10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2007; 3(1):57-63. doi:10.7150/ijbs.3.57 This issue Cite
Short Research Communication
Prion-derived copper-binding peptide fragments catalyze the generation of superoxide anion in the presence of aromatic monoamines
Graduate School of Environmental Engineering, The University of Kitakyushu, Kitakyushu 808-0135, Japan
Received 2006-8-1; Accepted 2006-11-6; Published 2006-11-9
Abstract
Objectives: Studies have proposed two opposing roles for copper-bound forms of prion protein (PrP) as an anti-oxidant supporting the neuronal functions and as a pro-oxidant leading to neurodegenerative process involving the generation of reactive oxygen species. The aim of this study is to test the hypothesis in which putative copper-binding peptides derived from PrP function as possible catalysts for monoamine-dependent conversion of hydrogen peroxide to superoxide in vitro.
Materials and methods: Four peptides corresponding to the copper (II)-binding motifs in PrP were synthesized and used for analysis of peptide-catalyzed generation of superoxide in the presence of Cu (II) and other factors naturally present in the neuronal tissues.
Results: Among the Cu-binding peptides tested, the amino acid sequence corresponding to the Cu-binding site in the helical region was shown to be the most active for superoxide generation in the presence of Cu(II), hydrogen peroxide and aromatic monoamines, known precursors or intermediates of neurotransmitters. Among monoamines tested, three compounds namely phenylethylamine, tyramine and benzylamine were shown to be good substrates for superoxide-generating reactions by the Cu-bound helical peptide.
Conclusions: Possible roles for these reactions in development of prion disease were suggested.
Keywords: aromatic monoamine, copper, prion, redox, superoxide
1. Introduction
Prion protein (PrP) from the brains of animals with transmissible spongiform encephalopathies (TSE) is present as partially protease-resistant form while that in uninfected brains presents as fully sensitive form [1]. In general, the former represents the infectious scrapie isoform of PrP (PrPSc) and the latter represents the intrinsic cellular PrP (PrPC). Such deposition of abnormal protein fibrils is a prominent pathological feature of many different “protein conformational” diseases, including not only “prion” dementias but also some important neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease and motor neuron disease [2]. In the cases of β-amyloid which accumulates in the brain in Alzheimer's disease and of α-synuclein accumulating in Lewy bodies in Parkinson's disease, there are good evidences that the toxic mechanism involves the production of reactive oxygen species (ROS) such as H2O2 and hydroxyl radicals (HO·), supporting the view that one of the fundamental molecular mechanisms underlying the pathogenesis of cell death in neurodegenerative diseases could be the direct production of ROS during formation of the abnormal protein aggregates [2,3].
As generalized in Fig. 1, PrPs from mammals have six to seven putative Cu-binding sites consisted of 4 distinct sequences. Opazo et al. [4] have propounded the importance of metals in neurobiology and proposed two opposing roles for copper-bound PrP as an anti-oxidant required for the neuronal functions and as a pro-oxidant leading to neurodegenerative process. To date, anti-oxidative roles of PrPC are well recognized. It has been shown that E. coli cells expressing PrP sequence (octapeptide repeats region) acquired the resistance to Cu and Cu-dependent oxidative damages, indicating that PrPC possibly contributes to protection of cells from free Cu-catalyzed generation of ROS such as HO· [5]. According to Wong et al. [6], Cu-bound PrPC shows a superoxide dismutase (SOD)-like activity in vitro, and its expression likely contributes to the cellular response to oxidative damages to the cells. Sauer et al. [7] have shown in tumor spheroids that an increase in ROS stimulates the production of PrPC and other ROS scavenging enzymes such as Cu, Zn-SOD and catalase, while ROS-lowering treatments effectively down-regulate the expression of PrPC, implying that PrPC expression in tumor spheroids is regulated by the internal redox state to meet the requirement to protect the cells from ROS. Interestingly, a drastic depress in SOD-like activity has been shown in the affinity-purified total PrP preparation isolated from scrapie-infected mouse brains suggesting that prion disease results in denature of PrP thus no longer active in ROS removal [6].
However, contrary to above reports, there are views questioning the proposed SOD-like activity of PrPs. Jones et al. [8] have reported that recombinant PrP exhibits no detectable dismutase activity above baseline levels measured for copper (II) ions.
