Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2019; 15(1):34-43. doi:10.7150/ijbs.28879 This issue Cite
Research Paper
E-cadherin is Required for the Homeostasis of Lgr5+ Gastric Antral Stem Cells
1. State Key Laboratory of Proteomics, Beijing Proteome Research Center, National Center for Protein Sciences, Beijing Institute of Lifeomics, Beijing 102206, China.
2. State Key Laboratory of Membrane Biology, College of Life Sciences, Tsinghua University, Beijing, 100084, China.
Received 2018-7-31; Accepted 2018-9-28; Published 2019-1-1
Abstract
Lgr5-expressing stem cells contribute to the epithelial turnover of the gastric antrum. However, the mechanism controlling the homeostasis of Lgr5+ antral stem cells is not fully understood. Here, we demonstrate the key role of E-cadherin in the homeostasis of Lgr5+ gastric antral stem cells. The deletion of E-cadherin in these cells results in their apoptosis, thereby leading to a marked decrease in their number. A reduced Lgr5+ stem cell pool caused by the loss of E-cadherin impairs gastric antral epithelial homeostasis in vivo and organoid growth in vitro. Furthermore, p53 contributes to the apoptosis of Lgr5+ stem cells following E-cadherin loss, while the simultaneous deletion of p53 rescues the phenotype in E-cadherin mutants. Our study reveals the critical pro-survival function of E-cadherin in Lgr5+ gastric antral stem cells and the key role of the Lgr5+ stem cell pool in the maintenance of gastric epithelial homeostasis.
Keywords: E-cadherin, gastric epithelium, Lgr5+ stem cells, apoptosis, homeostasis
Introduction
The adult gastric epithelium is organized into two distinct anatomical and functional domains: the proximal corpus and the distal pyloric antrum [1]. Both domains, which continuously self-renew throughout life, are fuelled by stem cells that constantly generate transit-amplifying cells and various differentiated cells. However, markers for functional stem cells vary. In the adult antral epithelium, Lgr5-expressing cells were the first well-described stem cells [2]. These multipotent Lgr5+ cells located at the base of each antral gland are actively proliferating and capable of long-term self-renewal. Each antral gland contains, on average, eight Lgr5+ stem cells, which adopt a stochastic symmetrical division model to maintain a balanced stem cell population [3]. A later study revealed that Sox2-expressing cells continuously produce various cell types, implying their self-renewal and differentiation potentials [4]. Furthermore, using CCK2R transgenic mice, Hayakawa and colleagues demonstrated that CCK2R+ cells give rise to differentiated multi-lineage cells [5]. In addition, a Runx1 enhancer element (eR1) was recently identified to mark a new stem cell population in the gastric corpus and antrum [6], while axin2, a canonical Wnt signalling target, was identified as a new marker of antral stem cells [7]. Although these heterogeneous cell populations contribute to the long-term self-renewal of the antral epithelium under normal homeostatic conditions, whether antral stem cells are essential to maintain the homeostasis of the antral epithelium has not been definitively established.
Previous in vivo studies using genetically engineered mice have identified that several signalling pathways are involved in the homeostatic behaviours of Lgr5+ antral stem cells [2, 8, 9]. Wnt signalling activation following adenomatous polyposis coli (Apc) deletion in Lgr5+ antral stem cells leads to a marked increase in the number of Lgr5+ stem cells [2]. Furthermore, Notch activation promotes the proliferation and competition of Lgr5+ antral stem cells, thereby inducing tissue expansion via gland fission [8]. Moreover, our previous study has shown that gastric Lgr5+ stem cells that have lost Smad4 and PTEN expression give rise to invasive gastric adenocarcinoma, while the homeostasis of these mutant Lgr5+ stem cells appears normal [9]. Nevertheless, the mechanisms underlying Lgr5+ stem cell behaviours are not fully understood.
E-cadherin, a transmembrane protein, links intercellular adhesion to the cytoskeleton [10]. A body of in vivo evidence has demonstrated that E-cadherin is implicated in maintaining stem cell homeostasis [11-13]. In the Drosophila ovary, E-cadherin-mediated cell adhesion is required for anchoring germline stem cells to their niche [11]. In mouse neural stem cells [12] and dental epithelial stem cells [13], the ablation of E-cadherin promotes stem cell differentiation and consequently exhausts the stem cell pool. In Mist1-expressing cells in the gastric corpus isthmus, Mist1+ cells with E-cadherin deficiency produce mucous-containing atypical cells, resembling the early pathognomonic features of the human signet ring cell carcinoma [14]. Nevertheless, the role of E-cadherin in the homeostasis of gastric antral stem cells remains unclear.
Here, we determine whether E-cadherin regulates Lgr5+ antral stem cell homeostasis and whether Lgr5+ antral stem cells are required for antral epithelial homeostasis.
