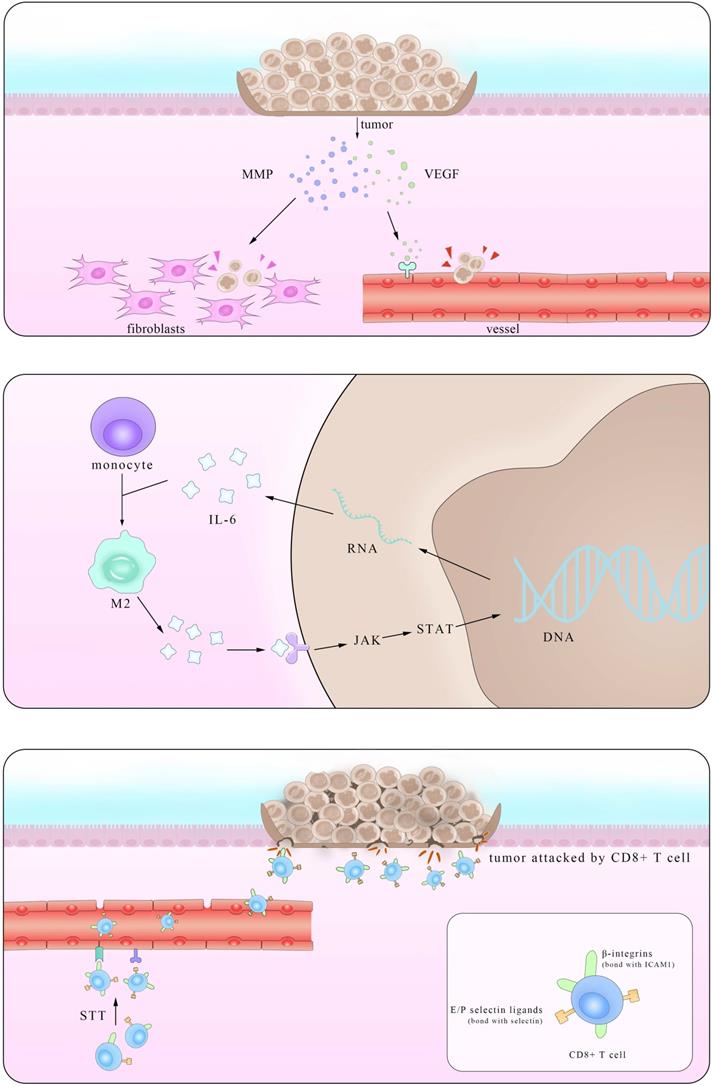
Epidemiological statistical results showed that patients with IBD have a higher risk of colorectal cancer (CRC), suggesting a close relationship between them [119]. In fact, major interactions between lymphocytes mediated by IL-6 show paramount resemblance and the distinction mainly lies in the immunosuppressive influence IL-6 exerts on Mφ and T cells in times of colorectal cancer which promote the immune evasion of tumors. It is reported that IL-6 secreted by colorectal cancer cells enhance the phagocytic capacity and migration of Mφ using a monocyte-macrophage THP-1 cell model and human peripheral monocytes [120]. These Mφ, derived from monocytes recruited also by IL-6, are actually M2-like tumor-associated macrophages (TAM) which could express a series of cytokines including IL-6 to promote tumor progression [121]. Another experiment demonstrated that IL-6 synergize with TGFβ1 induced the generation of CD8+CD25+Foxp3+ T cells (T8reg) ex vivo. With an ability to suppress CD4+ CD25- T cell proliferation and Th1 cytokine production, this kind of T8reg has strongly suppresses the function of immune system [122]. Studies also found the number of CD8+T cells show marked decrease in IL-6 overexpressed tumors due to impaired infiltration induced by IL-6 [123], consistent with the fact that this effect is significantly improved after STT, which will be depicted in details in Part 4.2.
An illustration of the IL-6-involved in cross-talk between intestinal bacteria and immune cells in both physiological and pathological conditions. IL-6 has close interactions with ENS within both enteric neurons and enteric nervous glia. The trans-presentation signaling pathway between DCs and naïve T cells is also showed here, suggesting a pivotal role of T cells in IL-6 mediated inflammatory process.
Working mainly via the trans-signaling pathway in the regulation of TGF-β [124], IL-6/JAK/STAT3 signaling axis is at the core of regulating many gene transcription and expression that play crucial roles in the generation and development of tumor [125]. And this axis exerts both inter-cellular and extra-cellular pro-tumor effects, coinciding with the results of previous clinical cases that increasing level of IL-6 in CRC patients indicates high mortality and bad prognosis [126, 127].
Various kinds of gene mutation are the radical reason of tumor generation and IL-6/JAK/STAT3 aggravate the influence by promoting excessive gene expression. Inter-cellular pro-tumor activities of IL-6 mainly concern mutant genes in charge of cell cycle, apoptosis and those proto-oncogenes and anti-oncogenes. There is a previous review mainly focusing on the role of STAT3 pathway [128], so here we just introduce p53 gene, the most frequently altered one in colorectal carcinomas, protect cells from canceration by inducing apoptosis and suppressing mitosis. However, in the presence of IL-6, p53 DNA exhibit methyltransferase and lose its viability, resulting in unlimited cell proliferation [129]. The activation of genetic transcription in many cells via both autocrine and paracrine like a chain action also leads to the deterioration of diseases, like that happens in the truncated Retinoid X receptor-α (tRXRα) murine which show an up-level in IL-6 concentration through NF-κB pathway. This enhancement was also found in UC patients, indicating an increasing susceptibility of colorectal cancer for them [130]. Apart from the inter-cellular impact, IL-6 promotes tumor invasion, agiogenesis and metastasis by enhancing the expression of a disintegrin metalloprotease 17 (ADAM17), vascular endothelial growth factor (VEGF) and matrix metalloproteinase (MMP). ADAM17 promotes the cleavage of IL-6R and thus enlarges the trans-signaling effects IL-6 exert [131]. Also considering previous reports that IL-6 potentiated the cross-talk between Mφ and tumors in other kinds of cancers, it is reasonable to inquire whether these happened in IBD and CRC [132]. Further investigations in answering these questions will benefit the clinical application of these achievements.
