10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2005; 1(2):80-86. doi:10.7150/ijbs.1.80 This issue Cite
Review
Dissecting Nck/Dock Signaling Pathways in Drosophila Visual System
McGill Centre for Research in Neuroscience, and Department of Neurology and Neurosurgery, McGill University Health Centre, 1650 Cedar Avenue, Montreal, Quebec H3G 1A4, Canada
Received 2004-10-1; Accepted 2005-2-1; Published 2005-4-1
Abstract
The establishment of neuronal connections during embryonic development requires the precise guidance and targeting of the neuronal growth cone, an expanded cellular structure at the leading tip of a growing axon. The growth cone contains sophisticated signaling systems that allow the rapid communication between guidance receptors and the actin cytoskeleton in generating directed motility. Previous studies demonstrated a specific role for the Nck/Dock SH2/SH3 adapter protein in photoreceptor (R cell) axon guidance and target recognition in the Drosophila visual system, suggesting strongly that Nck/Dock is one of the long-sought missing links between cell surface receptors and the actin cytoskeleton. In this review, I discuss the recent progress on dissecting the Nck/Dock signaling pathways in R-cell growth cones. These studies have identified additional key components of the Nck/Dock signaling pathways for linking the receptor signaling to the remodeling of the actin cytoskeleton in controlling growth-cone motility.
Keywords: axon guidance, neuronal target recognition, signal transduction, fly visual system.
1. Introduction
The normal function of the nervous system relies on a complex and precise network of neuronal connections. To establish a connection with its target cell during development, a neuron sends out an axon, which often travels over relatively long distance towards the target region in response to guidance cues present in the surrounding environment (i.e. axon guidance) [1]. After reaching the target region, the axon shuts down motility in response to specific targeting signals and subsequently selects a specific target cell within the target field for establishing synaptic connections (i.e. target recognition). The movement of an axon is directed by the growth cone, a sensorimotor structure located at the leading edge of a growing axon. The growth cone possesses sophisticated signaling systems that allow cell surface receptors to communicate with the actin cytoskeleton, thus rapidly converting extracellular guidance and targeting signals into directed movement or the arrest of outgrowth [2, 3]. Accumulated evidence supports that the signaling mechanisms linking growth-cone receptors to the actin cytoskeleton are evolutionarily conserved and appear to be utilized in many other biological processes that require dynamic remodeling of the actin cytoskeleton [2, 3]. Thus, the dissection of the growth-cone signaling systems not only helps to understand the mechanism of axon guidance and target recognition during neural development, but also sheds light on the general signaling mechanisms underlying the restructuring of the actin cytoskeleton in response to extracellular signals.
During past two decades, a combination of biochemical, immunological and genetic studies in both vertebrates and invertebrates have led to significant progress on the understanding of the molecular mechanism of axon guidance and target recognition [1, 2, 3]. A number of growth-cone receptors that detect short-range or long-range cues have been identified. The mechanisms by which these receptors transduce the guidance and targeting signals into the changes in the actin cytoskeleton are also beginning to emerge.
In this review, I will focus on discussing the recent progress on the dissection of the Nck/Dock signaling pathways for regulating photoreceptor (R cell) axon guidance and target recognition in the Drosophila visual system. The wiring of neural network in the Drosophila visual system has been proven to be a powerful model system for genetic dissection of axon guidance and target recognition [4, 5]. The pattern of R-cell-to-brain connectivity in the fly visual system is relatively simple and can be easily visualized. Moreover, the development of the Drosophila compound eye has been extensively studied. A large collection of sophisticated molecular and genetic tools are available for detailed phenotype analysis of genes that encode for signaling proteins expressed in R-cell growth cones. Furthermore, the powerful fly genetics and the recent completion of the genome project greatly facilitate the identification and characterization of genes in Drosophila.
2. The Establishment of R-cell Connectivity in the Developing Fly Visual System
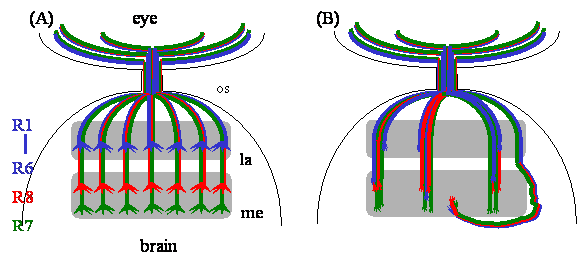
The Drosophila adult visual system is comprised of the compound eye and the optic lobe. The compound eye consists of ~750 ommatidia, or single eye unit. Each ommatidium contains eight different photoreceptor neurons (R cells), which are numbered R1-R8. R cells are born at third-instar larval stage, when precursor cells in the eye-imaginal disc begin to differentiate into R cells [6]. Within each ommatidium, the R8 precursor cell differentiates first, followed by R1-R7 cells. The pioneer R8 axon migrates toward the posterior edge of an eye-imaginal disc, where it converges with axons from other ommatidia and subsequently enters the optic stalk, a structure that connects the eye disc and the brain (Fig. 1A). After exiting the optic stalk, the R8 axon migrates over the superficial layer of the optic lobe, the lamina, and subsequently terminates in the deeper medulla layer. Within each ommatidium, the axons projected from later born R1-R7 cells form a single fascicle with the pioneer R8 axon, and thus follow the route of the R8 axon until reaching a region in between two layers of lamina glial cells (i.e. epithelial and marginal glia). Here R1-R7 axons have to make a decision, whether to stop within the lamina or to migrate further into the medulla. In response to a stop signal from lamina glial cells [7], R1-R6 axons terminate in the lamina (Fig. 1A). During pupation, R1-R6 growth cones undergo further stereotyped rearrangements and establish synaptic connections with lamina cartridge neurons [8, 9]. In contrast, R7 growth cones extend further into the medulla and terminate within a layer (i.e. M7) that is deeper than the R8 terminal field (i.e. M3) (Fig.1A).