Results
E-cadherin is indispensable for the development of the embryonic gastric epithelium
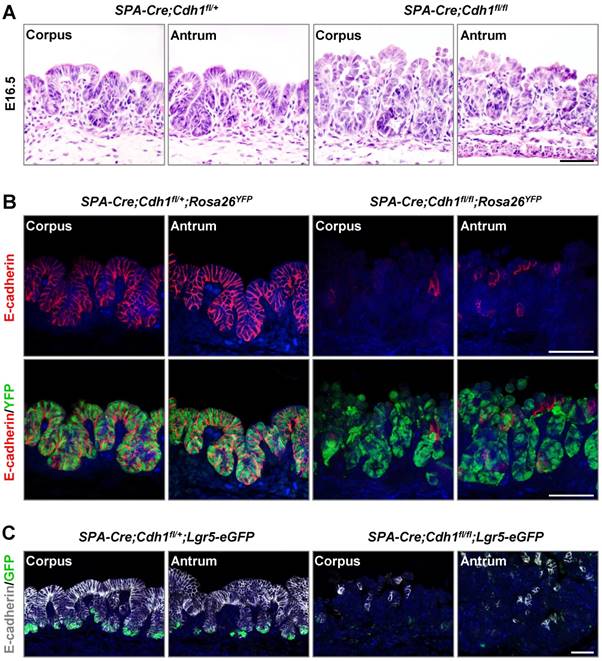
To investigate the role of E-cadherin in the homeostasis of the gastric epithelium, we first deleted the cadherin 1 gene (Cdh1, encoding E-cadherin) in mouse gastric epithelial cells by crossing Cdh1fl/fl mice [15] with SPA-Cre transgenic mice [16, 17]. The SPA-Cre mouse line began to show Cre activity throughout the gastric epithelium at embryonic day 16.5 (E16.5), as showed by being lack of E-cadherin expression at E16.5, but being intact at E15.5 (Figure S1A). At E16.5, just after the deletion of E-cadherin, SPA-Cre;Cdh1fl/fl mice displayed a disordered epithelial architecture and abnormal cell shapes throughout the gastric corpus and antrum (Figure 1A). Strikingly, SPA-Cre;Cdh1fl/fl mice could survive to adulthood. Histological analysis revealed the glandular epithelia in SPA-Cre;Cdh1fl/fl corpus and antrum being replaced by E-cadherin-sufficient, Keratin 14-expressing keratinocytes that possibly migrated from adjacent forestomach (Figure S1B).
To precisely mark gastric epithelial cells that lacked E-cadherin expression, we further bred SPA-Cre;Cdh1fl/fl mice with a Cre reporter mouse line: Rosa26-LoxP-Stop-LoxP-YFP (Rosa26YFP) [18]. Consistently, the deletion of E-cadherin led to a significantly disordered gastric epithelium and a loose organization of epithelial cells (Figure 1B), similar to the phenotype obtained when deleting E-cadherin throughout the entire intestinal epithelium [19]. To examine the putative effect of E-cadherin deletion on gastric Lgr5+ cells, which emerge during the embryonic stage [2], we crossed SPA-Cre;Cdh1fl/fl mice with Lgr5-eGFP-IRES-CreERT2 mice[2], in which Lgr5-expressing cells were monitored by GFP expression. In sharp contrast to control SPA-Cre; Cdh1fl/+; Lgr5-eGFP-IRES-CreERT2 mice, where GFP- positive Lgr5+ cells resided at the base of the glands in both the corpus and the antrum, GFP-expressing Lgr5+ cells were hardly observed in SPA-Cre; Cdh1fl/fl; Lgr5-eGFP-IRES-CreERT2 mice (Figure 1C). These results suggest that E-cadherin is essential for the development of the gastric epithelium and may play a specific role in the maintenance of gastric Lgr5+ cells.
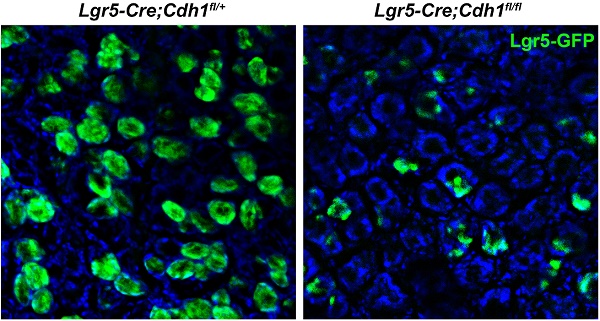
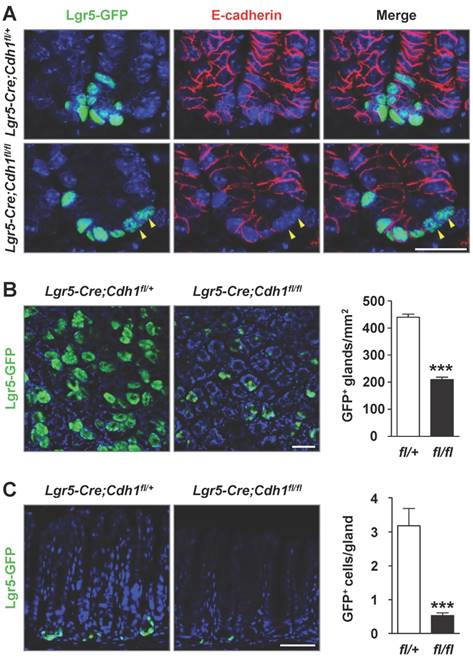
E-cadherin loss in Lgr5+ stem cells in the adult gastric antrum leads to a dramatic decrease in the number of Lgr5+ stem cells
To explore the cell-autonomous function of E-cadherin in Lgr5+ stem cells in the adult gastric antrum, we sporadically deleted E-cadherin within the antral Lgr5+ stem cells by breeding Cdh1fl/fl mice with Lgr5-eGFP-IRES-CreERT2 (Lgr5-Cre) mice. To this end, tamoxifen (Tam) was administered to 8-10-week-old mice. After three consecutive days of Tam treatment, double immunofluorescence staining showed that some Lgr5+ antral stem cells lacked E-cadherin expression in Lgr5-Cre;Cdh1fl/fl mice (Figure 2A). To improve the efficiency of E-cadherin deletion, Tam was administered every other day for 7 consecutive weeks, which had no detrimental effect on the gastric antrum (Figure S2). By directly observing the whole-mount GFP fluorescence, we found that at 10 days after continued Tam treatment, the number of Lgr5-GFP+ glands in Lgr5-Cre;Cdh1fl/fl mice were comparable to that in control mice, while began to reduce at 20 days of continued Tam treatment (Figure S3A and S3B). After 7 weeks, E-cadherin deletion in the Lgr5+ stem cells led to a 52.5% reduction in Lgr5-GFP+ glands in the antrums of Lgr5-Cre;Cdh1fl/fl mice (Figure 2B). Even in the remaining Lgr5-GFP+ glands, Lgr5+ cell clusters typically consisted of singlets or doublets and were much reduced compared to those in control Lgr5-Cre;Cdh1fl/+ mice (Figure 2B). Consistently, the analysis of sections confirmed that the deletion of E-cadherin significantly lowered the average number of Lgr5+ cells per gland by 83.3% (Figure 2C).