Anti-tumor effects of IL-6
Based on its pro-tumor effects, many primarily developed pharmaceuticals with completely IL-6 blockade were tested and used. However, these drugs improved symptoms at the cost of unpredictable side effects, presaging a multifaceted correlation among IL-6, tumor and body immune system. In fact, it was not until recent decades that the function of IL-6 in restraining tumor growth with hyperthermia came to be noticed. Daniel et al. found that by using CT26 colorectal tumor, after treated with systemic thermal therapy (STT), marked lower tumor volume and a larger number of TUNEL+ apoptotic cells were detected in tumor vicinity. In the meantime, statistical analysis showed higher vessel density and more gathering cytotoxic T cells accompanied with similar IL-6 level alterations, suggesting the existence of close relationship between them. Then, to further block the function of several mediates related to the trafficking of CD8+T cells respectively, the results clarified the kernel role E/P-selectin and ICAM-1 played in the transference. To elucidate the exact part IL-6 participated, researchers established IL-6-/- mice and verified comparably decreased E/P-selectin and ICAM-1 in contrast to the WT group after STT treatment. Some studies have corroborated the positive relevance between the expression level of E/P-selectin as well as ICAM-1 and tumor metastasis. Together with the findings here we can draw the conclusion that IL-6 inhibits tumor growth by promoting the expression of E/P-selectin and ICAM-1 via JAK/STAT3 pathway and consequently facilitating T cells infiltration. Notably, this effect was also witnessed in co-culture with STT treated mice tissues [133]. Albeit a great breakthrough it may be, much unknown questions limited its clinical application. With many anti-tumor drugs targeted IL-6 as well as its downstream receptors, it is reasonable to query that to what degree and in what condition that the effect IL-6 induced by the thermal treatment can win against the one it plays in the pro-tumor activities.
In brief, the two main ways of IL-6 are not limited to intestine but also in various kinds of cancers, here, we just summarize the researches of IL-6 and its tumor-related functions specifically in the gut (as shown in Table 1). Also its dual functions are shown visually in Figure 3 below.
Being associated with Parkinson's disease
Parkinson's disease (PD) is a common neurodegenerative disease occurred mostly in the aged. The main pathological manifestations witnessed in PD are the degeneration of dopaminergic (DA) neurons in the pars compacta of substantia nigra (SNpc) and marked reduction of DA level in corpus striatum. Similar to the close relationship between the nervous system and immune system in the intestine mentioned above, tremendous investigation have demonstrated that brain inflammation is one of the plausible explanations to the lesions of the dopaminergic neurons.
Pattern of dual mechanism of pro-/anti-tumor IL-6. The left part stands for the anti-tumor effect of IL-6 in promoting the secretion of VEGF and MMP in tumors and consequently enhancing their metastasis. The right part shows that after STT, higher expression levels of E/P selectin and ICAM1 facilitate T cells trafficking, thus restricting tumor progression.
Key factors in IL-6 related gastrointestinal tumor
| Natural inhibitors | Inflammation initiators | Synthetical inhibitors |
|---|---|---|
| Ly6/Plaur domain-containing 8 (LYPD8) [134] | Monocarboxylate transporter 4 (MCT4) [135] | Tocilizumab (TCZ) [136] |
| 6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid (M1) [137] | Glucagon-like peptide-1 (GLP-1) [138] Glucose‑dependent insulinotropic polypeptide (GIP) incretins [138] | Silibinin [139] [herbal] |
| Corticotrophin-releasing hormone (CRH)/CRHR1 [140] | KIOM-MA/MA128 [141] [herbal] | |
| Toll‐like receptor 4 [142] | Cocoa [143] | |
| Sonic hedgehog (SHH) signaling [144] | IL-6 monoclonal antibody (mAb) [145] | |
| Probiotic agent VSL#3 [146]; Balsalazide (BSZ) [146] |
Based on the fact that some typical symptoms in PD like increasing colon transit time and constipation [147] resemble those in IBD, indicating the deficits in the intestinal immune barrier, and that some of them appear even earlier in the intestine than the motor ones finally manifest, researchers postulated the existence of brain-gut-axis modulation system in PD [148]. Also concerning the direction by which the disease spreads, two views exist among scholars, which are from brain to the gut or vice versa [149].
The brain-gut-axis, an inevitable pathway for both directions of signaling, consists of both neural and humoral mediated pathways in acceptance to immune signals. The interactions between IL-6 and nerves have been discussed above in part 3.1 so here we put the emphasis other mediates included in the humoral pathway. With bacteria metabolites and other cytokines and cells able to cross the brain-blood-barrier (BBB) serving as neurotransmitters or neuromediators, the brain receives signals from the gut and send instructive projections back. As discussed before, IL-6 has close interactions with bacteria and play significant roles in the process of inflammation. However, without the ability to cross the BBB, the alteration of IL-6 concentration in the brain results largely from astrocytes and microglia in response to inflammation. And IL-6 in the intestine pass the inflammatory signal to the brain via other cells and molecules bearing the function of diffusing the inflammation, including CD4+T cells, Th17 cells and C-reactive protein (CRP), all of which form close link with IL-6 during inflammation [150]. Of them the α-synuclein (α-SYN) may be the most direct one for its unique characteristics in nervous system, and it can go through the BBB from peripheral nervous system and blood in times of inflammation as well as in the opposite direction. Studies also found increase of α-SYN in both CNS and ENS in times of inflammation, but most experiments just focus on the relationship of the concentration of α-SYN monomers together with its toxic oligomers and IL-6 in the CNS, few researches we found on intestinal IL-6 include the one conducted by Gruden et al., which demonstrated a positive connection of serum level between them [151]. In this way we deem IL-6 to be pro-inflammatory like loudspeakers aggravating the state of illness and setting up a vicious spiral. Another study found the adoption of a fibrillar form of α-SYN after treated with LPS and increasing IL-6 level correlated with TLR-4 [152]. Apart from the role in leading to apoptosis, IL-6 can sometimes be related to the plerosis of neurons and promote the neuronal survival after being treated [153]. Yet the concrete connection between inflammation and α-SYN remains vague and whether IL-6 predominates in the process like that in CNS requires further investigation.