3. Dreadlocks (Dock) Is Specifically Required for R-cell Axon Guidance and Target Recognition in the Fly Visual System
The Dreadlocks (dock) gene was originally identified by Garrity and Rao in the Zipursky group in a forward genetic screen for mutations that disrupted R-cell axonal projection pattern but not affected R-cell differentiation and cell fate determination [10]. While loss of dock did not affect R-cell growth-cone motility, it caused abnormal axonal fasciculation, altered local retinotopic array, abnormal completed wiring pattern in the medulla, dramatic reduction in the size of R-cell growth cones, and the mistargeting of R1-R6 growth cones into the medulla layer (Fig. 1B). The dock gene encodes for a SH2/SH3 adapter protein consisting exclusively of three N-terminal Src homology 3 (SH3) domains and a single C-terminal Src homology 2 (SH2) domain [10]. Unlike Drk, another well-known SH2/SH3 adapter protein in Drosophila that links the receptor tyrosine kinase (RTK) signaling to the classical Ras pathway in regulating DNA synthesis and gene expression [11, 12], however, Dock is not required for R-cell differentiation and fate determination determination [10].
The Dock protein is highly homologous to the human proto-oncogene Nck [13]. Indeed, expression of a human Nck transgene in R cells almost fully rescued the dock phenotype [14], suggesting strongly that Nck is the functional homolog of Dock in vertebrates. The structure of the Dock protein and the specific axonal projection phenotype support a model in which Nck/Dock links the guidance and targeting signals from growth-cone receptors to the actin cytoskeleton. By analogy with the action of Drk, it was proposed that Dock mediates growth-cone signaling by binding simultaneously to activated growth-cone receptors and downstream effector proteins [10]. Indeed, biochemical studies on Nck demonstrated that Nck binds via its SH2 domain to specific phosphotyrosine-containing motifs on activated cell surface receptors and simultaneously binds via its SH3 domains to a number of target proteins carrying a conserved PXXP motif, the consensus SH3-binding site [15]. Interestingly, many of Nck SH3-binding proteins, for instance, the Ste20-like serine/threonine kinase Pak (p21-activated kinase) and Wiskott-Aldrich syndrome protein (WASP), have been implicated previously in regulating actin dynamics in cultured cells [16, 17, 18, 19]. However, the biological function of Nck as well as the in vivo relevance of the physical interactions between Nck and its binding partners had not been determined at the time when the dock gene was identified. The identification of Dock thus not only serves as an excellent starting point for dissecting the mechanism of R-cell axon guidance and target recognition, but also provides a great opportunity for understanding the signaling mechanisms by which cell surface receptors communicate with the actin cytoskeleton in general.
As the first step towards understanding the Dock signaling pathway in R-cell growth cones, Rao and Zipursky performed mutagenesis analysis to determine the domain requirements for the function of Dock [14]. Single point mutation was introduced into the SH2 domain and each SH3 domain to inactivate the binding capacity. These mutant constructs were introduced into flies by germ-line transformation to generate mutant transgenic lines, which were then tested for their ability to rescue the dock null mutant phenotype. Surprisingly, it was found that the SH2 domain is not essential for rescuing the defects in R-cell guidance and growth-cone morphology, which appears to be due to the redundancy between the SH2 domain and two SH3 domains (i.e. SH3-1 and SH3-3) [14]. This is in marked contrast to the action of Drk and its homolog Grb2 in mammals and Sem-5 in C-elegans. The SH2 domain of Drk/Grb2/Sem-5 binds to tyrosine-phosphorylated receptor tyrosine kinases and is indispensable for the function of Drk/Grb2/Sem-5 in cell differentiation and proliferation [20]. Interestingly, while the SH2 domain is redundant with the SH3 domains for R-cell axon guidance and growth-cone expansion, it is essential for the establishment of neuronal connectivity in the inner optic lobe (i.e. lobula and lobula plate) [14]. It also appears likely that this functional redundancy between SH2 and SH3 domains does not exist for the role of Dock in regulating the lamina-specific targeting of R1-R6 axons (see below). These data argue strongly that Dock utilizes different combinations of domains in mediating distinct signaling events in different cellular processes or different cell types. Indeed, it has been shown that Dock binds to different sets of cell surface receptors in different cell types [21, 22, 23]. In R cells, Dock appears to be required in at least two distinct signaling pathways, one for R-cell axon guidance and growth-cone expansion, and another for R1-R6 growth-cone targeting (see below).