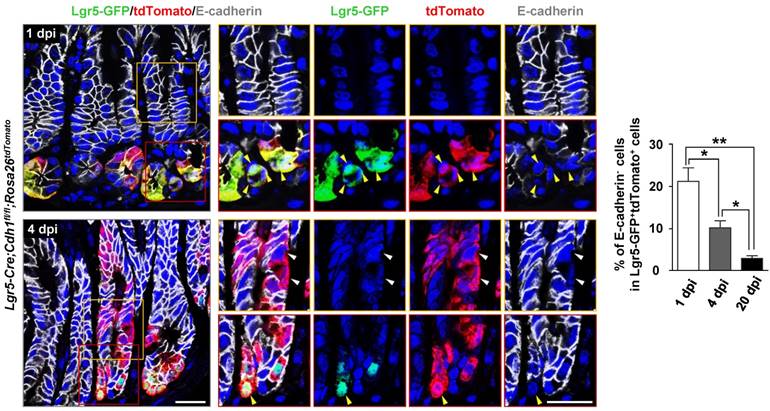
To further verify the role of E-cadherin in Lgr5+ stem cell homeostasis, we examined the behaviour of Lgr5+ stem cells in a short-term lineage tracing system by crossing Lgr5-Cre;Cdh1fl/fl mice with Rosa26-LoxP-Stop-LoxP-tdTomato (Rosa26tdTomato) mice [20], where Rosa26tdTomato labels Lgr5-Cre-expressing cells and their progenies upon Tam induction. At 1 day post-injection (1 dpi) after a 3-day Tam treatment, tdTomato expression was confined at the base of the gland, where Lgr5+ stem cells reside (Figure 3 and Figure S4A). At 1 dpi, 21.2% of the labelled Lgr5+ antral stem cells (GFP+/tdTomato+) lacked E-cadherin expression in Lgr5-Cre;Cdh1fl/fl;Rosa26tdTomato mice. At 4 dpi, however, the percentage of E-cadherin-null Lgr5+ stem cells (GFP+/tdTomato+/E-cadherin-) significantly dropped to 10.2%, and further to 3.3% at 20 dpi (Figure 3). Taken together, these data indicate that E-cadherin is required for maintaining the Lgr5+ antral stem cell pool.
E-cadherin is required for the survival of Lgr5+ antral stem cells
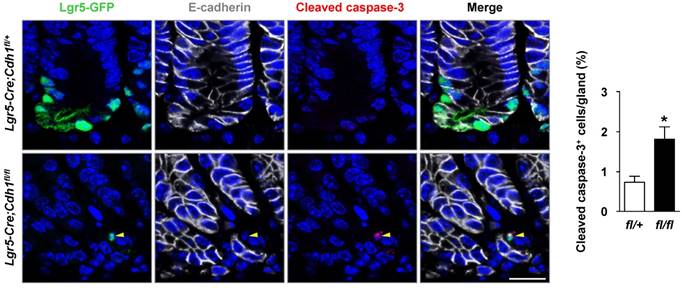
The loss of Lgr5+ antral stem cells induced by the deletion of E-cadherin might be due to apoptosis, accelerated stem cell differentiation, or both. We first investigated whether E-cadherin loss in Lgr5+ stem cells led to apoptosis, thereby resulting in their significant reduction. Indeed, the immunostaining of cleaved caspase-3 showed a dramatic increase in apoptosis in E-cadherin-deficient Lgr5+ stem cells, both in the long and short-term E-cadherin deletion mice (Figure 4 and Figure S4B). This suggests that E-cadherin is necessary for the survival of Lgr5+ antral stem cells.
E-cadherin is required for the maintenance of the gastric embryonic epithelial architecture and Lgr5-expressing cell pool. (A) H&E staining of the gastric corpus and antrum in SPA-Cre;Cdh1fll+ and SPA-Cre;Cdh1fl/fl mice at E16.5. (B) Co-immunofluorescence staining (Co-IF) for YFP (green) and E-cadherin (red) in the gastric epithelium of SPA-Cre;Cdh1fll+;Rosa26YFP and SPA-Cre;Cdh1fl/fl;Rosa26YFP mice. (C) Co-IF for Lgr5-GFP (green) and E-cadherin (white) in the gastric epithelium of SPA-Cre;Cdh1fll+;Lgr5-eGFP-IRES-CreERT2 and SPA-Cre;Cdh1fl/fl;Lgr5-eGFP-IRES-CreERT2 mice. Nuclei are dyed using DAPI (blue). Scale bars: 50 μm.