Although the concrete link between IL-6 and PD disease is yet to be found, we still hypothesize that IL-6 in the GI tract is strongly associated with PD through the modification of immunologic function and bacterial related factors. Careful analysis of these findings may provide a novel theraputic effect of the tricky problem.
Conclusion
Owing to its various functions in almost the whole body, it is hard to restrict its level in the single intestine so the improvement here may be at the cost of defection elsewhere. Also on account of sophisticating environment of intestine, sorely alteration in IL-6 is likely to only alleviate the symptom and fundamental solution calls for a healthy diet. Despite the limitations, more clinical trials probably offer patients better choices with less side-effect.
In this review, we summarize the functions of IL-6 in intestinal immune barrier, which has extensive correlation with both nervous system and immune system. With its conflicting roles in inflammation and tumor, these will put forward possible direction for further investigation.
Box 1: Key points
- Since its first clone and structural measurement in 1986, the functions of IL-6 in different organs have been gradually unveiled;
- The signaling pathway of IL-6 can be generally classified into three types, classic signaling, trans-signaling and trans-presentation;
- IL-6 contributes a lot to the maintenance of intestinal homeostasis by participating in the immune-epithelial-bacteria cross-talk and maintaining mucosal integrity;
- IL-6 has double roles in the process of intestinal inflammation and tumor, but the precise balance remains difficult to control;
- Unexpectedly exerting an influence on the peripheral nervous system, IL-6 within the intestine has potential effects on the Parkinson's disease.
Abbreviations
GI: Gastrointestinal; BSF-2: B-cell stimulatory factor 2; NBRF: National Biomedical Research Foundation Database; Genetic Sequence Data Bank: GSDB; IL-6: Interleukin-6; LAB: lactobacillus; IgA: immunoglobulin A; SCFAs: short chain fatty acids; MUC2: mucin2; TLRs: toll-like receptors; TJ: tight junction; JAM: junctional adhesion molecule; ZO: zonula occludens; JNK: c-Jun N-terminal kinase; Cdx2: caudal-related homeobox-2; CD: Crohn disease; UC: ulcerative colitis; HS/R: hemorrhagic shock and resuscitation; WT: wild type; KO: knock out; sPLA2: secretory phospholipase A2; PL: phospholipid; PE: phosphatidylethanolamine; SM: sphingomyelin; PC: phosphatidylcholine; LPC: lysophosphatidylcholine; Tregs: T regular cells; IL-6R: IL-6 receptor; gp130: glycoprotein130; sIL-6R: soluble IL-6R; STAT3: signal transducer and activator of transcription; SHP-2: Src homology 2 domain-containing protein tyrosine phosphatase-2; ERK: extracellular-signal-regulated kinase; MAPK: mitogen-activated protein kinase; PI3K: phosphoinositol-3 kinase; PkB: protein kinase B; SOCS 3: suppressor of cytokine signaling; PIAS: protein inhibitors of activated STAT; TGF-β: transforming growth factor β; ERK: extracellular signal-regulated kinases; VIP: vasoactive intestinal peptide; CRF: corticotropin-releasing factor; IBS: irritable bowel syndrome; uro2: urocortin 2; LPS: Lipopolysaccharide; HPA: hypothalamic pituitary adrenal axis; LMMP: longitudinal muscle myenteric plexus; RORγt: retinoid-related orphan receptor γt; CDAI: Crohn's Disease Activity Index; ICAM1: intercellular cell adhesion molecule-1; EPCR: endothelial protein C receptor; TM: thrombomodulin; VEC: vascular endothelial cell; Mφ: macrophages; CCL-20: CC-chemokine-ligand-20; CAC: colitis-associated colorectal cancer; CCR-6: CC-chemokine-receptor-6; tRXRα: truncated Retinoid X receptor-α; ADAM17: a disintegrin metalloprotease 17; VEGF: vascular endothelial growth factor; MMP: matrix metalloproteinase; STT: systemic thermal therapy.
Acknowledgements
Funding
National Natural Science Foundation of China, No. 81673671 and No. 81274173.
Authors' contributions
Guo YX and Wang BY contributed equally to the writing of this manuscript; Guo YX, Wang BY, Yang ZJ and Wang FF wrote the manuscript; Guo YX, Hua RX, Wang TT and Wang BY designed the illustrations; Gao L and Shang HW analyzed the data; Xu JD revised the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mahdavi Abhari F, Pirestani M, Dalimi A. Anti-amoebic activity of a cecropin-melittin hybrid peptide (CM11) against trophozoites of Entamoeba histolytica. Wien Klin Wochenschr. 2019;131:427-34
2. Nancy A. Louis PWL. The Intestinal Immune Barrier. NeoReviews. 2009;10:e180
3. Zhang Z, La Placa D, Nguyen T, Kujawski M, Le K, Li L. et al. CEACAM1 regulates the IL-6 mediated fever response to LPS through the RP105 receptor in murine monocytes. BMC Immunol. 2019;20:7
4. Kozak W, Kluger MJ, Soszynski D, Conn CA, Rudolph K, Leon LR. et al. IL-6 and IL-1β in Fever: Studies Using Cytokine-Deficient (Knockout) Micea. Annals of the New York Academy of Sciences. 1998;856:33-47
5. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-8
6. Kaur S, Bansal Y, Kumar R, Bansal G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg Med Chem. 2020;28(5):115327
7. Aden K, Breuer A, Rehman A, Geese H, Tran F, Sommer J. et al. Classic IL-6R signalling is dispensable for intestinal epithelial proliferation and repair. Oncogenesis. 2016;5:e270
8. Toshio Hirano KY, Hisashi Harada. Complementary DNA for a novel human interleukin(BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73-76
9. Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006;8(Suppl 2):S2