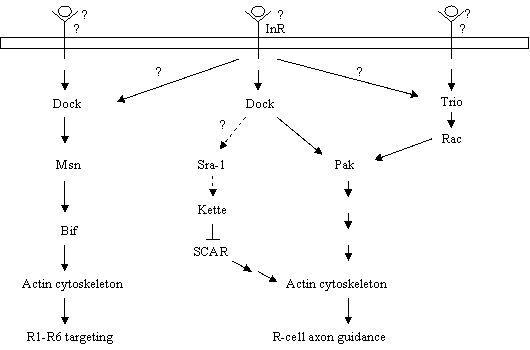
4. The DInR-Dock-Pak Signaling Pathway for R-cell Axon Guidance and Growth-cone Expansion
To understand the action of Dock in R-cell growth cones, it is necessary to identify cell surface receptors that function upstream of Dock, and intracellular effectors that are regulated by Dock-linked signals during R-cell growth-cone guidance and target recognition. In Drosophila, Dock has been shown to physically associate with at least three cell surface receptors, including the fly homologs of human insulin receptor InR [23], the Down syndrome cell adhesion molecule (DSCAM) [21] and the repulsive guidance receptor Roundabout (Robo) [22]. While it has been shown that InR is the upstream receptor for Dock in R-cell growth cones [23], DSCAM and Robo appear to regulate Dock during the pathfinding of larval photoreceptor axons and the medline crossing of embryonic CNS axons, respectively [21, 22]. The mammalian genome contains three InR genes, which have been shown to be required for the control of metabolic activity as well as learning and memory in the nervous system [24, 25, 26, 27, 28]. Fly possesses a single InR gene, which is involved in regulating cell growth, longevity and female fertility [29, 30, 31]. Using the cytoplasmic domain of DInR as a bait in a yeast two-hybrid screen, Pick and colleagues identified Dock as a DInR-interacting protein [23]. DInR associates with Dock in adult flies, and is highly expressed in R-cell axons and their growth cones. dinr loss-of-function mutants displayed defects in R-cell axon guidance and growth-cone morphology that are almost identical to that in dock mutants [23]. These defects appear not to be caused by a general defect in R-cell growth and differentiation. Chico, the Drosophila insulin receptor substrate (IRS) homolog functioning downstream of DInR in mediating cell-growth signaling [32], is not required for R-cell axon guidance [23]. The in vivo relevance of the physical interaction between DInR and Dock is further supported by the observed genetic interaction between dinr and dock during R-cell axon guidance [23]. Interestingly, DInR appears to bind to both SH2 and SH3 domains of Dock in a yeast two-hybrid assay [23], which is consistent with the observed functional redundancy between SH2 and SH3 domains of Dock for R-cell axon guidance [14]. These observations thus build a compelling case for that DInR functions upstream of Dock during R-cell axon guidance. Given the fact that InR is required for learning and memory in mammals, it is likely that a similar interaction between Nck and InR also exists in the mammalian nervous system.
The search for downstream effectors of Dock in R-cell growth cones benefits greatly from previous biochemical studies on its mammalian homolog Nck, which led to the identification of more than twenty Nck-binding proteins [15]. Among them, the Ste20-like serine/threonine kinase Pak is particularly interesting. In cultured cells, it has been shown that Nck recruits Pak to the plasma membrane [33]. Subsequently, Pak is activated through its association with GTP-bound active form of Rho family small GTPases Rac and Cdc42 [34], key regulators of the actin cytoskeleton [35]. Pak binds to the 2nd SH3 domain of Nck, which in Dock is essential for R-cell axon guidance. In mammals, the six Pak isoforms can be divided into two groups [36]. The polypeptide of the group I (i.e. Paks 1-3) Pak carries a N-terminal PXXP motif for binding to Nck, a CRIB motif (Cdc42/Rac interactive binding) for binding to small GTPases Rac and Cdc42, and a C-terminal serine/threonine kinase domain. Three pak-related genes are found in the fly genome, including pak [37], mbt (mushroom bodies tiny) [38] and pak3. Among them, Pak is most closely related to the vertebrate group I Pak and also contains the consensus N-terminal PXXP motif responsible for binding to Nck. Indeed, Zipursky and colleagues demonstrated a direct association of Pak with Dock [39]. To determine if Pak and Dock functions in the same pathway in vivo, they took a reverse genetic approach to examine the role of Pak during R-cell growth-cone guidance [39]. Indeed, they found that mutations in the pak gene locus caused severe defects in R-cell axon guidance and growth-cone expansion that are almost indistinguishable from that in dock mutants. The function of Pak in R-cell growth cones requires the kinase domain, the Dock-binding PXXP motif, and the CRIB domain for binding to GTP-bound Rac and Cdc42 [39]. That expression of a membrane-tethered version of Pak substantially rescued the defects in R-cell axon guidance and growth-cone expansion in dock null mutants [39], suggesting strongly that the action of Dock in R-cell axon guidance is to target Pak to the plasma membrane. Once reaching the plasma membrane, like the situation of the vertebrate Pak [34, 40], the fly Pak may then be activated by GTP-bound Rac and Cdc42 to regulate the remodeling of the actin cytoskeleton within R-cell growth cones.
