10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2009; 5(3):256-264. doi:10.7150/ijbs.5.256 This issue Cite
Research Paper
The Role of SRC-1 in Murine Prostate Carcinogenesis Is Nonessential due to a Possible Compensation of SRC-3/AIB1 Overexpression
1. Department of Molecular and Cellular Biology, Baylor College of Medicine, One Baylor Plaza, Houston, TX, USA
2. Institute of Biosciences and Technology, Texas A&M Health Science Center, 2121 W. Holcombe Boulevard, Houston, TX, USA
Received 2009-2-4; Accepted 2009-3-11; Published 2009-3-14
Abstract
The androgen and androgen receptor (AR)-regulated gene expression plays important roles in normal prostate and prostate cancer development, and AR transcriptional control of genes is mediated by transcriptional coactivators, including the three members of the steroid receptor coactivator (SRC) family, SRC-1 (NCOA1), SRC-2 (TIF2/GRIP1/NCOA2) and SRC-3 (AIB1, ACTR/RAC3/NCOA3). SRC-1 and SRC-3 are overexpressed in multiple human endocrine cancers and knockdown of either one of them in prostate cancer cell lines impedes cellular proliferation. Knockout of SRC-3 in mice suppresses the progression of spontaneous prostate carcinogenesis. In this study, we investigated SRC-1 contribution to prostate cancer in vivo by deleting the SRC-1 gene in TRAMP mice, which contain the probasin promoter-driven SV40 T/t antigen transgene. In assessing tumor mass of mice at various ages, we found that initiation and progression of prostate cancer induced by SV40 T/t antigens were unaltered in SRC-1-/- mice versus WT mice. Primary tumor histology and metastasis to distant lymph nodes were also similar in these mice at all time points assessed. These results demonstrate that the role of SRC-1 in mouse prostate carcinogenesis is nonessential and different from the essential contribution of SRC-3 that is required for prostate cancer progression and metastasis in mice. Interestingly, we observed that during prostate tumorigenesis SRC-1 expression was relatively constant, while SRC-3 expression was significantly elevated. Therefore, the loss of SRC-1 function may be compensated by SRC-3 overexpression during prostate tumorigenesis in SRC-1-/- mice.
Keywords: SRC-1, SRC-3, AIB1, prostate cancer
INTRODUCTION
Prostate cancer is the most common malignancy among American men and second leading cause of cancer death (1). Early in their development, prostate tumors require androgen stimulation for growth and survival. Therein they respond to androgen deprivation therapy. Following remission, however, tumors frequently recur in an androgen-independent form refractory to current treatment modalities (2). Understanding the development and progression of prostate cancer through androgen-dependent and independent stages is essential for devising novel targeted therapeutic strategies.
The androgen receptor (AR) signaling pathway is involved in development and progression of prostate cancer (3). Androgen receptor is a member of the nuclear receptor superfamily, a collection of proteins with hormone-activated transcriptional activities. Transcriptional control by nuclear receptors depends not only on the presence and concentration of appropriate hormone but also on regulation by coactivator molecules. Coactivators associate with hormone-bound receptors at the gene site and recruit general transcription machinery. Therefore, coactivator expression level and activity may profoundly alter the transcriptional activities of nuclear receptors (4, 5). The best-characterized coactivators comprise the steroid receptor coactivator (SRC) family. Its three members are 160-kDa proteins termed SRC-1 (NCOA1), SRC-2 (TIF2/GRIP1/NCOA2) and SRC-3 (AIB1/p/CIP/RAC3/ACTR/TRAM-1/NCOA3). Extensive studies in knockout mice demonstrate involvement of the SRC family in regulating normal development and physiology. Genetic disruption of SRC-1 in mice results in partial steroid hormone resistance in reproductive organs such as uterus, prostate, testis, and mammary gland (5). Deletion of SRC-2 causes reproductive impairment and hypofertility in both male and female mice (6, 7). SRC-3 null (SRC-3-/-) mice exhibit somatic growth retardation, female reproductive dysfunction and mammary gland growth reduction (8, 9). Therein, SRC family members have critical roles in development and maintenance of normal tissues.