10. Wennerås C, Hagberg L, Andersson R, Hynsjö L, Lindahl A, Okroj M. et al. Distinct inflammatory mediator patterns characterize infectious and sterile systemic inflammation in febrile neutropenic hematology patients. PloS one. 2014;9:e92319
11. Comini L, Pasini E, Bachetti T, Dreano M, Garotta G, Ferrari R. Acute haemodynamic effects of IL-6 treatment in vivo: involvement of vagus nerve in NO-mediated negative inotropism. Cytokine. 2005;30:236-42
12. Kawashima T, Ikari N, Kouchi T, Kowatari Y, Kubota Y, Shimojo N. et al. The molecular mechanism for activating IgA production by Pediococcus acidilactici K15 and the clinical impact in a randomized trial. Sci Rep. 2018;8:5065
13. Vinderola G, Matar C, Perdigon G. Role of Intestinal Epithelial Cells in Immune Effects Mediated by Gram-Positive Probiotic Bacteria: Involvement of Toll-Like Receptors. Clinical and diagnostic laboratory immunology. 2005;12:1075
14. Han SK, Kim DH. Lactobacillus mucosae and Bifidobacterium longum Synergistically Alleviate Immobilization Stress-Induced Anxiety/Depression in Mice by Suppressing Gut Dysbiosis. Journal of microbiology and biotechnology. 2019;29:1369-74
15. Wang JJ, Zhang QM, Ni WW, Zhang X, Li Y, Li AL. et al. Modulatory effect of Lactobacillus acidophilus KLDS 1.0738 on intestinal short-chain fatty acids metabolism and GPR41/43 expression in β-lactoglobulin-sensitized mice. Microbiol Immunol. 2019;63:303-15
16. Li S, Fu C, Zhao Y, He J. Intervention with α-Ketoglutarate Ameliorates Colitis-Related Colorectal Carcinoma via Modulation of the Gut Microbiome. BioMed research international. 2019;2019:8020785
17. Wang Y, Wang K, Han GC, Wang RX, Xiao H, Hou CM. et al. Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis. Mucosal immunology. 2014;7:1106-15
18. Tsay TB, Yang MC, Sun JT, Chen PH, Lin YS, Shih MH. et al. Enteric bacterial loads are associated with interleukin-6 levels in systemic inflammatory response syndrome patients. Formosan Journal of Surgery. 2016;49:208-16
19. Ding Y, Yan Y, Chen D, Ran L, Mi J, Lu L. et al. Modulating effects of polysaccharides from the fruits of Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food & function. 2019;10:3671-83
20. Polakowski CB, Kato M, Preti VB, Schieferdecker MEM, Ligocki Campos AC. Impact of the preoperative use of synbiotics in colorectal cancer patients: A prospective, randomized, double-blind, placebo-controlled study. Nutrition (Burbank, Los Angeles County, Calif). 2019;58:40-6
21. Hegazy SK, El-Bedewy MM. Effect of probiotics on pro-inflammatory cytokines and NF-kappaB activation in ulcerative colitis. World journal of gastroenterology. 2010;16:4145-51
22. Qu H, Zhang Y, Chai H, Gao ZY, Shi DZ. Effects of microbiota-driven therapy on inflammatory responses in elderly individuals: A systematic review and meta-analysis. PloS one. 2019;14:e0211233
23. Ashizawa Y, Kuboki S, Nojima H, Yoshitomi H, Furukawa K, Takayashiki T. et al. OLFM4 Enhances STAT3 Activation and Promotes Tumor Progression by Inhibiting GRIM19 Expression in Human Hepatocellular Carcinoma. Hepatol Commun. 2019;3:954-70
24. Kuhn KA, Schulz HM, Regner EH, Severs EL, Hendrickson JD, Mehta G. et al. Bacteroidales recruit IL-6-producing intraepithelial lymphocytes in the colon to promote barrier integrity. Mucosal immunology. 2018;11:357-68
25. Coyne CB, Bergelson JM. CAR: a virus receptor within the tight junction. Adv Drug Deliv Rev. 2005;57:869-82
26. Nagai M, Yaoita E, Yoshida Y, Kuwano R, Nameta M, Ohshiro K. et al. Coxsackievirus and adenovirus receptor, a tight junction membrane protein, is expressed in glomerular podocytes in the kidney. Lab Invest. 2003;83:901-11
27. Xu Z, Waeckerlin R, Urbanowski MD, van Marle G, Hobman TC. West Nile virus infection causes endocytosis of a specific subset of tight junction membrane proteins. PLoS One. 2012;7:e37886
28. Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. The Journal of biological chemistry. 2011;286:31263-71
29. Dahan S, Roda G, Pinn D, Roth-Walter F, Kamalu O, Martin AP. et al. Epithelial: lamina propria lymphocyte interactions promote epithelial cell differentiation. Gastroenterology. 2008;134:192-203
30. Sakamoto H, Mutoh H, Sugano K. Expression of Claudin-2 in intestinal metaplastic mucosa of Cdx2-transgenic mouse stomach. Scandinavian journal of gastroenterology. 2010;45:1273-80
31. Miller TL, McGee DW. Epithelial cells respond to proteolytic and non-proteolytic detachment by enhancing interleukin-6 responses. Immunology. 2002;105:101-10
32. Dann SM, Spehlmann ME, Hammond DC, Iimura M, Hase K, Choi LJ. et al. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. Journal of immunology (Baltimore, Md: 1950). 2008;180:6816-26
33. Liu D, Jiang XY, Zhou LS, Song JH, Zhang X. Effects of Probiotics on Intestinal Mucosa Barrier in Patients With Colorectal Cancer after Operation: Meta-Analysis of Randomized Controlled Trials. Medicine. 2016;95:e3342
34. Uciechowski P, Dempke W.C.M. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology. 2020;98:131-137
35. Satoh Y, Ishikawa K, Ono K, Vollrath L. Quantitative light microscopic observations on Paneth cells of germ-free and ex-germ-free Wistar rats. Digestion. 1986;34:115-21
36. Clarke LL, Gawenis LR, Bradford EM, Judd LM, Boyle KT, Simpson JE. et al. Abnormal Paneth cell granule dissolution and compromised resistance to bacterial colonization in the intestine of CF mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G1050-8
37. Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci. 2002;59:156-70