A previous study has reported a fate model of gastric Lgr5+ stem cells in which more than 90% of Lgr5+ stem cells symmetrically divide to generate either two Lgr5+ stem cells or two Lgr5- differentiated progenies [3]. We next investigated whether E-cadherin-deficient Lgr5+ stem cells would adopt a bias towards generating more Lgr5-negative differentiated progenies, thereby contributing to the exhaustion of E-cadherin-deficient Lgr5+ stem cells. After seven weeks of Tam treatment in Lgr5-Cre;Cdh1fl/fl mice, E-cadherin-deficient epithelial glands or patches were hardly observed throughout the entire antral epithelium (Figure S6A). Furthermore, we monitored the dynamics of Lgr5+ stem-cell-derived non-stem progenies in the short-term lineage tracing of Lgr5-Cre;Cdh1fl/fl mice. Non-stem progenies (GFP-/ tdTomato+) of Lgr5+ stem cells with E-cadherin expression were rarely observed above the Lgr5+ stem cell compartment at 1 dpi, but rapidly expanded upward at 4 dpi (Figure 3 and Figure S4A). Remarkably, non-stem progenies lacking E-cadherin expression only accounted for 7.5% of all non-stem progenies at 4 dpi, and even dropped to 4.1% at 20 dpi in the mosaic E-cadherin-deficient antrum (Figure 3). Additionally, we found that the non-stem progenies of E-cadherin-deficient Lgr5+ stem cells (E-cadherin-/ tdTomato+) expressed Chromogranin A (ChrgA), a marker for endocrine cells, and Tff2, a marker for mucous neck cells at 20 dpi after Tam treatment (Figures S5A and S5B), indicating that E-cadherin in Lgr5+ stem cells was dispensable for specific lineage commitment. On the other hand, we found that E-cadherin-deficient Lgr5+ stem cells were still capable of proliferation (Figure S5C), highlighting the protective role of E-cadherin in the survival of Lgr5+ gastric antral stem cells.
E-cadherin ablation in Lgr5+ antral stem cells results in a significant decrease in the number of Lgr5+ stem cells. (A) Co-IF for Lgr5-GFP (green) and E-cadherin (red) in the gastric antrum of Lgr5-Cre;Cdh1fll+ and Lgr5-Cre;Cdh1fl/fl mice. Yellow arrowheads indicate Lgr5-GFP+E-cadherin- stem cells. (B) Direct imaging for endogenous Lgr5-GFP fluorescence on whole mount tissues (left) and quantification of Lgr5-GFP-positive glands per mm2 (right). (C) Lgr5-GFP fluorescence on 5 μm frozen sections (left) and quantification of Lgr5-GFP-positive cells per gland (right). Lgr5-Cre;Cdh1fl/+ (n = 4) and Lgr5-Cre;Cdh1fl/fl (n = 6) mice after 7 weeks of Tam treatment. The data are presented as means ± SEM, ***P < 0.001. Nuclei are dyed using DAPI (blue). Scale bars: 20 μm (A), 50 μm (B and C).
The E-cadherin-null-induced loss of Lgr5+ stem cells impairs the homeostasis of the antral epithelium
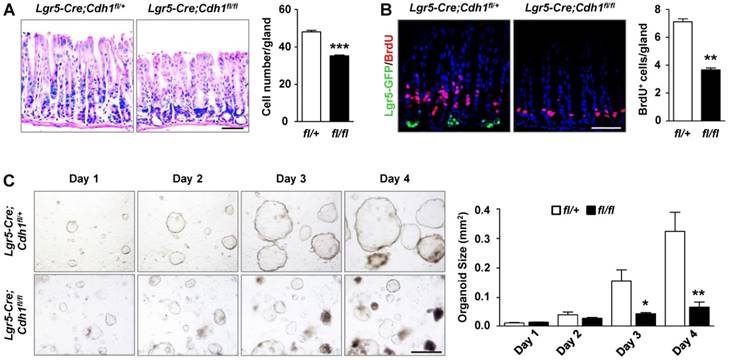
The reduced number of Lgr5+ stem cells in E-cadherin mutants offered us an opportunity to examine the function of Lgr5+ stem cells during the homeostasis of the antral epithelium. Remarkably, the height of the antral epithelium in the E-cadherin- deficient antrum decreased by 26.5%, as measured by the average epithelial cell number per gland (Figure 5A). Meanwhile, the overall number of proliferative cells, labelled with BrdU, dramatically decreased by 48.5% in E-cadherin-deficient mice compared to that in control mice (Figure 5B). Additionally, we found that the impaired Lgr5+ stem cell pool had no effect on the differentiation of antral cells, as evidenced by markers for mucous neck cells (Tff2), endocrine cells (ChrgA), and surface mucous cells (Ulex Europaeus Agglutinin, UEA) (Figure S6B). These results suggest that Lgr5+ stem cells are crucial for maintaining the homeostasis of the antral epithelium.
To corroborate the effect of Lgr5+ stem cells on homeostasis in the antral gland, we used the gland organoid model which reflects stem cell function in vitro. In contrast with the gradually expanding and budding organoids in control mice, organoid growth in Lgr5-Cre;Cdh1fl/fl mice was greatly inhibited, and some organoids began to shrink and collapsed within 4 days of 4-hydroxytamoxifen (4H-Tam) treatment (Figure 5C). Direct tdTomato fluorescence imaging confirmed that the vast majority of the shrunken Lgr5-Cre;Cdh1fl/fl organoids had resulted from Cre-mediated recombination (Figure S7). Collectively, these in vivo and in vitro results indicate that E-cadherin is indispensable for the maintenance of the Lgr5+ stem cell pool, thereby contributing to the antral epithelial homeostasis.