It is generally accepted that, like amyloid-β peptide, prion induces apoptotic cell death in neuronal tissues [9]. A PrP amino acid sequence 106-126 covering a putative Cu-binding site, likely forms the region causing neuronal apoptosis since a synthetic Cu-bound peptide corresponding to PrP 106-126 is highly fibrillogenic and highly toxic to neuronal cells as if behaving as a PrPSc mimic [9,10].
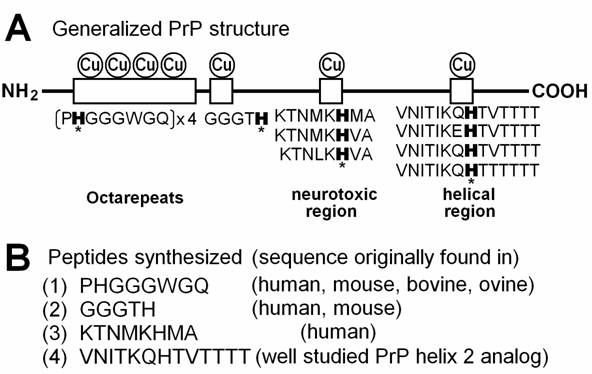
Copper-binding motifs in PrP protein. (A) Putative copper-binding domains present in PrP protein and variations in amino acid sequences required for binding to copper. Histidine residues possibly involved in anchoring of copper are marked with asterisks. (B) Four peptide sequences corresponding to copper-binding motifs in PrP. (1) The most characterized Cu-binding sequence repeated 4 times in human, mouse and ovine, 6 times in bovine was used. (2) An additional Cu binding sequence found in the basic region in human and mouse PrPs immediately after the octarepeats. (3) PrPs from mammals and chicken show similar sequences, but human sequence was tested. (4) Well characterized analog for PrP helix 2 sequence was used [33]. The PrP helix 2 sequences varied among species but the Cu-anchoring H residue is highly conserved in various mammals such as mouse, bovine, ovine, tammar wallaby, brush-tailed possum, and Brazilian opossum.
Through in vitro studies with electron spin resonance spectroscopy, Allsop and his colleagues have shown that H2O2 (detected with a spin trap after conversion to HO· via Fenton reaction) is produced during the early stages of protein aggregation associated with neurodegenerative diseases involving β-amyloid, α-synuclein and toxic forms of PrP [11-13]. Especially Cu-loaded PrP 106-126, the neurotoxic region, likely catalyzes the robust generation of H2O2 [12].
In addition to the Cu-loaded PrP peptide-dependent formation of H2O2, the present study reports the production of superoxide anion (O2•-), another key member of ROS in biological systems, by Cu-binding PrP fragments in the presence of certain co-factors. Previously, the author has proposed a hypothetical model mechanism in which Cu-bound form of PrP catalyzes the generation of ROS in the presence of some natural co-factors at specific occasions in the neuronal tissues [14]. The author has made a speculation that aromatic monoamines may form a group of such co-factors possibly contributing to the PrP-dependent oxidative burst [14] since Cu-bound PrP has high affinity and catalytic activity for oxidation of some aromatic monoamines such as dopamine which is abundantly present in neuronal tissues [15]. The aim of the present study is to test the hypothesis in which putative Cu-binding peptides derived from PrP function as possible catalysts for monoamine-dependent conversion of H2O2 to O2•- in vitro.
2. Materials and methods
Peptides
Four peptides corresponding to each Cu-binding sequence in PrP protein were synthesized to examine the behavior of such Cu-binding domains in chemiluminescence analysis of peptide-mediated oxidative burst. The peptides were obtained from the custom peptide service department of Sigma Genosis Japan (Ishikari, Hokkaido). The amino acid sequences of the peptides chemically synthesized and purified on high pressure liquid chromatography were (1) PHGGGWGQ (purity, 95.72%), (2) GGGTH (purity, 98.98%), (3) KTNMKHMA (purity, 98.85%) and (4) VNITKQHTVTTTT (purity, 99.02%).
Chemicals
2-Methyl-6-phenyl-3,7-dihydroimidazo[1,2-a]pyrazin-3-one (Cypridina luciferin analog, CLA), a chemiluminescence reagent specific to O2•-, was purchased from Tokyo Kasei Kogyo Co. (Tokyo, Japan). Phenylethylamine (PEA), tyramine hydrochloride, and tryptamine hydrochloride were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan). Benzylamine, dopamine hydrochloride, and serotonin were from Nakalai Tesque Inc. (Kyoto, Japan). Other chemicals used in this study were of reagent grade purchased from Sigma (St. Louis, MO, USA).