Several groups, including our own, have demonstrated important roles for SRC proteins in multiple cancers (10-15). We exploited SRC-3 -/- mice to determine that SRC-3 deficiency suppresses mammary gland tumor development induced either by oncogenes or chemical carcinogens (11, 12, 16). We identified an association between SRC-3 and prostate cancer in the SV40-induced transgenic adenocarcinoma of the mouse prostate (TRAMP) model, noting increase in SRC-3 expression in prostatic luminal epithelial cells during tumorigenesis. To further investigate the relationship, we crossed TRAMP and SRC-3-/- mice. Remarkably, prostate cancer progression observed in these bigenic animals was markedly delayed and much more differentiated than those observed in WT/TRAMP mice. In vitro studies demonstrating the requirement of SRC-3 for prostate cancer cell proliferation and survival confers our findings (10, 14). In addition, SRC-3 directly regulates transcription of matrix metalloproteinases, MMP-2, MMP-9 and MMP-13, to potentiate cancer cell invasion and metastasis (15, 16).
SRC-1 has also been linked to prostate cancer. A clinical study showed increased SRC-1 protein in hormone-refractory prostate tumors compared with benign prostatic hyperplasia or androgen-dependent tumors (17). A second study showed increased SRC-1 expression in localized androgen dependent prostate tumors correlates with increased metastases to distant lymph nodes (18). Moreover, in vitro analyses demonstrate that SRC-1 knockdown represses the activation of AR target genes and reduces AR-dependent cellular proliferation (18).
In the current study, we investigate the role of SRC-1 in an in vivo model of prostate cancer. We created a bigenic mouse model in which SRC-1-/- mice were crossed with TRAMP mice. Surprisingly, inactivation of SRC-1 did not inhibit prostate cancer initiation and progression, as tumors in bigenic mice were morphologically similar to those of WT/TRAMP mice. These results are at odds with our findings in the SRC-3-/- TRAMP bigenic and also contradict published in vitro and clinical findings. Interestingly, we observed an increase in SRC-3 expression in the prostate tumors of both WT/TRAMP and SRC-1-/-/TRAMP mice. Therefore, SRC-3 may compensate for the absent SRC-1 in promoting prostate tumorigenesis in this model. In conclusion, our analysis of prostate carcinogenesis in TRAMP model with a SRC-1 null background demonstrates that SRC-1 is not an essential coactivator to drive prostate cancer initiation and progression.
MATERIALS AND METHODS
Mice. SRC-1-/- mice were initially generated as described (5), and subsequently backcrossed into a C57BL/6J strain background. TRAMP mice with 50% C57BL/6J and 50% 129SvEV genetic contribution and harboring the Probasin-SV40 T/t transgene were produced as previously reported (19). To generate SRC-1-/-/TRAMP mice, SRC-1-/- mice in C57BL/6J genetic background were crossed with 50% C57BL/6J-50% 129SvEV TRAMP mice. The offspring were backcrossed with C57BL/6J SRC-1-/- mice three times to generate 93.75% C57BL/6J-6.25% 129SvEV (experimental) SRC-1-/-/TRAMP and (control) SRC-1+/+ (WT)/TRAMP mice. This breeding strategy ensured all experimental mice were hemizygous for the TRAMP transgene. Genotyping was performed via PCR-based screening assay on DNA extracted from ear tip biopsy by proteinase K digestion. For TRAMP mice, primer sequences were 5'-CCGGTCGACCGGAAGCTTCCACAAGTGCATTTA (forward) and 5'-CTCCTTTCAAGACCTAGAAGGTCCA (reverse). SRC-1 knockout mice were genotyped as described previously (5).
Tissue examination and Histology. TRAMP and bigenic mice were weighed, anesthetized and sacrificed at 8, 12, 18, 24 and 30 weeks of age. All major organs were inspected for evidence of tumors, while lymph nodes were assessed for metastases. The entire genitourinary (GU) tract, consisting of the bladder, urethra, seminal vesicles, ampullary gland, and prostate, was excised and dissected under low power microscope. The wet weights of the GU tract, seminal vesicles, and prostate were recorded. Tissues were fixed in 10% buffered formalin for 12 hr at 4oC, dehydrated in sequentially increasing ethanol concentrations, processed and embedded in paraffin blocks. Sections of 5 μm thickness were stained with hematoxylin and eosin (H&E), and examined under a light microscope. Histopathology was determined according to the GEM grading classification scheme in which prostatic intraepithelial neoplasia (PIN), well-differentiated adenocarcinoma (WDA), moderately-differentiated adenocarcinoma (MDA) and poorly-differentiated adenoarcinoma (PDA) are qualitatively and quantitatively scored (20).