Deletion of p53 rescues the E-cadherin deletion-induced apoptosis in Lgr5+ stem cells
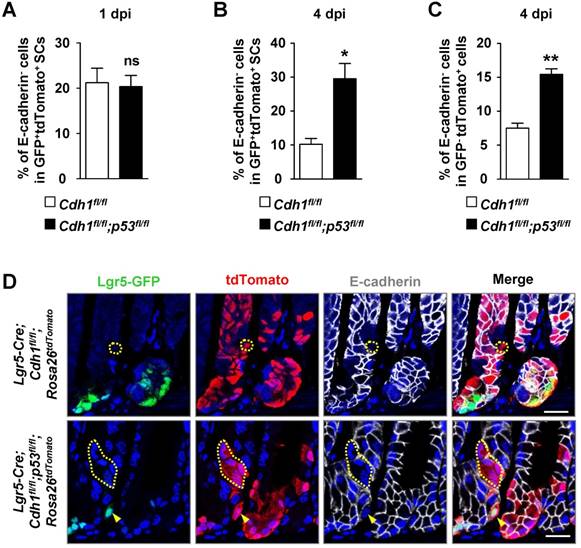
Finally, we tested the hypothesis that the pro-survival function of E-cadherin was mediated via p53, a key effector of apoptosis. We found the expression of p53 in some E-cadherin-deficient Lgr5+ stem cells, indicating that p53 was partially activated in E-cadherin-deficient Lgr5+ stem cells (Figure S8). To functionally examine the role of p53 in E-cadherin-loss-induced apoptosis in Lgr5+ stem cells, additional deletion of p53 in Lgr5+ stem cells was obtained by crossing Lgr5-Cre;Cdh1fl/fl;Rosa26tdTomato mice with p53fl/fl mice [21]. The Cdh1 and p53 double mutant mice exhibited a recombination rate comparable to that in E-cadherin single mutant mice at 1 dpi (20.3% versus 21.2%, Figure 6A). At 4 dpi, however, the percentage of E-cadherin-deficient Lgr5+ stem cells and non-stem progenies in Cdh1 and p53 double mutant mice was 29.5% and 15.4% respectively; these percentages were much higher than 8.2%, observed in E-cadherin-deficient Lgr5+ stem cells, and 6.3% observed in E-cadherin-deficient non-stem progenies in E-cadherin single mutant mice (Figure 6B, C and D). More importantly, We detected the apoptosis in Lgr5-Cre;Cdh1fl/fl;p53fl/fl mice at 4 dpi after Tam treatment, and found no apoptosis in E-cadherin-deficient Lgr5+ stem cells following p53 deletion, in contrast to the apoptotic Lgr5+ stem cells in Lgr5-Cre;Cdh1fl/fl mice (Figure S4B). These observations indicate that the E-cadherin-regulated survival of Lgr5+ antral stem cells is dependent on p53.
Discussion
The cellular dynamics of Lgr5+ gastric antral stem cells are well-established, but the mechanism that regulates Lgr5+ stem cell homeostasis is not fully understood. Here, we demonstrate that E-cadherin deletion in Lgr5+ gastric antral stem cells induces their apoptosis, thereby leading to a significant reduction in Lgr5+ stem cell number. E-cadherin was identified as a previously unappreciated regulator of stem cell survival. Moreover, the pro-survival function of E-cadherin has been demonstrated in various epithelial cells [15, 19], but not in stem cells. E-cadherin loss within neural and dental epithelial stem cells promotes the differentiation of stem cells [12, 13], while in the gastric corpus isthmus, Mist1+ cells lacking E-cadherin may differentiate into abnormal mucous-producing atypical cells [14].
Reduced percentage of E-cadherin-deficient Lgr5+ stem cells in short-term lineage tracing assays. Co-IF for Lgr5-GFP (green), tdTomato (red), and E-cadherin (white), and quantification of E-cadherin-negative cells in Lgr5-GFP+tdTomato+ stem cells in the gastric antrum of Lgr5-Cre;Cdh1fl/fl;Rosa26tdTomato (n = 3) mice at 1, 4, and 20 dpi. The data are presented as the means ± SEM, **P < 0.01, *P < 0.05. Yellow arrowheads indicate Lgr5-GFP+tdTomato+E-cadherin- stem cells, while white ones indicate Lgr5-GFP-tdTomato+E-cadherin- progenies. Nuclei are dyed using DAPI (blue). Scale bars: 20 μm.
E-cadherin loss in Lgr5+ stem cells lead to their apoptosis. Co-IF for Lgr5-GFP (green), E-cadherin (white), and cleaved caspase-3 (red), and quantification of cleaved caspase-3+ cells per gland in the gastric antrum of Lgr5-Cre;Cdh1fl/+ (n = 4) and Lgr5-Cre;Cdh1fl/fl (n = 6) mice after 7 weeks of Tam treatment. The data are presented as means ± SEM, *P < 0.05. Yellow arrowheads indicate Lgr5-GFP+E-cadherin- cleavedcaspase-3+ cells. Nuclei are dyed using DAPI (blue). Scale bars: 20 μm.
The loss of Lgr5+ stem cells following E-cadherin deficiency impairs the gastric antral homeostasis. (A) H&E staining and quantification of the number of epithelial cells per gland in Lgr5-Cre;Cdh1fl/+ (n = 4) and Lgr5-Cre;Cdh1fl/fl (n = 6) antrum after 7 weeks of Tam treatment. (B) Co-IF for Lgr5-GFP (green) and BrdU (red), and quantification of BrdU-positive cells per gland in Lgr5-Cre;Cdh1fl/+ (n = 4) and Lgr5-Cre;Cdh1fl/fl (n = 6) antrum after 7 weeks of Tam treatment. (C) Morphology of antral organoids after treatment with 4H-Tam for 1, 2, 3, and 4 days, and quantification of organoid size at the different time points in the antrum of Lgr5-Cre;Cdh1fl/+ (n = 3) and Lgr5-Cre; Cdh1fl/fl mice (n = 3). The data are presented as means ± SEM, ***P < 0.001, **P < 0.01, *P < 0.05. Nuclei are dyed using DAPI (blue). Scale bars: 50 μm (A and B), 100 μm (E).