Detection of superoxide
The peptides and other chemicals were dissolved in phosphate buffered saline and generation of O2•- was monitored by chemiluminescence of CLA with a CHEM-GLOW Photometer (American Instrument Co., MD., USA) equipped with a pen recorder, and expressed as relative luminescence units (rlu) as previously described [16]. CLA-chemiluminescence specifically indicates the generation of O2•- (and 1O2 with a lesser extent) but not that of O3, H2O2 or HO• [17]. According to our previous study, the signal for 1O2 can be minimized by avoiding the use of high concentration of organic solvents such as ethanol over 2% (v/v) in the culture media [18]. Thus the induced chemiluminescence recorded here reflects the generation of O2•- rather than 1O2.
3. Results
Aromatic monoamine-dependent oxidative burst catalyzed by the helical Cu-bound peptide
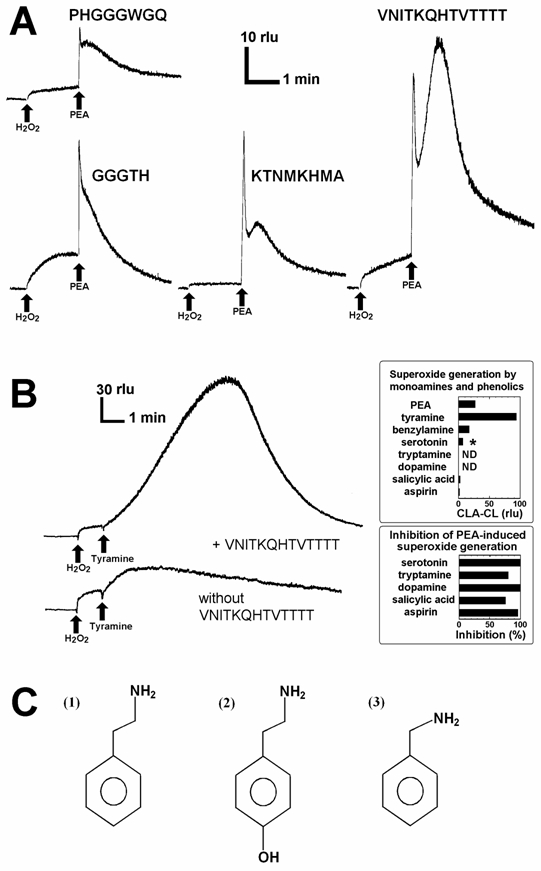
Since earlier studies suggested the high affinity of PrP towards dopamine and related monoamines [15], direct interactions between PrP-derived peptides and some aromatic monoamines leading to generation of O2•- was examined in phosphate buffered saline containing 5 µM CLA, by measuring the O2•--specific chemiluminescence. However, we observed no increase in O2•--dependent chemiluminescence in the reaction mixture simply consisted of peptides and aromatic monoamines (data not shown). Then, addition of other co-factors such as CuSO4 and H2O2 were examined by analogy to the catecholamine-dependent oxidative burst-catalyzing enzymes such as copper-amine oxidases, monoamine oxidases and monoamine-oxidizing peroxidases. Here, generation of O2•- was examined by adding CLA, peptide, Cu, H2O2 and aromatic monoamines to phosphate buffered saline in this order. In the reaction mixture (200 µl) containing helical peptide (VNITKQHTVTTTT), Cu2+, H2O2, and phenylethylamine (PEA) with an approximate molar ratio of 1 : 3 : 3 : 10, a robust increase in the O2•--dependent chemiluminescence was observed (Fig. 2A). The observed burst of O2•- production was a biphasic event consisting of the acute spike and the gradual increase in O2•-. As discussed below, the PEA-induced acute spike can be observed in the Cu-H2O2 solution lacking peptides, only the later phase with gradual large increase in chemiluminescence must be attributed to the helical peptide-dependent reactions. Other peptides except for GGGTH also showed catalytic activity leading to biphasic O2•- production with lesser extents. Here again, only the secondary phase of oxidative burst must be considered as the consequences of peptide chemistry. Among the peptides, the activity of VNITKQHTVTTTT peptide sequence was outstanding thus is of great interests.