Immnuohistochemistry. All immunohistochemical staining was performed on 5 μm de-parafinized sections. Antigen retrieval was carried out by incubating the slides in 0.01 M citric acid buffer (pH 6.0) using microwave method. Slides were then cooled and washed successively with PBS and deionized water. Next, endogenous peroxidase activity was inactivated by incubation in methanol containing 3% hydrogen peroxide. Sections were subsequently incubated overnight at 4oC with following primary antibodies: Mouse monoclonal anti-T antigen (BD Transduction Laboratory) (for detection of SV40 large T-antigen), goat polyclonal anti-SRC-1 (Santa Cruz) and rabbit polyclonal anti-SRC-3 (Cell Signaling) antibodies. Biotinylated secondary antibodies, rabbit anti-mouse for TRAMP, horse anti-goat for SRC-1 and goat anti-rabbit for SRC-3 were used, and were each diluted 1:600. The Avidin Biotin Complex kit (Vector Laboratories) was used for chromophore-mediated detection.
Immunoblotting analysis. Prostate samples designated for Western blot analysis were lysed in RIPA buffer and prepared as described (13). The following primary antibodies were used for detection: rabbit polyclonal anti-SRC-1 (Santa Cruz) and rabbit polyclonal anti-AIB1 (SRC-3) (gift from Dr. R. Wu, Baylor College of Medicine, Houston, TX). Horse radish peroxydase (HRP)-conjugated (goat) anti-rabbit secondary antibodies were used.
RESULTS
Loss of SRC-1 does not suppress prostate cancer tumorigenesis
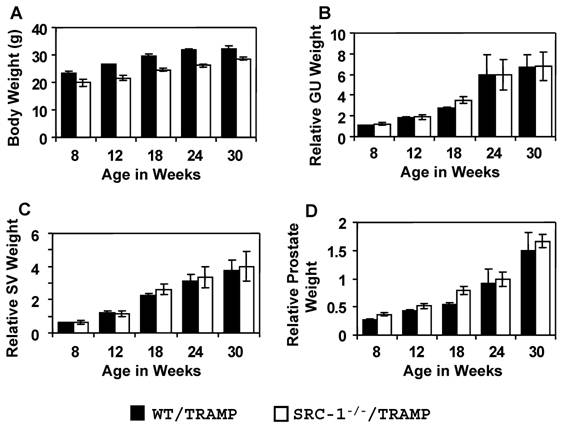
To study the contribution of SRC-1 to prostate cancer, we generated SRC-1-/-/TRAMP mice and compared formation and progression of genitourinary tumors versus WT/TRAMP mice. We monitored tumor growth in each genotype, collecting tissue samples at 8, 12, 18, 24, and 30 weeks of age. At each time point, we assessed total body weight and individual weights of GU tract, prostate and seminal vesicles. At all time points, SRC-1-/-/TRAMP mice had slightly lower body weight than WT/TRAMP mice (Fig. 1A). Relative GU tract weight (normalized to body weight) increased progressively with age in both WT/TRAMP and SRC-1-/-/TRAMP mice. No statistically significant difference was observed between the two genotypes (Fig. 1B). Similarly, relative weights of prostate and seminal vesicle (each normalized to body weight) were also unchanged between WT/TRAMP and SRC-1-/-/TRAMP mice at each time point assessed (Fig. 1, C and D). Upon sacrifice, we inspected the GU tract under low power dissecting microscope and collected all identifiable tumors. No tumors were grossly visible in either genotype at the 8, 12 and 18-week time points. At the 24-week time point, 6/8 SRC-1-/-/TRAMP and 7/9 WT/TRAMP mice developed tumors. At 30 weeks, 9/9 SRC-1-/-/TRAMP and 7/7 WT/TRAMP mice had large GU tract tumors. Tumors collected at both the 24 and 30-week time points had similar gross morphology between the WT/TRAMP and SRC-1-/-/TRAMP mice. Therefore, gross anatomical assessment suggests prostate tumorigenesis and local cancer progression are similar in SRC-1-/-/TRAMP and WT/TRAMP mice.