In vertebrates, GTP-bound active form of Rac and Cdc42 can both bind to Pak and stimulate its kinase activity [40]. Interestingly, later studies demonstrated that Rac but not Cdc42 is the major activator of Pak in R-cell growth cones [41, 42]. The fly genome contains three Rac-related sequences, including Rac1, Rac2 and Mtl (Mig-two-like), which appear to have largely overlapping function during development. While loss of all three Rac genes caused a dock- and pak-like R-cell growth-cone guidance phenotype [42], mutations in Cdc42 appeared to mainly affect R-cell differentiation and cell survival [43]. Consistent with that Rac is the major activator of Pak in R-cell growth cones, mutations in the trio gene encoding for a guanine nucleotide exchange factor promoting GTP/GDP exchange on Rac but not Cdc42, also caused a pak-like R-cell axon guidance phenotype [41]. A role for trio in R-cell axon guidance was firstly demonstrated by Dickson and colleagues, who performed an elegant and simple eye-specific genetic screen to search for genes that are required autonomously during R-cell growth-cone guidance [44]. The fly Trio protein contains a N-terminal conserved domain, six Spectrin repeats, two GEF domains and a SH3 domain, all of which are also present in its homolog Trio in vertebrates and UNC-73 in C-elegans [45, 46, 47]. Unlike the vertebrate Trio [45], however, the fly Trio does not contain the domain for binding to Lar, a receptor tyrosine phosphatase [48]. The action of Trio for promoting GTP/GDP exchange on small GTPases is dependent on its GEF domains consisting of a Dbl homology (DH) domain and a pleckstrin homology (PH) domain, which are folded together to form a pocket for small GTPases. Interestingly, only GEF 1, which promotes GTP/GDP exchange on Rac but not Cdc42 and Rho, is essential for R-cell growth-cone guidance [41]. Reducing the dosage of trio enhanced a weak dock loss-of-function phenotype, while reducing the dosage of pak enhanced a weak trio phenotype [41]. Moreover, A trio gain-of-function eye phenotype could be fully suppressed by loss of three Rac genes [42]. These studies suggest strongly that Trio functions upstream of Rac and Pak during R-cell axon guidance.
Thus, the combination of above biochemical and genetic studies supports a model in which Pak is activated sequentially by two signals (Fig. 2). First, the activation of InR by one signal leads to the targeting of the Dock-Pak complex to the plasma membrane. Subsequently, the activation of Trio by another signal leads to an increase in the local concentration of GTP-bound Rac, which stimulates the activity of the membrane-associated Pak, thus allowing Pak to regulate actin dynamics during R-cell growth-cone guidance. Several basic questions still remain to be addressed. First, the ligand that binds and activates DinR during R-cell axon guidance is unknown. In Drosophila, seven insulin-like genes have been identified [49]. It will be of interest to determine if any of them acts as a guidance cue for InR in the fly visual system. If not, it will be necessary to search for other ligands that activate InR during R-cell axon guidance. Second, the upstream signal that activates Trio remains elusive. In mammals, Trio can associate directly with the receptor tyrosine phosphatase Lar [45]. Although the fly Trio does not contain the binding site for Lar, it does interact with Lar genetically during motor axon guidance in embryos [50] as well as the targeting of R7 axons in the visual system [51]. It is possible that Trio is also activated by a Lar-like receptor during R-cell axon guidance. Third, the mechanism by which Pak regulates actin dynamics in R-cell growth cones is unknown. It has been shown that the vertebrate Pak phosphorylates a number of cytoskeletal regulators, for instance, myosin light chain kinase and LIM kinase [36]. It would be of interest to determine if their fly homologs are also the substrates of Pak in R-cell growth cones.