The cases of tyramine instead of PEA added to the reaction mixture containing 5 μM CLA, 0.3 µM helical peptide, 0.9 µM CuSO4, and 1 mM H2O2 are shown in Fig. 2B. Effects of various aromatic amines namely PEA, tyramine, dopamine, serotonin, tryptamine, benzylamine, on generation of O2•- in peptide-Cu-H2O2 system were compared (Fig. 2B, upper inset). Among the aromatic monoamines, only three monoamines, PEA, tyramine, and benzylamine induced the generation of O2•-, and the structures of such active monoamines are shown in Fig. 2C. In addition, effects of two salicylates known to stimulate the conversion of H2O2 to O2•- by human methemoglobin [19,20] and various peroxidases [20], were also tested (Fig. 2B, upper inset). However, these salicylates were shown to be poor substrates for the generation of O2•- in this system. Interestingly, five related compounds inactive in induction of O2•- namely, serotonin, tryptamine, dopamine, salicylate and aspirin were shown to interfere with the PEA-induced O2•--generating reaction (Fig. 2B, lower inset), opening the ways for chemical and clinical modulation or manipulation of the monoamine-dependent oxidative burst in the PrP rich neuronal tissues.
As the helical peptide was shown to be most active among four Cu-binding peptides derived from PrP as the catalyst for PEA-dependent generation of O2•-, three other peptides less active towards PEA were shown to be also poorly active even towards tyramine and benzylamine (data not shown).
Among, three active monoamines, PEA is the most abundant species in neuronal tissues since PEA is a key precursor for many of catecholamine neurotransmitters. It is likely that reaction involving PEA is most likely to occur in the neuronal tissues compared to the reactions involving tyramine or benzylamine, thus following assays were carried out using PEA as a possible substrate.
Requirement for co-factors
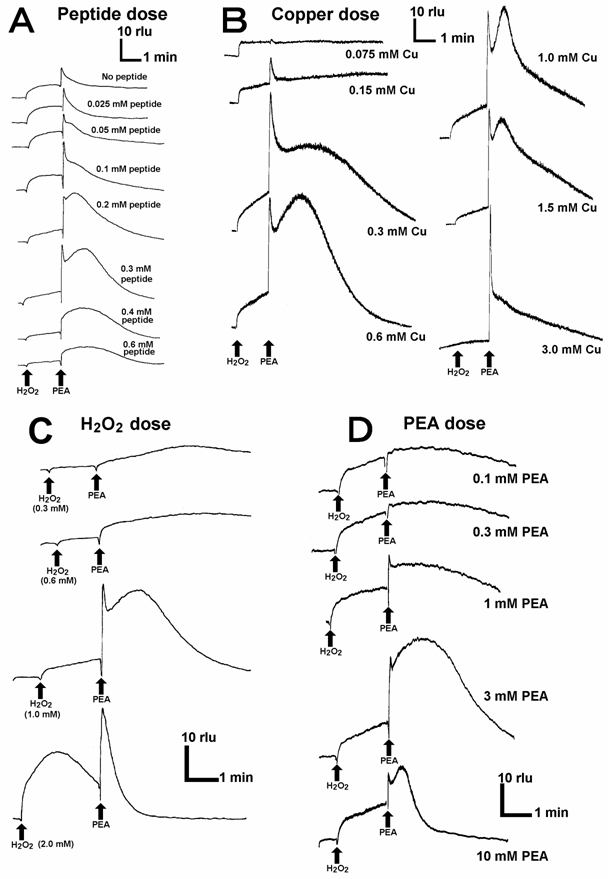
In the reaction mixtures lacking either of 5 components namely CLA, helical peptide, Cu, H2O2 and aromatic monoamines, no drastic increase in the second phase of O2•--dependent chemiluminescence was observed, suggesting that any component is necessarily required for the reaction leading to generation and detection of O2•-. Typical results for peptide-Cu-H2O2-PEA system are shown (Fig. 3). Effects of the concentrations of helical peptide (Fig. 3A), copper (Fig. 3B), H2O2 (Fig. 3C), and PEA (Fig. 3D) on O2•- generation were examined. All components of the reaction were shown to act in concentration-dependent manners, further confirming that each component is necessary for the O2•- generating reaction and thus each component can be a limiting factor. Since the requirement for H2O2 was confirmed, the type of reaction catalyzed by the Cu-supplemented PrP helical sequence is rather similar to the aromatic monoamine oxidation reactions leading to O2•- generation catalyzed by H2O2-requiring plant peroxidases [21, 22] or human methemoglobin [19].