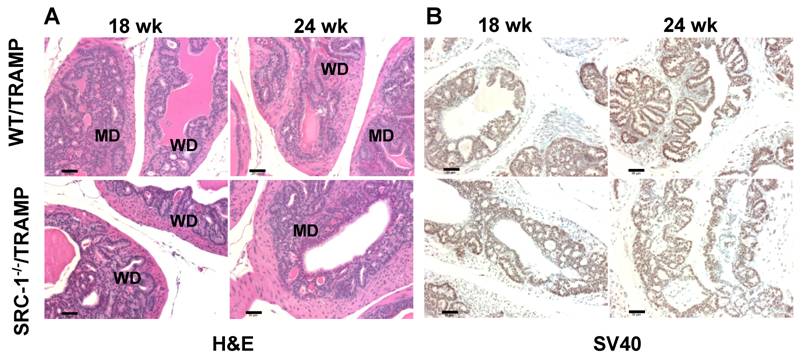
In order to ascertain the impact of SRC-1 deletion on tumor morphology, we performed histopathological assessment of prostates from SRC-1-/-/TRAMP and WT/TRAMP mice at 8, 12, 18, 24, and 30-week time points. 5 μm H&E-stained sections from mice of each genotype were subjected to morphological (GEM) analysis for cancer progression, taking into account relative abundance of PIN, WDA, MDA, and PDA tissues. While WT/TRAMP appeared at 12 weeks to show a subtly advanced cancer versus SRC-1-/-/TRAMP, both genotypes had similar morphology at all subsequent time points (Fig. 2). Therefore, the histological analysis further indicates similar prostate tumor progression between SRC-1-/-/TRAMP and WT/TRAMP mice.
Prostate tumor growth in SRC-1-/-/TRAMP and WT/TRAMP mice. Body weight (panel A), genitourinary weight (panel B), seminal vesicle weight (panel C) and prostate weight (panel D) were recorded at sacrifice and the mean (± s.e.m.) relative organ weights are represented as a function of age and genotype. Relative weights are calculated by normalizing the organ weight to body weight. WT/TRAMP mice: 8 weeks, n=6; 12 weeks, n=10; 18 weeks, n=10; 24 weeks, n=9; 30weeks, n=7; SRC-1-/-/TRAMP mice: 8 weeks, n=3; 12 weeks, n=8; 18 weeks, n=10; 24 weeks, n=8; 30weeks, n=11.
A) H&E stained histologic sections of the WT/TRAMP and SRC-1-/-/TRAMP prostate tissues at different tumorigenic stages (Pathologic grades: PIN, prostatic intraepithelial neoplasia; WD, well-differentiated adenocarcinoma; MD, moderately differentiated adenocarcinoma; PD, poorly differentiated adenocarcinoma; PHY, phylloides-like cancer). Images were taken at x200 magnification and the scale bars represent 50 μm in length. B) Immunohistochemical analysis of SV40 T antigen expression in WT/TRAMP and SRC-1-/-/TRAMP prostate tissues. Only dorsal lobes are shown here. The slides were counterstained with hematoxylin. Images were taken at x200 magnification and the scale bars represent 50 μm in length. Brown color, T antigen immunoreactivity. Note that T antigen expression levels are similar between SRC-1-/-/TRAMP and WT/TRAMP prostates.