5. The Dock-Msn-Bif Signaling Pathway for R1-R6 Growth-cone Targeting
Loss of dock not only caused defects in R-cell axon guidance and growth-cone expansion, it also disrupted R1-R6 growth-cone targeting [10]. In dock mutants, many R1-R6 axons bypassed the lamina, their appropriate target layer, and extended into the medulla instead (Fig. 1B). By contrast, R1-R6 connectivity remains largely normal in pak and trio mutants [39, 41]. These observations suggest the presence of additional signaling proteins that interact with Dock during R1-R6 growth-cone targeting. Biochemical studies on Nck show that in addition to Pak, Nck also binds to another Ste20-like serine/threonine kinase NIK (Nck-interacting kinase) in cultured cells [52]. NIK belongs to the GCK subfamily of Ste20-like serine/threonine kinases [53, 54], and has a homolog Misshapen (Msn) in Drosophila [55] and Mig-15 in C. elegans [56], both have been shown to be required for cell shape changes during development. Like other GCK subfamily members, NIK/Msn/Mig-15 is comprised of an N-terminal kinase domain, and a C-terminal regulatory region containing binding sites for Nck but not for small GTPases. By taking a combination of biochemical and genetic approaches, we demonstrate that Msn functions downstream of Dock to regulate R1-R6 growth-cone targeting [57]. Dock binds via its first and second SH3 domains to the Proline-rich sequence on Msn [57, 58]. Loss of msn, like loss of dock, caused many R1-R6 growth cones to project aberrantly through the lamina into the medulla [57]. Conversely, overexpression of Msn led to premature growth-cone termination before they reached their normal lamina termination site. In addition to its physical association with Dock, msn also interacts genetically with dock during R-cell projections [57]. These data suggest strongly that Dock-mediated R1-R6 targeting requires the activation of Msn, which may phosphorylate one or more cytoskeletal regulators leading to the lamina-specific termination of R1-R6 growth cones.
In a search for the suppressors of a premature growth-cone termination phenotype induced by overexpression of Msn, we identified Bifocal (Bif), a putative cytoskeletal regulator [59], as a downstream target of Msn in R-cell growth cones [60]. The bif gene was originally cloned by the Chia group [59]. Mutations in bif caused abnormal R-cell morphology. This observation, together with that Bif co-localizes with F-actin during development, led to the suggestion that Bif is a cytoskeletal regulator [59]. The potential role for Bif in R-cell growth cones was examined by genetic analysis [60]. Interestingly, like loss of dock and msn, mutations in bif also caused many R1-R6 growth cones to project aberrantly into the medulla. Like overexpression of Msn, overexpression of Bif also induced early growth-cone termination. Biochemical studies show that Msn directly associates with Bif and phosphorylates it [60]. Although it is unclear if Bif binds directly to F-actin, it promotes F-actin formation and co-localizes with F-actin in cultured cells. The effect of Bif on the actin cytoskeleton in cultured cells could be modulated significantly in the presence of Msn [60]. These studies support a model in which a Dock-linked targeting signal activates Msn, which phosphorylates Bif. Bif, in turn, directly or indirectly modulates the actin-cytoskeleton within R-cell growth cones leading to the termination of R1-R6 growth cones within the lamina (Fig. 2).
The molecular nature of the stop signal and the upstream receptor for Dock during R1-R6 growth-cone targeting remains elusive. DinR has been shown to function upstream of Dock during R-cell axon guidance and growth-cone expansion (see above). It would be of interest to determine if DinR also interacts with Dock in specifying R1-R6 targeting. Since lamina glial cells appear to be essential for R1-R6 growth-cone targeting [7], future genetic analysis of genes required in lamina glial cells for R1-R6 termination may help to identify the glial-derived protein that functions as a stop signal for R1-R6 growth cones in the lamina.
6. Dock-Sra-1-Kette, A Third Path to the Actin Cytoskeleton in R-cell Growth Cones?
In addition to its interaction with pak, msn and trio, dock also displays genetic interaction with kette in R-cell growth cones [61]. The kette gene encodes for a member of the evolutionarily conserved NAP-1/Hem2/GEX3 family proteins [61, 62]. Although it is unclear if Dock and Kette physically interact with each other in R-cell growth cones, their mammalian homologs Nck and NAP-1(Nck-interacting protein 1) do form an in vivo complex [63]. The association between Nck and NAP-1 is indirect and appears to be mediated by PIR/Sra1 (specifically Rac associated protein 1), which may bind directly to the 1st SH3 domain of Nck [64, 65]. NAP-1/Kette and PIR/Sra1 are present in a large protein complex also comprising of Abi (Abelson-interactor) [66, 67], SCAR/WAVE1 [68, 69], and HSPC300 [70]. Biochemical studies show that the formation of this large complex prevents SCAR/WAVE1 from activating the Arp2/3 complex that initiates actin nucleation [70]. Consistently, genetic analysis in Drosophila demonstrates that reducing the dosage of scar largely suppresses the kette axon guidance phenotype in the fly embryo [71]. In addition to its inhibitory effect on the activity of SCAR/WAVE1, NAP1/Kette is also required for the stability of SCAR/WAVE1 [72, 73]. In Drosophila cultured cells, reducing the expression of Kette with RNAi treatment led to the degradation of the SCAR protein. Interestingly, it has been shown that the binding of Nck to PIR/Sra1 and NAP-1/Kette can release SCAR/WAVE1 from the inhibitory complex, leading to the activation of the Arp2/3 complex [70]. Similarly, Dock may interact with PIR/Sra1 and NAP1/Kette in R-cell growth cones to regulate the activity of SCAR, thus providing an additional mechanism for Dock to regulate actin dynamics during R-cell growth-cone guidance (Fig. 2). Future studies will be necessary to examine the exact role of Kette in R-cell growth cones, and determine the potential cross-talk between the Kette pathway and other Dock-linked pathways.