Here, the Cu-loaded peptide-dependent O2•- generation was shown to be fueled by H2O2 added to the reaction mixture. Actually, supply of H2O2 at the micro-domains on Cu-bound whole PrP is possible since Cu-loaded PrP 106-126, the neurotoxic region, is known to catalyze the production of H2O2 [12]. Despite of weak O2•--generating activity of KTNMKHMA corresponding to the neurotoxic sequence (Fig. 2A), this region may contribute to the oxidative burst by producing H2O2.
Superoxide-generating reactions catalyzed by the Cu-supplied PrP peptide fragments. (A) Typical traces of O2•--dependent chemiluminescence increase catalyzed by four putative Cu-binding PrP fragments are shown. Each reaction mixture contained CLA (5 μM), either peptide sequence as indicated (0.3 mM each), CuSO4 (0.9 mM), H2O2 (1 mM) and PEA (3 mM). (B) Comparison of the actions of aromatic monoamines. Reaction mixtures contained 5 μM CLA, 0.3 mM helical peptide, 0.9 mM CuSO4, 1 mM H2O2, and 3 mM of aromatic monoamies or salicylates. Typical traces for tyramine-induced oxidative burst with and without helical peptide are shown. The O2•--generating activities of related compounds were compared in upper inset. Asterisk, O2•- can be observed in serotonin-Cu solution lacking the peptide suggesting that observed chemilumienscence is not reflecting the peptide dependent generation of O2•-. The lower inset shows that five compounds shown to be inactive in oxidative burst induction, in turn inhibits the PEA-dependent O2•- generation. Prior to addition of PEA to the reaction mixture, each inhibitory compound (3 mM) was added. (C) Structures of aromatic monoamines active in Cu-loaded PrP helical peptide-dependent production of O2•-. (1) Phenylethylamine (PEA), (2) tyramine, (3) benzylamine.
Effects of the concentrations of peptide-, copper-, H2O2-, and PEA on superoxide generation. (A) Effect of the helical peptide concentration. (B) Effect of CuSO4 concentration. (C) Effect of H2O2 concentration. (D) Effect of PEA concentration. Basically, the reaction mixtures contained constant amounts of CLA (5 μM), the helical peptide sequence (0.3 mM, exception in A), CuSO4 (0.9 mM, exception in B), H2O2 (1 mM, exception in C) and PEA (3 mM, exception in D).
4. Discussion
Cu-binding sites on PrP
Importance of metalloproteins in neurobiology has been shown both as oxidant and antioxidant in neurodegenerative processes [4]. Cu is an essential trace element but its redox reactivity leads to the risk of damage to the cells and tissues, especially in neurodegenerative diseases such as Menkes' and Wilson's diseases occurring via disorders of Cu metabolism, and Alzheimer's disease and 'prion' diseases, the two major conformational diseases, as documented [23-25, etc]. The likely roles for Cu in induction of critical steps in the apoptotic pathways leading to neurodegeneration is outlined in the above reviews.
Recent studies have shown that PrPs can form a novel group of Cu-binding proteins [26,27]. Human PrP has four Cu-binding sites in the “octarepeats” region (PrP 60-91) in which amino acid sequence PHGGGWGQ appears four times and each repeat possibly binds single Cu2+ at physiological neutral and basic range of pH [28]. In general, the most relevant copper binding mode would involve formation of a 4:1 octarepeat:Cu complex involving coordination of one single Cu molecule by the 4 histidines on the 4 octarepeat segments of a single PrP molecule [29]. In chicken, the Cu-binding sites analogous to the octarepeats are known as hexa-repeats with each repeat consisting of the amino acid sequence HNPGYP and here again His residues play a key role in anchoring of Cu [30]. Morante et al. [31] showed that partial occupancy of Cu on bovine PrP is manifested by binding of Cu to PrP in the intermolecular or inter-octarepeat orientations while total occupancy of Cu is manifested by intra-repeat binding of Cu to the octarepeat region.