Furthermore, we found that p53 is involved in apoptosis in Lgr5+ cells following E-cadherin loss, as the deletion of p53 in E-cadherin mutants rescued the populations of E-cadherin-deficient Lgr5+ stem cells and their non-stem cell progenies. Our finding that p53 functions as a downstream effector of E-cadherin in Lgr5+ antral stem cells parallels that in breast epithelial cells [22] and gastric parietal cells [23], where apoptosis occurring upon E-cadherin deletion could be blocked following the loss of p53. Previous study has demonstrated that in gastric parietal cells, the synergistic tumor suppressor role of E-cadherin and p53 in the development of diffuse-type gastric cancer (DGC) [23]. Co-deletion of p53 in E-cadherin-deficient parietal cells not only abrogated E-cadherin-deficiency-induced apoptosis, but also promoted cell growth [23]. Similarly, in Mist1+ stem cells, concurrent deletion of E-cadherin and p53 under the condition of chronic inflammation induced by Helicobacter felis infection could result in DGC formation within 9 months [14]. These above results indicated that DGC could originate from both stem and differentiated cells. However, both parietal cells and Mist1+ stem cells are predominantly located at gastric corpus. Therefore, the cell-of-origin for DGC in gastric antrum, where DGC also occurred in human, remains unknown. Additionally, our previous study has confirmed that Lgr5+ stem cells in gastric antrum are the cellular origin of invasive intestinal-type gastric cancer in mice with PTEN and Smad4 deletion [9]. Future efforts are needed to detect the long-term effect of Lgr5+ stem cells mutant with E-cadherin and p53.
Our finding demonstrates that the Lgr5+ population is required for gastric antral epithelial homeostasis. Antral Lgr5-expressing cells are identified as actively cycling stem cells, with a turnover time of 10-14 days in the antral epithelium, implying an important role for Lgr5+ stem cells in the homeostasis of the antral epithelium [2]. However, the role of the Lgr5+ population in the maintenance of the antral epithelium has not been established. A recent study identified Axin2 as a new marker of the long-lived stem cell population in the gastric antrum [7]. Axin2+ cells are divided into two populations: Axin2+Lgr5+ cells at the base, and Axin2+Lgr5- cells above the lower isthmus. Upon the depletion of Axin2+Lgr5+ cells, Axin2+Lgr5- cells give rise to new Lgr5+ cells at the base, indicating the potential for interconversion between the different stem cell populations[7]. However, because the depletion of the Axin2+Lgr5+ population in that study was transient, it remains unknown whether the regeneration of the entire gland by Axin2+Lgr5- cells depends on the Lgr5+ cell population [7]. Here, we provide in vivo evidence that Lgr5+ stem cells are required for the homeostasis of the gastric antral epithelium. The ablation of Lgr5+ stem cells caused by E-cadherin loss-induced apoptosis severely compromises the homeostasis of the antral epithelium. Furthermore, in vitro organoid growth supports the essential role of the Lgr5+ stem cell pool. The crucial function of the Lgr5+ population under normal antral homeostatic conditions is not concordant with that in the gastric corpus and the intestinal epithelium, where Lgr5 marks reserve stem cells [24] and mitotically active stem cells [25], respectively. Moreover, the specific ablation of the Lgr5+ population in Lgr5DTR mice is tolerated in the gastric corpus [24] and intestine [26] during homeostatic self-renewal. The negligible effect of the ablation of Lgr5+ cells on the homeostasis of the gastric corpus and intestines may be attributable to the activation of other stem cell populations, as shown by the increase in the number of Bmi1-expressing stem cells in intestine [27] and Troy-expressing stem cells in the gastric corpus [28]. Nevertheless, further studies are needed to clarify the interrelations among Lgr5+, Axin2+, and other cell types in the gastric antrum under stress and normal homeostatic conditions.
Material and Methods
Animals
Animal experiments were approved by the Animal Experimentation Committee of the Beijing Institute of Lifeomics. The mice used in this study were as follows: Lgr5-eGFP-IRES-CreERT2, SPA-Cre, Cdh1fl/fl, p53fl/fl, Rosa26-LoxP-Stop-LoxP-YFP, and Rosa26-LoxP-Stop-LoxP-tdTomato. SPA-Cre mice at E15.5, E16.5, and 2 months and Lgr5-Cre mice at 8-10 weeks regardless of sexes were used. At least 3 mice per genotype were used.
Tamoxifen and BrdU treatment
The activity of Lgr5-Cre was induced using Tam (T5648, Sigma Aldrich, St Louis, MO). Tam was dissolved in 10% ethanol and 90% corn oil and was injected intraperitoneally at 100 mg kg-1. For short-term lineage tracing, mice were injected for 3 consecutive days and harvested at 1, 4, and 20 dpi. For long-term induction, mice were injected with Tam every other day for 10 days, 20 days and 7 consecutive weeks. Mice were injected intraperitoneally with 100 mg kg-1 BrdU (35002, Sigma Aldrich,) 2 hours prior to harvesting.