7. Concluding Remarks
A decade ago, biochemical and genetic studies of the well-known SH2/SH3 adapter protein Grb2/Drk/Sem-5 greatly advanced our knowledge of the signaling mechanisms by which the signals are conveyed from cell surface receptors to the nuclei in regulating DNA synthesis and gene expression during cell growth and differentiation [20]. Similarly, recent studies on the signaling of the Nck/Dock SH2/SH3 adapter in R-cell growth cones have shed light on the molecular mechanism of another equally important cellular process, the communication between cell surface receptor and the actin cytoskeleton. Over the past eight years after the implication of Dock in R-cell axon guidance and target recognition, significant progress has been made in defining the signaling pathways by which Nck/Dock links growth-cone receptors to the actin cytoskeleton. These studies have led to the identification of several key players in the pathways, most of which are evolutionarily conserved. Some have also been shown to play a role in the mammalian nervous system. For instance, mutations in the brain-specific Pak isoform Pak3 cause nonsyndromic X-linked mental retardation in humans [74], which may be due to an early axon guidance defect during development. The mammalian InR has also been implicated in learning and memory [25, 26, 27, 28], which may reflect a role for InR in the wiring of neural network in mammals. Thus, in addition to continuously fill the gaps in the Nck/Dock signaling pathways for R-cell axon guidance and target recognition in Drosophila, a major challenge for the future is to define the role of Nck/Dock and its interacting proteins in the establishment of neuronal connectivity in mammals.
Conflict of interests
None declared.
References
1. Tessier-Lavigne M. et al. The molecular biology of axon guidance. Science. 1996Nov15 ;274(5290):1123-33
2. Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002Dec6 ;298(5600):1959-64
3. Guan KL. et al. Signalling mechanisms mediating neuronal responses to guidance cues. Nat Rev Neurosci. 2003Dec ;4(12):941-56
4. Clandinin TR. et al. Making connections in the fly visual system. Neuron. 2002Aug29 ;35(5):827-41
5. Tayler TD. et al. Axon targeting in the Drosophila visual system. Curr Opin Neurobiol. 2003Feb ;13(1):90-5
6. Tomlinson A. et al. Cell fate in the Drosophila ommatidium. Dev Biol. 1987 ;166:264-75
7. Poeck B. et al. Glial cells mediate target layer selection of retinal axons in the developing visual system of Drosophila. Neuron. 2001Jan ;29(1):99-113
8. Meinertzhagen IA. et al. The development of the optic lobe. In: (ed.) Bate M, Martinez-Arias A. The Development of Drosophila melanogaster. New York: Cold Spring Harbor Press. 1993:1363-1491
9. Clandinin TR. et al. Afferent growth cone interactions control synaptic specificity in the Drosophila visual system. Neuron. 2000Nov ;28(2):427-36
10. Garrity PA. et al. Drosophila photoreceptor axon guidance and targeting requires the dreadlocks SH2/SH3 adapter protein. Cell. 1996May31 ;85(5):639-50
11. Olivier JP. et al. A Drosophila SH2-SH3 adaptor protein implicated in coupling the sevenless tyrosine kinase to an activator of Ras guanine nucleotide exchange, Sos. Cell. 1993Apr9 ;73(1):179-91
12. Simon MA. et al. An SH3-SH2-SH3 protein is required for p21Ras1 activation and binds to sevenless and Sos proteins in vitro. Cell. 1993Apr9 ;73(1):169-77
13. Lehmann JM. et al. Nck, a melanoma cDNA encoding a cytoplasmic protein consisting of the src homology units SH2 and SH3. Nucleic Acids Res. 1990Feb25 ;18(4):1048-
14. Rao Y. et al. Domain requirements for the Dock adapter protein in growth- cone signaling. Proc Natl Acad Sci U S A. 1998Mar3 ;95(5):2077-82
15. Li W. et al. Nck/Dock: an adapter between cell surface receptors and the actin cytoskeleton. Oncogene. 2001Oct1 ;20(44):6403-17
16. Chen W. et al. The Caenorhabditis elegans p21-activated kinase (CePAK) colocalizes with CeRac1 and CDC42Ce at hypodermal cell boundaries during embryo elongation. J Biol Chem. 1996Oct18 ;271(42):26362-8
17. Miki H. et al. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. Embo J. 1996Oct1 ;15(19):5326-35
18. Symons M. et al. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell. 1996Mar8 ;84(5):723-34
19. Sells MA. et al. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997Mar1 ;7(3):202-10
20. Pawson T. Tyrosine kinase signalling pathways. Princess Takamatsu Symp. 1994 ;24:303-22
21. Schmucker D. et al. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000Jun9 ;101(6):671-84
22. Fan X. et al. Slit stimulation recruits Dock and Pak to the roundabout receptor and increases Rac activity to regulate axon repulsion at the CNS midline. Neuron. 2003Sep25 ;40(1):113-27