38. Jeffery V, Goldson AJ, Dainty JR, Chieppa M, Sobolewski A. IL-6 Signaling Regulates Small Intestinal Crypt Homeostasis. Journal of immunology (Baltimore, Md: 1950). 2017;199:304-11
39. Hirano T, Taga T, Nakano N, Yasukawa K, Kashiwamura S, Shimizu K. et al. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2). Proceedings of the National Academy of Sciences. 1985;82:5490
40. Yasukawa K, Hirano T, Watanabe Y, Muratani K, Matsuda T, Nakai S. et al. Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. The EMBO journal. 1987;6:2939-45
41. Lin XH, Guo JL, Wen YQ, Li YX, Wei DD, Yang RL. et al. Role of IgG plasma cells in the change of protein C system in ulcerative colitis. Acta physiologica Sinica. 2017;69:172-82
42. Perez-Berezo T, Franch A, Castellote C, Castell M, Perez-Cano FJ. Mechanisms involved in down-regulation of intestinal IgA in rats by high cocoa intake. J Nutr Biochem. 2012;23:838-44
43. Del Giudice M, Gangestad SW. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. 2018;70:61-75
44. Boulanger MJ, Chow DC, Brevnova EE, Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science (New York, NY). 2003;300:2101-4
45. Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX. et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57-62
46. Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clinical science (London, England: 1979). 2012;122:143-59
47. Li Y, Deuring J, Peppelenbosch MP, Kuipers EJ, de Haar C, van der Woude CJ. IL-6-induced DNMT1 activity mediates SOCS3 promoter hypermethylation in ulcerative colitis-related colorectal cancer. Carcinogenesis. 2012;33:1889-96
48. Li Y, de Haar C, Chen M, Deuring J, Gerrits MM, Smits R. et al. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut. 2010;59:227-35
49. Felix A. Montero-Julian, Brailly H, Sautès C, Joyeux I, Tartour E. Characterization of Soluble gpl3O Released by Melanoma Cell Lines: A Polyvalent Antagonist of Cytokines from the Interleukin 6 Family. Clinical Cancer Research. 1997;3:1443-51
50. Moritz RL, Ward LD, Tu GF, Fabri LJ, Ji H, Yasukawa K. et al. The N-terminus of gp130 is critical for the formation of the high-affinity interleukin-6 receptor complex. Growth Factors. 1999;16:265-78
51. Heink S, Yogev N, Garbers C, Herwerth M, Aly L, Gasperi C. et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic T(H)17 cells. Nature immunology. 2017;18:74-85
52. Larauche M, Kiank C, Tache Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society. 2009;60(Suppl 7):33-46
53. Alshamsan A. Induction of tolerogenic dendritic cells by IL-6-secreting CT26 colon carcinoma. Immunopharmacol Immunotoxicol. 2012;34:465-9
54. Herbeuval JP, Lelievre E, Lambert C, Dy M, Genin C. Recruitment of STAT3 for production of IL-10 by colon carcinoma cells induced by macrophage-derived IL-6. J Immunol. 2004;172:4630-6
55. Sun Q, Liu Q, Zheng Y, Cao X. Rapamycin suppresses TLR4-triggered IL-6 and PGE(2) production of colon cancer cells by inhibiting TLR4 expression and NF-kappaB activation. Mol Immunol. 2008;45:2929-36
56. Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54:1481-91
57. Rühl A, Franzke S, Collins S M, Stremmel W. Interleukin-6 expression and regulation in rat enteric glia cells. AJP Gastrointestinal and Liver Physiology. 2001;280:G1163-71
58. Burgueño JF, Barba A, Eyre E, Romero C, Neunlist M, Fernández E. TLR2 and TLR9 modulate enteric nervous system inflammatory responses to lipopolysaccharide. Journal of neuroinflammation. 2016;13:187
59. Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122-30
60. Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9:125-34
61. Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458-62
62. Schäfer KH, Mestres P, März P, Rose-John S. The IL-6/sIL-6R fusion protein hyper-IL-6 promotes neurite outgrowth and neuron survival in cultured enteric neurons. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 1999;19:527-32
63. Soufflet F, Biraud M, Rolli-Derkinderen M, Lardeux B, Trang C, Coron E. et al. Modulation of VIPergic phenotype of enteric neurons by colonic biopsy supernatants from patients with inflammatory bowel diseases: Involvement of IL-6 in Crohn's disease. Neurogastroenterology and motility. 2018;30:e13198
64. Chen L, Yuan W, Chen Z, Wu S, Ge J, Chen J. et al. Vasoactive intestinal peptide represses activation of tumor-associated macrophages in gastric cancer via regulation of TNFα, IL-6, IL-12 and iNOS. Int J Oncol. 2015;47:1361-70
65. Kelles A, Janssens J, Tack J. IL-1beta and IL-6 excite neurones and suppress cholinergic neurotransmission in the myenteric plexus of the guinea pig. Neurogastroenterology and motility. 2000;12:531-8
66. Rühl A, Hurst S, Collins SM. Synergism between interleukins 1 beta and 6 on noradrenergic nerves in rat myenteric plexus. Gastroenterology. 1994;107:993-1001
67. O'Malley D, Liston M, Hyland NP, Dinan TG, Cryan JF. Colonic soluble mediators from the maternal separation model of irritable bowel syndrome activate submucosal neurons via an interleukin-6-dependent mechanism. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2010;300:G241-G52
68. O'Malley D, Dinan TG, Cryan JF. Altered expression and secretion of colonic interleukin-6 in a stress-sensitive animal model of brain-gut axis dysfunction. Journal of neuroimmunology. 2011;235:48-55
69. Buckley MM, O'Halloran KD, Rae MG, Dinan TG, O'Malley D. Modulation of enteric neurons by interleukin-6 and corticotropin-releasing factor contributes to visceral hypersensitivity and altered colonic motility in a rat model of irritable bowel syndrome. The Journal of physiology. 2014;592:5235-50