In vitro studies have shown that the actual least motif in the octarepeats necessarily required for binding of Cu consists of 4 amino acids HGGG [28] or 5 amino acids HGGGW [32]. Although there are additional Cu-binding sites on PrP such as amino acid regions 92-96 (GGGTH) [27], 124-126 (KHM) [10] and 180-193 (VNITIKQHTVTTTT) [33], and all studies suggested that His residue in each region (or each repeat unit) plays a key role in anchoring of Cu (Fig. 1). Based on above knowledge, four peptides corresponding to Cu-binding domains on PrP, namely PHGGGWGQ (repeated in PrP 60-91), GGGTH (PrP 92-96), KTNMKHMA (PrP 120-127) and VNITKQHTVTTTT (helical, PrP 180-193 analog) were synthesized in the present study and the helical Cu-binding motif was shown to be the most active in monoamine-dependent generation of O2•-. Although the octarepeat-dependent oxidative burst (second phase) was about one forth of the helical peptide-dependent one, this amino acid octet is repeated 4 times in human PrP and 6 times in bovine PrP, its contribution to oxidative burst in whole protein can be greater than that observed for the single repeat peptide.
Redox regulations
The amino-terminal part of PrP (23-98) is rich in amino acid residues susceptible to oxidation, such as His, Lys, Arg, and Pro, thus PrP is sensitive to oxidative changes such as production of ROS and the conformational changes in PrP may be readily induced by redox changes [15, 34]. In addition to the above amino acid residues on PrP, Met residues may be good targets for attacks by ROS. Recently, H2O2-dependent oxidation of Met residues has been reported for recombinant Syrian hamster PrP (29-231) [35]. The likely target residues include Met109, Met112, Met129 and Met134 and the susceptibility to oxidation of each Met residue was shown to be a function of accessibility to the solvent. While Cu-catalyzed oxidation leads to extensive aggregation of PrP, the H2O2-dependent Met oxidation on PrP leads only to a modest increase in β-sheet structure.
According to recently proposed model, there may be an intermediate structure of PrP between PrPC and PrPSc, to be formed from PrPC in response to cellular redox changes thus designated as PrPRDX [36]. An in vitro study demonstrated that PrPRDX of recombinant hamster PrP is catalytically active and capable of further conversion of PrPC to PrPRDX. At the end, PrPRDX accumulates and disulfide bonds are formed among each PrPRDX molecule, and lastly formation of PrPSc is completed. This attractive model successfully explained how pathogenic form of PrP could be propagated by the presence of an intermediate PrP.
Note that the PrPRDX originally proposed by Lee and Eisenberg [36] was described to be in the oligomeric form distinct from the inert SH swapped dimeric form. Due to the nature of experimental design aimed to produce the redox-activated PrP oligomers at once from the SH-opened PrP monomers produced by exposing the bulk of PrP to the stepwise reduction and oxidation, their model (domain-swapping model) lacks the idea on the very initial event possibly occurring on a single molecule. Here the author wish to mention that PrP-catalyzed generation of O2•- (that may attack the SH residues on PrP) as a possible redox step(s) that converts the monomeric native PrPC to the catalytically active monomeric PrPRDX intermediate which in turn acts as the seed for further production of PrPRDX from PrPC, and to elongate the oligomers. The latest report by Shiraishi et al. [37] supports the view that the Cu bound to PrP undergoes catalytic cycling in the presence of catecholamines and causes the oxidation of protein, possibly involving the ROS-generating reactions which is sensitive to copper chelators, catalase and also to SOD, implying the involvements of H2O2 and O2•-. Possible contribution of PrP-catalyzed redox changes such as oxidation of catecholamines accompanying productions of H2O2 and O2•-, in aid of PrPRDX generation is worth of further examination.
Abbreviations
CLA: Cypridina luciferin analog; HO·: hydroxyl radicals; MAO: monoamine oxidase; O2•-: superoxide anion; PEA: phenylethylamine; PrP: prion protein; PrPC: intrinsic cellular PrP; PrPSc: scrapie isoform of PrP; PrPRDX: PrP redox intermediate; rlu: relative luminescence units; ROS: reactive oxygen species; SOD: superoxide dismutase; TSE: transmissible spongiform encephalopathies.
Conflict of interest
Declared none.