23. Song J. et al. Axons guided by insulin receptor in Drosophila visual system. Science. 2003Apr18 ;300(5618):502-5
24. Baskin DG. et al. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 1999Nov27 ;848(1-2):114-23
25. Lannert H. et al. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci. 1998Oct ;112(5):1199-208
26. Wickelgren I. Tracking insulin to the mind. Science. 1998 ;280(5363):517-9
27. Craft S. et al. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry. 1999Dec ;56(12):1135-40
28. Zhao W. et al. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J Biol Chem. 1999Dec3 ;274(49):34893-902
29. Fernandez R. et al. The Drosophila insulin receptor homolog: a gene essential for embryonic development encodes two receptor isoforms with different signaling potential. Embo J. 1995Jul17 ;14(14):3373-84
30. Chen C. et al. The Drosophila insulin receptor is required for normal growth. Endocrinology. 1996Mar ;137(3):846-56
31. Tatar M. et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001Apr6 ;292(5514):107-10
32. Bohni R. et al. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell. 1999Jun25 ;97(7):865-75
33. Galisteo ML. et al. The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J Biol Chem. 1996Aug30 ;271(35):20997-1000
34. Lu W. et al. Mechanism of activation of Pak1 kinase by membrane localization. Oncogene. 1999Jan21 ;18(3):797-806
35. Dickson BJ. Rho GTPases in growth cone guidance. Curr Opin Neurobiol. 2001 ;11(1):103-10
36. Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003 ;72:743-81
37. Harden N. et al. A Drosophila homolog of the Rac- and Cdc42-activated serine/threonine kinase PAK is a potential focal adhesion and focal complex protein that colocalizes with dynamic actin structures. Mol Cell Biol. 1996May ;16(5):1896-908
38. Melzig J. et al. A protein related to p21-activated kinase (PAK) that is involved in neurogenesis in the Drosophila adult central nervous system. Curr Biol. 1998Nov5 ;8(22):1223-6
39. Hing H. et al. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell. 1999Jun25 ;97(7):853-63
40. Manser E. et al. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994Jan6 ;367(6458):40-6
41. Newsome TP. et al. Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell. 2000Apr28 ;101(3):283-94
42. Hakeda-Suzuki S. et al. Rac function and regulation during Drosophila development. Nature. 2002Mar28 ;416(6879):438-42
43. Genova JL. et al. Functional analysis of Cdc42 in actin filament assembly, epithelial morphogenesis, and cell signaling during Drosophila development. Dev Biol. 2000May1 ;221(1):181-94
44. Newsome TP. et al. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000Feb ;127(4):851-60
45. Debant A. et al. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci U S A. 1996May28 ;93(11):5466-71
46. Steven R. et al. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell. 1998Mar20 ;92(6):785-95
47. Lin MZ. et al. Orchestral maneuvers in the axon: trio and the control of axon guidance. Cell. 2000Apr28 ;101(3):239-42
48. Streuli M. et al. A new member of the immunoglobulin superfamily that has a cytoplasmic region homologous to the leukocyte common antigen. J Exp Med. 1988Nov1 ;168(5):1523-30
49. Brogiolo W. et al. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001Feb20 ;11(4):213-21
50. Bateman J. et al. The guanine nucleotide exchange factor trio mediates axonal development in the Drosophila embryo. Neuron. 2000Apr ;26(1):93-106
51. Maurel-Zaffran C. et al. Cell-autonomous and -nonautonomous functions of LAR in R7 photoreceptor axon targeting. Neuron. 2001Oct25 ;32(2):225-35
52. Su YC. et al. NIK is a new Ste20-related kinase that binds NCK and MEKK1 and activates the SAPK/JNK cascade via a conserved regulatory domain. Embo J. 1997Mar17 ;16(6):1279-90
53. Sells MA. et al. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell. Biol. 1997 ;7:162-7
54. Dan I. et al. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001May ;11(5):220-30
55. Treisman JE. et al. misshapen encodes a protein kinase involved in cell shape control in Drosophila. Gene. 1997Feb20 ;186(1):119-25
56. Poinat P. et al. A conserved interaction between beta1 integrin/PAT-3 and Nck-interacting kinase/MIG-15 that mediates commissural axon navigation in C. elegans. Curr Biol. 2002Apr16 ;12(8):622-31
57. Ruan W. et al. The SH2/SH3 adaptor protein dock interacts with the Ste20-like kinase misshapen in controlling growth cone motility. Neuron. 1999Nov ;24(3):595-605
58. Su YC. et al. The Ste20 kinase misshapen regulates both photoreceptor axon targeting and dorsal closure, acting downstream of distinct signals. Mol Cell Biol. 2000Jul ;20(13):4736-44