70. O'Malley D, Cryan JF, Dinan TG. Crosstalk between interleukin-6 and corticotropin-releasing factor modulate submucosal plexus activity and colonic secretion. Brain Behav Immun. 2013;30:115-24
71. Dunn AJ. Effects of cytokines and infections on brain neurochemistry. Clin Neurosci Res. 2006;6:52-68
72. Li A, Xiong J, Chen Z. IL-6, TNF-α, and iNOS is associated with decreased colonic contraction in rats with multiple organ dysfunction syndrome. Journal of Surgical Research. 2012;178:e51-e7
73. Chang XW, Qin Y, Jin Z, Xi TF, Yang X, Lu ZH. et al. Interleukin-6 (IL-6) mediated the increased contraction of distal colon in streptozotocin-induced diabetes in rats via IL-6 receptor pathway. International journal of clinical and experimental pathology. 2015;8:4514-24
74. Manning J, Buckley MM, O'Halloran KD, O'Malley D. In vivo neutralization of IL-6 receptors ameliorates gastrointestinal dysfunction in dystrophin-deficient mdx mice. Neurogastroenterology and motility. 2016;28:1016-26
75. Tsuchida Y, Hatao F, Fujisawa M, Murata T, Kaminishi M, Seto Y. et al. Neuronal stimulation with 5-hydroxytryptamine 4 receptor induces anti-inflammatory actions via α7nACh receptors on muscularis macrophages associated with postoperative ileus. Gut. 2011;60:638-47
76. Wehner S, Schwarz NT, Hundsdoerfer R, Hierholzer C, Tweardy DJ, Billiar TR. et al. Induction of IL-6 within the rodent intestinal muscularis after intestinal surgical stress. Surgery. 2005;137:436-46
77. Schwarz NT, Engel B, Eskandari MK, Kalff JC, Grandis JR, Bauer AJ. Lipopolysaccharide preconditioning and cross-tolerance: The induction of protective mechanisms for rat intestinal ileus. Gastroenterology. 2002;123:586-98
78. Ni Y, Liu M, Yu H, Chen Y, Liu Y, Chen S. et al. Desmethylbellidifolin From Gentianella acuta Ameliorate TNBS-Induced Ulcerative Colitis Through Antispasmodic Effect and Anti-Inflammation. Front Pharmacol. 2019;10:1104
79. Nishiyama K, Aono K, Fujimoto Y, Kuwamura M, Okada T, Tokumoto H. et al. Chronic kidney disease after 5/6 nephrectomy disturbs the intestinal microbiota and alters intestinal motility. J Cell Physiol. 2019;234:6667-78
80. Cicenia A, Santangelo F, Gambardella L, Pallotta L, Iebba V, Scirocco A. et al. Protective Role of Postbiotic Mediators Secreted by Lactobacillus rhamnosus GG Versus Lipopolysaccharide-induced Damage in Human Colonic Smooth Muscle Cells. Journal of clinical gastroenterology. 2016;50(Suppl 2):S140-S4
81. Killoran KE, Miller AD, Uray KS, Weisbrodt NW, Pautler RG, Goyert SM. et al. Role of innate immunity and altered intestinal motility in LPS- and MnCl2-induced intestinal intussusception in mice. American journal of physiology Gastrointestinal and liver physiology. 2014;306:G445-G53
82. Zhang L, Hu L, Chen M, Yu B. Exogenous interleukin-6 facilitated the contraction of the colon in a depression rat model. Digestive diseases and sciences. 2013;58:2187-96
83. Ihara E, Beck PL, Chappellaz M, Wong J, Medlicott SA, MacDonald JA. Mitogen-activated protein kinase pathways contribute to hypercontractility and increased Ca2+ sensitization in murine experimental colitis. Mol Pharmacol. 2009;75:1031-41
84. de Buhr MF, Mahler M, Geffers R, Hansen W, Westendorf AM, Lauber J. et al. Cd14, Gbp1, and Pla2g2a: three major candidate genes for experimental IBD identified by combining QTL and microarray analyses. Physiol Genomics. 2006;25:426-34
85. Kim HS, Cheon JH, Jung ES, Park J, Aum S, Park SJ. et al. A coding variant in FTO confers susceptibility to thiopurine-induced leukopenia in East Asian patients with IBD. Gut. 2017;66:1926-35
86. Hindryckx P, Laukens D, D'Amico F, Danese S. Unmet Needs in IBD: the Case of Fatigue. Clin Rev Allergy Immunol. 2018;55:368-78
87. Watson KL Jr, Kim SC, Boyle BM, Saps M. Prevalence and Impact of Functional Abdominal Pain Disorders in Children With Inflammatory Bowel Diseases (IBD-FAPD). J Pediatr Gastroenterol Nutr. 2017;65:212-7
88. Matsunaga H, Hokari R, Kurihara C, Okada Y, Takebayashi K, Okudaira K. et al. Omega-3 polyunsaturated fatty acids ameliorate the severity of ileitis in the senescence accelerated mice (SAM)P1/Yit mice model. Clinical and experimental immunology. 2009;158:325-33
89. Matsumoto S, Hara T, Mitsuyama K, Yamamoto M, Tsuruta O, Sata M. et al. Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. Journal of immunology (Baltimore, Md: 1950). 2010;184:1543-51
90. Atreya R, Neurath MF. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol. 2005;28:187-96
91. Ivanov II, McKenzie BS Zhou L, Tadokoro CE Lepelley A, Lafaille JJ et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121-33
92. Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y. et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29-39
93. Bamidele AO, Svingen PA, Sagstetter MR, Sarmento OF, Gonzalez M, Braga Neto MB. et al. Disruption of FOXP3-EZH2 Interaction Represents a Pathobiological Mechanism in Intestinal Inflammation. Cellular and molecular gastroenterology and hepatology. 2019;7:55-71
94. Persson Emma K, Uronen-Hansson H, Semmrich M, Rivollier A, Hägerbrand K, Marsal J. et al. IRF4 Transcription-Factor-Dependent CD103(+)CD11b(+) Dendritic Cells Drive Mucosal T Helper 17 Cell Differentiation. Immunity. 2013;38:958-69
95. Danese S, Vermeire S, Hellstern P, Panaccione R, Rogler G, Fraser G. et al. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn's disease (ANDANTE I and II). Gut. 2019;68:40-8
96. Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. Journal of immunology (Baltimore, Md: 1950). 2000;164:4878-82