Immunohistochemical and immunofluorescence staining analysis
Stomach tissues were harvested and fixed in 4% paraformaldehyde (PFA) before paraffin embedding. H&E staining, immunohistochemical staining (IHC), and immunofluorescence staining (IF) were carried out on 5 µm paraffin-embedded sections. Antibodies were as follows: GFP (1:200, Cat. no. 2956, CST, Danvers, MS), BrdU (1:300, Cat. no. ab6326, Abcam, Cambridge, UK), Ki67 (1:1000, Cat. no. ab15580, Abcam), E-cadherin (1:300, Cat. no. 610181, BD Biosciences, Franklin Lakes, NJ), cleaved caspase-3 (1:200, Cat. no. 9661S, CST), TRITC-labelled UEA (1:100; Cat. no. L4889, Sigma), ChrgA (1:100, Cat. no. ZA-0066, Zhongshanjinqiao, Beijing, China), Tff2 (1:500, R33452, Sigma), FITC-labelled DBA (1:100, L9142, Sigma), RFP (1:500, Cat. no. 600-401-379, Rockland, Limerick, PA; tdTomato can be recognized by the RFP antibody), p53 (1:100, Cat. no. 2524S, CST,). The immunofluorescence staining was visualized with TSA regents (Fluorescein, FP1168; Cyanine3, FP1170; Cyanine5, FP1171; PerkinElmer, Waltham, MA) and nuclear counterstaining was performed using DAPI. IF was observed under confocal microscopy (LSP 880, Zeiss, Jena, Germany). IHC was visualized with DAB (1:20, Cat. no. ZLI-9018, Zhongshanjinqiao).
p53 deletion rescues the phenotype of Lgr5+ stem cells that have lost E-cadherin expression. (A) Quantification of E-cadherin-negative cells in Lgr5-GFP+tdTomato+ stem cells in the gastric antrum of Lgr5-Cre;Cdh1fl/fl;Rosa26tdTomato (n = 3) and Lgr5-Cre;Cdh1fl/fl;p53fl/fl;Rosa26tdTomato mice (n = 3) at 1 dpi. (B-C) Quantification of E-cadherin-negative cells (B) in Lgr5-GFP+tdTomato+ stem cells or (C) in Lgr5-GFP-tdTomato+ progenies in the gastric antrum of Lgr5-Cre;Cdh1fl/fl;Rosa26tdTomato (n = 3) and Lgr5-Cre;Cdh1fl/fl;p53fl/fl;Rosa26tdTomato mice (n = 3) at 4 dpi. (D) Co-IF for Lgr5-GFP (green), tdTomato (red), and E-cadherin (white) in the gastric antrums of Lgr5-Cre;Cdh1fl/fl;Rosa26tdTomato and Lgr5-Cre; Cdh1fl/fl;p53fl/fl;Rosa26tdTomato mice at 4 dpi. The data are presented as means ± SEM, ***P < 0.001, **P < 0.01, *P < 0.05, ns = not significant. Yellow arrowheads indicate the E-cadherin-deficient Lgr5+ stem cells, while dotted lines indicate E-cadherin-deficient Lgr5-negative progenies. Nuclei are dyed using DAPI (blue). Scale bars: 20 μm.
Direct GFP imaging by confocal microscopy
Harvested gastric antral tissues were fixed in 4% PFA for 1 hour. For the whole mount analysis of endogenous GFP, gastric tissues removed from the muscle layer were mounted upside down. For the frozen section, tissues were embedded in O.C.T. (4583, Sakura tissue-Tek, Tokyo, Japan) and then cut into sections using a Cryostat microtome (77200186 Issue 5, Thermo, Waltham, MA). Nuclear counterstaining was performed using DAPI and endogenous GFP was directly imaged under confocal microscopy (LSP 880, Zeiss).
Gastric organoid culture
Isolated gastric antral glands were mixed with Matrigel (BD Bioscience) and cultured in medium (Advanced DMEM/F12 supplemented with B27, N2, and N-Acetylcysteine (Invitrogen, Waltham, MA)) containing growth factors (50 ng ml-1 EGF (Peprotech, Rocky Hill, NJ), 100 ng ml-1 FGF10 (Peprotech), 500 ng ml-1 R-spondin1 (R&D Systems, Minneapolis, MN), 100 ng ml-1 Noggin (R&D), and 100 ng ml-1 Wnt3A (R&D)) as described previously [2]. 4H-Tam (100 nM, H7904, Sigma) was used after the first 24 hours and the medium was changed every 2 days. Organoids were observed and imaged every 24 hours.
Quantification
The quantifications of in vivo and in vitro data were performed using image Pro Plus 6.0 software, including the average number of Lgr5-GFP+ gland per mm2 and Lgr5-GFP+ cell per gland (Figure 2B and 2C), the cleaved caspase-3+ cell per gland (Figure 4), the total cell number per gland (Figure 5A), the average number of BrdU-positive cells per gland (Figure 5B) in Lgr5-CreERT2;Cdh1fl/+ and Lgr5-CreERT2;Cdh1fl/fl mice following 7-week Tam treatment; the percentage of E-cadherin-deficient cells in GFP+ tdTomato+ stem cells or in GFP- tdTomato+ cells in Lgr5-CreERT2;Cdh1fl/fl; Rosa26tdTomato (Figure 3) and Lgr5-CreERT2;Cdh1fl/fl; p53fl/fl; Rosa26tdTomato mice (Figure 6) at different time points following 3-day Tam treatment; and the organoid size (mm2) at different time points under 4H-Tam treatment.
Statistical analysis
The statistical analysis was performed using the GraphPad Prism 5 software. Results are presented as the mean ± SEM. A two-tailed unpaired Student's t-test was used to calculate P-values for all datasets. Statistical significance is indicated by P values: *P < 0.05, **P < 0.01 and ***P < 0.001.