59. Bahri SM. et al. The Drosophila bifocal gene encodes a novel protein which colocalizes with actin and is necessary for photoreceptor morphogenesis. Mol Cell Biol. 1997Sep ;17(9):5521-9
60. Ruan W. et al. Bifocal is a downstream target of the Ste20-like serine/threonine kinase misshapen in regulating photoreceptor growth cone targeting in Drosophila. Neuron. 2002Dec5 ;36(5):831-42
61. Hummel T. et al. The Drosophila HEM-2/NAP1 homolog KETTE controls axonal pathfinding and cytoskeletal organization. Genes Dev. 2000Apr1 ;14(7):863-73
62. Baumgartner S. et al. The HEM proteins: a novel family of tissue-specific transmembrane proteins expressed from invertebrates through mammals with an essential function in oogenesis. J Mol Biol. 1995Aug4 ;251(1):41-9
63. Kitamura T. et al. Molecular cloning of p125Nap1, a protein that associates with an SH3 domain of Nck. Biochem Biophys Res Commun. 1996Feb15 ;219(2):509-14
64. Kitamura Y. et al. Interaction of Nck-associated protein 1 with activated GTP-binding protein Rac. Biochem J. 1997 ;322(Pt 3):873-8
65. Kobayashi K. et al. p140Sra-1 (specifically Rac1-associated protein) is a novel specific target for Rac1 small GTPase. J Biol Chem. 1998Jan2 ;273(1):291-5
66. Dai Z. et al. Abi-2, a novel SH3-containing protein interacts with the c-Abl tyrosine kinase and modulates c-Abl transforming activity. Genes Dev. 1995Nov1 ;9(21):2569-82
67. Shi Y. et al. Abl-interactor-1, a novel SH3 protein binding to the carboxy-terminal portion of the Abl protein, suppresses v-abl transforming activity. Genes Dev. 1995Nov1 ;9(21):2583-97
68. Bear JE. et al. SCAR, a WASP-related protein, isolated as a suppressor of receptor defects in late Dictyostelium development. J Cell Biol. 1998Sep7 ;142(5):1325-35
69. Miki H. et al. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. Embo J. 1998Dec1 ;17(23):6932-41
70. Eden S. et al. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002Aug15 ;418(6899):790-3
71. Bogdan S. et al. Kette regulates actin dynamics and genetically interacts with Wave and Wasp. Development. 2003Sep ;130(18):4427-37
72. Kunda P. et al. Abi, Sra1, and Kette control the stability and localization of SCAR/WAVE to regulate the formation of actin-based protrusions. Curr Biol. 2003Oct28 ;13(21):1867-75
73. Rogers SL. et al. Molecular requirements for actin-based lamella formation in Drosophila S2 cells. J Cell Biol. 2003Sep15 ;162(6):1079-88
74. Allen KM. et al. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet. 1998Sep ;20(1):25-30
FIGURES
R-cell axon guidance and target recognition in the fly visual system. (A) In the wild-type fly visual system, R-cell axons form a highly organized projection pattern in the optic lobe. R1-R6 axons terminate in the lamina, while R7 and R8 axons project through the lamina into the medulla. R-cell growth cones expand significantly in size after reaching their appropriate target layer. (B) In dock mutants, R-cell axons form abnormal large bundles in both lamina and medulla. R-cell growth cones fail to expand. Some axons bypass the medulla. Many R1-R6 axons project aberrantly through the lamina into the medulla. Abbreviations: la, lamina; me, medulla; os, optic stalk.
Models for the action of the Nck/Dock signaling pathways in R-cell growth cones. During R-cell axon guidance and growth-cone expansion, Pak is activated by the combined action of an InR-Dock-linked signal and a Trio-Rac-linked signal, thus allowing Pak to modulate cytoskeletal changes. Dock may also link the guidance signal to the actin cytoskeleton via the Sra-1-Kette-SCAR pathway. During R1-R6 targeting, a Dock-linked stop signal activates Msn, which in turn phosphorylates Bif. Bif then restructures the actin cytoskeleton.
Author biography
Yong Rao received Ph. D. in 1994 from Department of Biochemistry, University of Toronto, Canada. He became a Postdoctoral fellow with Dr. S. Lawrence Zipursky in Howard Hughes Medical Institute, University of California, Los Angeles, USA between 1994-1998. He is currently an Assistant Professor in Centre for Research in Neuroscience, Department of Neurology and Neurosurgery, McGill University. He received the Young Investigator Award from American Peptide Society. Research Interests include axon guidance, neuronal target recognition, and neuronal migration.
![]() Corresponding address:
Corresponding address:
Yong Rao, McGill Centre for Research in Neuroscience, The Montreal General Hospital Research Institute, Room L7-136, 1650 Cedar Avenue, Montreal, Quebec H3G 1A4, Canada. Tel: 514-934-1934 ext. 42520. Fax: 514-934-8265. Email: yong.raoca

 Global reach, higher impact
Global reach, higher impact