97. Wang L, Walia B, Evans J, Gewirtz AT, Merlin D, Sitaraman SV. IL-6 induces NF-kappa B activation in the intestinal epithelia. Journal of immunology (Baltimore, Md: 1950). 2003;171:3194-201
98. Gren ST, Grip O. Role of Monocytes and Intestinal Macrophages in Crohn's Disease and Ulcerative Colitis. Inflammatory Bowel Diseases. 2016;22:1992-8
99. Goode EC, Warburton RC, Gelson WTH, Watson AJM. Activated Intestinal Macrophages in Patients with Cirrhosis Release NO and IL-6 that May Disrupt Intestinal Barrier Function. Gastroenterology. 2013;145:1481-4
100. Van Assche G, Barbara G, Deng Y, Lovato P, Gauldie J, Collins SM. Neurotransmitters modulate cytokine-stimulated interleukin 6 secretion in rat intestinal smooth muscle cells. Gastroenterology. 1999;116:346-53
101. Khan I, Collins SM. Expression of cytokines in the longitudinal muscle myenteric plexus of the inflamed intestine of rat. Gastroenterology. 1994;107:691-700
102. Li E, Zhou P, Petrin Z, Singer SM. Mast cell-dependent control of Giardia lamblia infections in mice. Infection and immunity. 2004;72:6642-9
103. Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H. et al. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. Journal of hepatology. 2013;58:911-21
104. Senchenkova EY, Komoto S, Russell J, Almeida-Paula LD, Yan LS, Zhang S. et al. Interleukin-6 mediates the platelet abnormalities and thrombogenesis associated with experimental colitis. The American journal of pathology. 2013;183:173-81
105. Hozumi H, Russell J, Vital S, Granger DN. IL-6 Mediates the Intestinal Microvascular Thrombosis Associated with Experimental Colitis. Inflammatory Bowel Diseases. 2016;22:560-8
106. Alipour B, Homayouni-Rad A, Vaghef-Mehrabany E, Sharif SK, Vaghef-Mehrabany L, Asghari-Jafarabadi M. et al. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: a randomized double-blind clinical trial. Int J Rheum Dis. 2014;17:519-27
107. Wunderlich CM, Ackermann PJ, Ostermann AL, Adams-Quack P, Vogt MC, Tran ML. et al. Obesity exacerbates colitis-associated cancer via IL-6-regulated macrophage polarisation and CCL-20/CCR-6-mediated lymphocyte recruitment. Nature communications. 2018;9:1646
108. Kuhn KA, Manieri NA, Liu TC, Stappenbeck TS. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS One. 2014;9:e114195
109. Yang R, Han X, Uchiyama T, Watkins SK, Yaguchi A, Delude RL. et al. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. American journal of physiology Gastrointestinal and liver physiology. 2003;285:G621-G9
110. Meng ZH, Dyer K, Billiar TR, Tweardy DJ. Distinct effects of systemic infusion of G-CSF vs. IL-6 on lung and liver inflammation and injury in hemorrhagic shock. Shock (Augusta, Ga). 2000;14:41-8
111. Kimizuka K, Nakao A, Nalesnik MA, Demetris AJ, Uchiyama T, Ruppert K. et al. Exogenous IL-6 inhibits acute inflammatory responses and prevents ischemia/reperfusion injury after intestinal transplantation. Am J Transplant. 2004;4:482-94
112. Baqar S, Pacheco ND, Rollwagen FM. Modulation of mucosal immunity against Campylobacter jejuni by orally administered cytokines. Antimicrob Agents Chemother. 1993;37:2688-92
113. Rollwagen FM, Yu ZY, Li YY, Pacheco ND. IL-6 Rescues Enterocytes from Hemorrhage Induced Apoptosisin vivoandin vitroby abcl-2Mediated Mechanism. Clinical Immunology and Immunopathology. 1998;89:205-13
114. Rollwagen FM, Li YY, Pacheco ND, Nielsen TB. Systemic bacteraemia following haemorrhagic shock in mice: alleviation with oral interleukin 6. Cytokine. 1996;8:121-9
115. Rollwagen FM, Li YY, Pacheco ND, Baqar S. Systemic sepsis following hemorrhagic shock: alleviation with oral interleukin-6. Military medicine. 1997;162:366-70
116. Sitaraman SV, Merlin D, Wang L, Wong M, Gewirtz AT, Si-Tahar M. et al. Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. The Journal of clinical investigation. 2001;107:861-9
117. Meng D, Nanthakumar NN. IL-6 and Mip-2 Are Important for Neutrophil and Macrophage Recruitment During the Recovery From Colitis in Bacteria-Depleted Bacteria. Gastroenterology. 2011;140:S-638
118. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878-88
119. Burstein E, Fearon ER. Colitis and cancer: a tale of inflammatory cells and their cytokines. The Journal of clinical investigation. 2008;118:464-7
120. Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098
121. Yeh KY, Wu TH, Wu TL. Colorectal cancer cell-derived interleukin-6 enhances the phagocytic capacity and migration of THP-1 cells. Cytokine. 2016;79:82-9
122. Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Ménégaux F. et al. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58:520-9
123. Li J, Xu J, Yan X, Jin K, Li W, Zhang R. Targeting Interleukin-6 (IL-6) Sensitizes Anti-PD-L1 Treatment in a Colorectal Cancer Preclinical Model. Medical science monitor: international medical journal of experimental and clinical research. 2018;24:5501-8
124. Becker C, Fantini MC, Wirtz S, Nikolaev A, Lehr HA, Galle PR. et al. IL-6 signaling promotes tumor growth in colorectal cancer. Cell cycle (Georgetown, Tex). 2005;4:217-20
125. Siveen KS, Sikka S, Surana R, Dai X, Zhang J, Kumar AP. et al. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta. 2014;1845:136-54
126. Song Z, Ren D, Xu X, Wang Y. Molecular cross-talk of IL-6 in tumors and new progress in combined therapy. Thorac Cancer. 2018;9:669-75
127. Waldner MJ, Foersch S, Neurath MF. Interleukin-6-a key regulator of colorectal cancer development. Int J Biol Sci. 2012;8:1248-53
128. Waldner MJ, Foersch S, Neurath MF. Interleukin-6-a key regulator of colorectal cancer development. Int J Biol Sci. 2012;8:1248-53