References
1. Jeffray M, McGovern G, Goodsir CM. et al. Sites of prion protein accumulation in scrapie-infected mouse spleen revealed by immuno-electron microscopy. J Pathol. 2000 ;191:323-32
2. Tabner BJ, Turnbull S, El-Agnaf O. et al. Production of reactive oxygen species from aggregating proteins implicated in Alzheimer's disease, Parkinson's disease and other neurodegenerative diseases. Curr Top Med Chem. 2001 ;1:507-17
3. Tabner BJ, El-Agnaf OMA, Turnbull S. et al. Hydrogen peroxide is generated during the very early stages of aggregation of the amyloid peptides implicated in Alzheimer's disease and familial British dementia. J Biol Chem. 2005 ;280:35789-92
4. Opazo C, Barria MI, Ruiz FH. et al. Copper reduction by copper binding proteins and its relation to neurodegenerative diseases. Biometals. 2003 ;16:91-8
5. Shiraishi N, Ohta Y, Nishikimi M. The octapeptide repeat region of prion protein binds Cu(II) in the redox inactive states. Biochem Biophys Res Commun. 2000 ;267:398-402
6. Wong BS, Brown DR, Pan T. et al. Oxidative impairment in scrapie-infected mice is associated with brain metals perturbations and altered antioxidant activities. J Neurochem. 2001 ;79:689-98
7. Sauer H, Dagdanova A, Hescheler J. et al. Redox-regulation of intrinsic prion expression in multicellular prostate tumor spheroids. Free Radic Biol Med. 1999 ;27:1276-83
8. Jones S, Batchelor M, Bhelt D. et al. Recombinant prion protein does not possess SOD-1 activity. Biochem J. 2005 ;392:309-12
9. Agostinho P, Oliveira CR. Involvement of calcineurin in the neurotoxic effects induced by amyloid-β and prion peptides. Eur J Neurosci. 2003 ;17:1189-96
10. Belosi B, Gaggelli E, Guerrini R. et al. Copper binding to the neurotoxic peptide PrP106-126: thermodynamic and structural studies. Chembiochem. 2004 ;5:349-59
11. Tabner BJ, Turnbull S, Fullwood NJ. et al. The production of hydrogen peroxide during early-stage protein aggregation: a common pathological mechanism in different neurodegenerative diseases? Biochem Soc Trans. 2005 ;33:548-50
12. Turnbull S, Tabner BJ, Brown DR. et al. Copper-dependent generation of hydrogen peroxide from the toxic prion protein fragment PrP106-126. Neurosci Lett. 2003 ;336:159-62
13. Turnbull S, Tabner BJ, Brown DR. et al. Generation of hydrogen peroxide from mutant forms of the prion protein fragment PrP 121-231. Biochemistry. 2003 ;42:7675-81
14. Furuichi T, Kawano T. Possible application of electron spin resonance to monitoring of prion diseases and hypotheses on oxidative action and propagation of copper-bound infectious protein. Bull Nippon Sport Sci Univ. 2005 ;35:71-80
15. Shiraishi N, Nishikimi M. Carbonyl formation on a copper-bound prion protein fragment, PrP23-98, associated with its dopamine oxidase activity. FEBS Lett. 2002 ;511:118-22
16. Kawano T, Kawano N, Hosoya H. et al. Fungal auxin antagonist hypaphorine competitively inhibits indole-3-acetic acid-dependent superoxide generation by horseradish peroxidase. Biochem Biophys Res Commun. 2001 ;288:546-51
17. Nakano M, Sugioka K, Ushijima Y. et al. Chemiluminescence probe with Cypridina luciferin analog, 2-methyl-6-phenyl-3,7-dihydroimidazo[1,2-a]pyrazin-3-one, for estimating the ability of human granulocytes to generate O2-. Anal Biochem. 1986 ;159:363-9
18. Yokawa K, Suzuki N, Kawano T. Ethanol-enhanced singlet oxygen-dependent chemiluminescence interferes with the monitoring of biochemical superoxide generation with a chemiluminescence probe, Cypridina luciferin analog. ITE Lett Batter New Technol Medic. 2004 ;5:49-52
19. Kawano T, Pinontoan R, Hosoya H. et al. Monoamine-dependent production of reactive oxygen species catalyzed by pseudoperoxidase activity of human hemoglobin. Biosci Biotechnol Biochem. 2002 ;66:1224-32