Abbreviations
Lgr5: leucine-rich repeat-containing g-protein coupled receptor5; eR1: Runx1 enhancer element; E16.5: embryonic day 16.5; Rosa26YFP: Rosa26-LoxP- Stop-LoxP-YFP; Lgr5-Cre: Lgr5-eGFP-IRES-CreERT2; Tam: tamoxifen; Rosa26tdTomato: Rosa26-LoxP-Stop-LoxP- tdTomato; dpi: days post injection; BrdU: bromodeoxyuridine; ChrgA: chromogranin A; UEA: ulex europaeus agglutinin; 4H-Tam: 4-hydroxytamoxifen; PFA: paraformaldehyde; H&E staining: hematoxylin and eosin staining; Co-IF: co-immunofluorescence staining; IF: immunefluorescence staining; IHC: immumohistochemical staining.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation (81772952, 81572717, 81272702, 31630093, 31430057, and 31571512), National Key Research and Development Program of China (2016YFC1300600), National Science and Technology Major Projects of Infectious Disease (2017ZX103304402), and the Beijing Nova Program (Z161100004916146).
Author contributions
Yuling Tang, Yan Teng, and Xiao Yang conceived and coordinated the study and wrote the paper. Yuling Tang, Jinliang Zhang, Xiubin Li, Chong Zhang, Yanxiao Wang and Jiaqian Xu performed the experiments and analysed data. Guan Yang and Yeguang Chen provided technical assistance. All authors reviewed the results and approved the final version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mills JC, Shivdasani RA. Gastric epithelial stem cells. Gastroenterology. 2011;140:412-24
2. Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH. et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25-36
3. Leushacke M, Ng A, Galle J, Loeffler M, Barker N. Lgr5(+) gastric stem cells divide symmetrically to effect epithelial homeostasis in the pylorus. Cell Rep. 2013;5:349-56
4. Arnold K, Sarkar A, Yram Mary A, Polo Jose M, Bronson R, Sengupta S. et al. Sox2+ Adult Stem and Progenitor Cells Are Important for Tissue Regeneration and Survival of Mice. Cell Stem Cell. 2011;9:317-29
5. Hayakawa Y, Jin G, Wang H, Chen X, Westphalen CB, Asfaha S. et al. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut. 2015;64:544-53
6. Matsuo J, Kimura S, Yamamura A, Koh CP, Hossain MZ, Heng DL. et al. Identification of Stem Cells in the Epithelium of the Stomach Corpus and Antrum of Mice. Gastroenterology. 2017;152(e14):218-31
7. Sigal M, Logan CY, Kapalczynska M, Mollenkopf HJ, Berger H, Wiedenmann B. et al. Stromal R-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature. 2017;548:451-5
8. Demitrack ES, Gifford GB, Keeley TM, Carulli AJ, VanDussen KL, Thomas D. et al. Notch signaling regulates gastric antral LGR5 stem cell function. EMBO J. 2015;34:2522-36
9. Li XB, Yang G, Zhu L, Tang YL, Zhang C, Ju Z. et al. Gastric Lgr5(+) stem cells are the cellular origin of invasive intestinal-type gastric cancer in mice. Cell Res. 2016;26:838-49
10. Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451-5
11. Song X, Zhu CH, Doan C, Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296:1855-7
12. Karpowicz P, Willaime-Morawek S, Balenci L, DeVeale B, Inoue T, van der Kooy D. E-Cadherin regulates neural stem cell self-renewal. J Neurosci. 2009;29:3885-96
13. Li CY, Cha W, Luder HU, Charles RP, McMahon M, Mitsiadis TA. et al. E-cadherin regulates the behavior and fate of epithelial stem cells and their progeny in the mouse incisor. Dev Biol. 2012;366:357-66
14. Hayakawa Y, Ariyama H, Stancikova J, Sakitani K, Asfaha S, Renz BW. et al. Mist1 Expressing Gastric Stem Cells Maintain the Normal and Neoplastic Gastric Epithelium and Are Supported by a Perivascular Stem Cell Niche. Cancer Cell. 2015;28:800-14
15. Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev. 2002;115:53-62
16. Guo SL, Ye H, Teng Y, Wang YL, Yang G, Li XB. et al. Akt-p53-miR-365-cyclin D1/cdc25A axis contributes to gastric tumorigenesis induced by PTEN deficiency. Nature communications. 2013;4:2544
17. Meng FW, Shi L, Cheng X, Hou N, Wang YL, Teng Y, Meng AM, Yang X. Surfactant protein A promoter directs the expression of Cre recombinase in brain microvascular endothelial cells of transgenic mice. Matrix Biol. 2007;26:54-57
18. Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Developmental Biology. 2001;1:1-4
19. Schneider MR, Dahlhoff M, Horst D, Hirschi B, Trulzsch K, Muller-Hocker J. et al. A key role for E-cadherin in intestinal homeostasis and Paneth cell maturation. PLoS One. 2010;5:e14325
20. Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133-40
21. Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14:994-1004
22. Derksen PW, Braumuller TM, van der Burg E, Hornsveld M, Mesman E, Wesseling J. et al. Mammary-specific inactivation of E-cadherin and p53 impairs functional gland development and leads to pleomorphic invasive lobular carcinoma in mice. Dis Model Mech. 2011;4:347-58
23. Shimada S, Mimata A, Sekine M, Mogushi K, Akiyama Y, Fukamachi H. et al. Synergistic tumour suppressor activity of E-cadherin and p53 in a conditional mouse model for metastatic diffuse-type gastric cancer. Gut. 2012;61:344-53
24. Leushacke M, Tan SH, Wong A, Swathi Y, Hajamohideen A, Tan LT. et al. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol. 2017;19:774-86
25. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003-7
26. Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD. et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255-9
27. Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915-20
28. Stange DEK. et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357-68
Author contact
![]() Corresponding authors: Yan Teng, Email: tengyanac.cn and Xiao Yang, Telephone/Fax: 8610-63895937, Email: yangxac.cn
Corresponding authors: Yan Teng, Email: tengyanac.cn and Xiao Yang, Telephone/Fax: 8610-63895937, Email: yangxac.cn

 Global reach, higher impact
Global reach, higher impact