129. Hodge DR, Peng B, Cherry JC, Hurt EM, Fox SD, Kelley JA. et al. Interleukin 6 supports the maintenance of p53 tumor suppressor gene promoter methylation. Cancer research. 2005;65:4673-82
130. Ye X, Wu H, Sheng L, Liu YX, Ye F, Wang M. et al. Oncogenic potential of truncated RXRα during colitis-associated colorectal tumorigenesis by promoting IL-6-STAT3 signaling. Nature communications. 2019;10:1463
131. Schumacher N, Rose-John S. ADAM17 Activity and IL-6 Trans-Signaling in Inflammation and Cancer. Cancers (Basel). 2019;11:1736
132. Huynh J, Etemadi N, Hollande F, Ernst M, Buchert M. The JAK/STAT3 axis: A comprehensive drug target for solid malignancies. Semin Cancer Biol. 2017;45:13-22
133. Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM. et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. The Journal of clinical investigation. 2011;121:3846-59
134. Xu J, Qian J, Zhang W, Chen E, Zhang G, Cao G. et al. LYPD8 regulates the proliferation and migration of colorectal cancer cells through inhibiting the secretion of IL6 and TNFalpha. Oncology reports. 2019;41:2389-95
135. Zhang S, Xu W, Wang H, Cao M, Li M, Zhao J. et al. Inhibition of CREB-mediated ZO-1 and activation of NF-κB-induced IL-6 by colonic epithelial MCT4 destroys intestinal barrier function. Cell proliferation. 2019;52:e12673
136. Ogata A, Kato Y, Higa S, Yoshizaki K. IL-6 inhibitor for the treatment of rheumatoid arthritis: A comprehensive review. Mod Rheumatol. 2019;29:258-67
137. Mishra P, Raj V, Bhadauria AS, Singh AK, Rai A, Kumar P. et al. 6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid attenuates colon carcinogenesis via blockade of IL-6 mediated signals. Biomedicine & pharmacotherapy. 2018;100:282-95
138. Fujita K, Tokuda H, Yamamoto N, Kainuma S, Kawabata T, Sakai G. et al. Incretins amplify TNF-α-stimulated IL-6 synthesis in osteoblasts: Suppression of the IκB/NF-κB pathway. International journal of molecular medicine. 2017;39:1053-60
139. Zheng R, Ma J, Wang D, Dong W, Wang S, Liu T. et al. Chemopreventive Effects of Silibinin on Colitis-Associated Tumorigenesis by Inhibiting IL-6/STAT3 Signaling Pathway. Mediators of inflammation. 2018;2018:1562010
140. Fang X, Hong Y, Dai L, Qian Y, Zhu C, Wu B. et al. CRH promotes human colon cancer cell proliferation via IL-6/JAK2/STAT3 signaling pathway and VEGF-induced tumor angiogenesis. Mol Carcinog. 2017;56:2434-45
141. Park KI, Kim DG, Lee BH, Ma JY. Fermented Herbal Formulas KIOM-MA128 Ameliorate IL-6-Induced Intestinal Barrier Dysfunction in Colon Cancer Cell Line. Mediators Inflamm. 2016;2016:6189590
142. Shi YJ, Zhao QQ, Liu XS, Dong SH, E JF, Li X. et al. Toll-like receptor 4 regulates spontaneous intestinal tumorigenesis by up-regulating IL-6 and GM-CSF. J Cell Mol Med. 2019;24:385-397
143. Saadatdoust Z, Pandurangan AK, Ananda Sadagopan SK, Mohd Esa N, Ismail A, Mustafa MR. Dietary cocoa inhibits colitis associated cancer: a crucial involvement of the IL-6/STAT3 pathway. J Nutr Biochem. 2015;26:1547-58
144. Kangwan N, Kim YJ, Han YM, Jeong M, Park JM, Go EJ. et al. Sonic hedgehog inhibitors prevent colitis-associated cancer via orchestrated mechanisms of IL-6/gp130 inhibition, 15-PGDH induction, Bcl-2 abrogation, and tumorsphere inhibition. Oncotarget. 2016;7:7667-82
145. Tellez-Banuelos MC, Haramati J, Franco-Topete K, Peregrina-Sandoval J, Franco-Topete R, Zaitseva GP. Chronic exposure to endosulfan induces inflammation in murine colon via beta-catenin expression and IL-6 production. Journal of immunotoxicology. 2016;13:842-9
146. Do EJ, Hwang SW, Kim SY, Ryu YM, Cho EA, Chung EJ. et al. Suppression of colitis-associated carcinogenesis through modulation of IL-6/STAT3 pathway by balsalazide and VSL#3. Journal of gastroenterology and hepatology. 2016;31:1453-61
147. Kupsky WJ, Grimes MM, Sweeting J, Bertsch R, Cote LJ. Parkinson's disease and megacolon: concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology. 1987;37:1253-5
148. Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson's disease. World J Gastroenterol. 2015;21:10609-20
149. Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson's disease. World journal of gastroenterology. 2015;21:10609-20
150. Liu YT, Sun MH, Cai CW, Ren C, Niu HC. Research progress on neural mechanism of peripheral inflammation in Parkinson's disease. Acta physiologica Sinica. 2019;71:732-740
151. Gruden MA, Yanamandra K, Kucheryanu VG, Bocharova OR, Sherstnev VV, Morozova-Roche LA. et al. Correlation between protective immunity to alpha-synuclein aggregates, oxidative stress and inflammation. Neuroimmunomodulation. 2012;19:334-42
152. Fitzgerald E, Murphy S, Martinson HA. Alpha-Synuclein Pathology and the Role of the Microbiota in Parkinson's Disease. Frontiers in Neuroscience. 2019;13:369
153. Zhang Y, He X, Wu X, Lei M, Wei Z, Zhang X. et al. Rapamycin upregulates glutamate transporter and IL-6 expression in astrocytes in a mouse model of Parkinson's disease. Cell death & disease. 2017;8:e2611
Author contact
![]() Corresponding author: Dr. Jingdong Xu, Department of Physiology, School of Basic Medical Sciences, Capital Medical University, Beijing, China, 10069. Tel.: (86)10-8391-1469; Fax: (86)10-8391-1469; E-mail: xu_jddedu.cn.
Corresponding author: Dr. Jingdong Xu, Department of Physiology, School of Basic Medical Sciences, Capital Medical University, Beijing, China, 10069. Tel.: (86)10-8391-1469; Fax: (86)10-8391-1469; E-mail: xu_jddedu.cn.
Received 2020-8-1
Accepted 2020-11-7
Published 2021-1-1