20. Kawano T. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep. 2003 ;2:829-37
21. Kawano T, Pinontoan R, Uozumi N. et al. Aromatic monoamine-induced immediate oxidative burst leading to an increase in cytosolic Ca2+ concentration in tobacco suspension culture. Plant Cell Physiol. 2000 ;41:1251-8
22. Kawano T, Pinontoan R, Uozumi N. et al. Phenylethylamine-induced generation of reactive oxygen species and ascorbate free radicals in tobacco suspension culture: mechanism for oxidative burst mediating Ca2+ influx. Plant Cell Physiol. 2000 ;41:1259-66
23. Rossi L, Lombardo MF, Ciriolo MR. et al. Mitochondrial dysfunction in neurodegenerative diseases associated with copper imbalance. Neurochem Res. 2004 ;29:493-504
24. Vassallo N, Herms J. Cellular prion protein function in copper homeostasis and redox signaling at the synapse. J Neurochem. 2003 ;86:538-44
25. Rotilio G, Carri MT, Rossi L. et al. Copper-dependent oxidative stress and neurodegeneration. IUBMB Life. 2000 ;50:309-14
26. Aronoff-Spencer E, Burns CS, Avdievich NI. et al. Identification of the Cu2+ binding sites in the N-terminal domain of the prion protein by EPR and CD spectroscopy. Biochemistry. 2000 ;39:13760-71
27. Burns CS, Aronoff-Spencer E, Legname G. et al. Copper coordination in the full-length, recombinant prion protein. Biochemistry. 2003 ;42:6794-803
28. Bonomo RP, Imperllizzeri G, Pappalardo G. et al. Copper II binding modes in the prion octapeptide PHGGWGGQ: a spectroscopic and voltammetric study. Chemistry. 2000 ;6:4195-202
29. Wells MA, Jelinska C, Hosszu LL. et al. Multiple forms of copper (II) coordination occur throughout the disordered N-terminal region of the prion protein at pH 7.4. Biochem J 2006. [Epub ahead of print]
30. Stanczak P, Luczkowski M, Juszczyk P. et al. Interactions of Cu2+ ions with chicken prion tandem repeats. Dalton Trans. 2004 ;14:2102-7
31. Morante S, Gonzalez-Iglesias R, Potrich C. et al. Inter- and intra-octarepeat Cu(II) sites geometries in the prion protein. J Biol Chem. 2004 ;279:11753-9
32. Burns CS, Aronoff-Spencer E, Dunham CM. et al. Molecular features of the copper binding sites in the octarepeat domain of the prion protein. Biochemistry. 2002 ;41:3991-4001
33. Brown DR, Guantieri V, Grasso G. et al. Copper(II) complexes of peptide fragments of the prion protein. Conformation changes induced by copper(II) and the binding motif in C-terminal protein region. J Inorg Biochem. 2004 ;98:133-43
34. Requena JR, Groth D, Legname G. et al. Copper-catalyzed oxidation of the recombinant SHa(29-231) prion protein. Proc Natl Acad Sci USA. 2001 ;98:7170-5
35. Requena JR, Dimitrova MN, Legname D. et al. Oxidation of methionine residues in the prion protein by hydrogen peroxide. Arch Biochem Biophys. 2004 ;432:188-95
36. Lee S, Eisenberg D. Seeded conversion of recombinant prion protein to a disulfide-bonded oligomer by a reduction-oxidation process. Nat Struct Biol. 2003 ;10:725-30
37. Shiraishi N, Inai Y, Bi W. et al. Fragmentation and dimerization of copper-loaded prion protein by copper-catalysed oxidation. Biochem J. 2005 ;387:247-55
Author contact
![]() Correspondence to: Prof. Tomonori Kawano Graduate School of Environmental Engineering, The University of Kitakyushu, Kitakyushu 808-0135, Japan. Tel: +81-93-695-3207, Fax: +81-93-695-3304. E-mail: kawanotomkitakyu-u.ac.jp
Correspondence to: Prof. Tomonori Kawano Graduate School of Environmental Engineering, The University of Kitakyushu, Kitakyushu 808-0135, Japan. Tel: +81-93-695-3207, Fax: +81-93-695-3304. E-mail: kawanotomkitakyu-u.ac.jp

 Global reach, higher impact
Global reach, higher impact