Ablation of SRC-1 does not inhibit prostate cancer metastasis
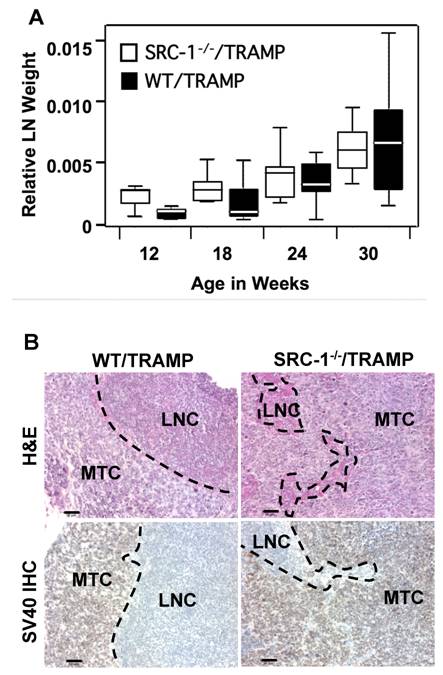
Clinical studies demonstrated that enhanced SRC-1 expression is associated with increased local invasiveness, metastatsis, and fatal disease progression (18). Therefore, we evaluated metastatic capacity of prostate tumors in SRC-1-/-/TRAMP versus WT/TRAMP mice. Specifically, we assessed periaortic lymph nodes, lungs, and liver since these organs are common sites of metastasis in the TRAMP model (19). Following sacrifice, periaortic lymph nodes were weighed and observed under the dissecting microscope. The relative (normalized to body weight) weight of the periaortic lymph nodes increased as a function of age in both SRC-1-/-/TRAMP and WT/TRAMP mice. Neither the overall rate of weight increase nor weights at individual time points were significantly different between the two genotypes (Fig. 3A). Moreover, we detected metastatic lesions in periaortic lymph nodes of both SRC-1-/-/TRAMP and WT/TRAMP mice. H&E staining of lymph node tissue confirmed presence of tumors while SV40 immunohistochmistry demonstrated tumors were prostate-originated metastases (Fig. 3B). Metastases were evident at weeks of age in both genotypes and we observed no significant difference in the number or morphology of these lesions at any subsequent time point. In sum, no significant difference was observed in lymph node size or metastatic tumor morphology between the two genotypes at any time between 8 and 36 weeks of age.
SRC-1 expression is relatively consistent throughout the stages of prostate cancer progression
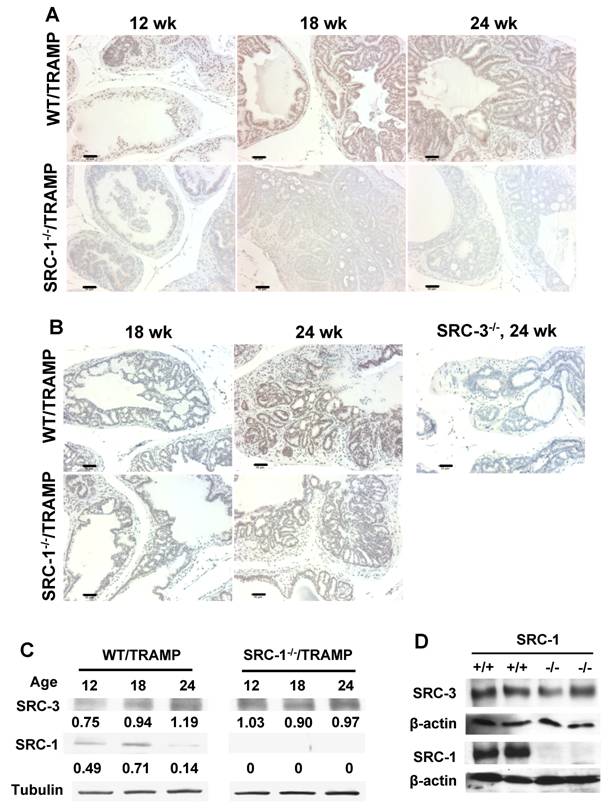
To check the SRC-1 expression patterns in TRAMP mouse prostate tissues, SRC-1 immunohistochemistry was performed. SRC-1 was found to be expressed in luminal epithelial cells and tumor cells of the prostate tissue and the expression was consistent with moderate level throughout the different stages of tumor progression (Fig. 4A). SRC-1 null prostate tissues were used as negative control (Fig. 4A). We also carried out immunoblotting analysis to obtain semi-quantitative SRC-1 level in TRAMP mice. SRC-1 level did not increase progressively as a function of age and prostate cancer progression. In fact, SRC-1 protein level decreased between 18 and 24 weeks of age (Fig. 4C). Protein lysates from SRC-1-null TRAMP prostates were used as negative controls (Fig. 4C). These results suggest that SRC-1 is not overexpressed during prostate carcinogenesis in mice.
A) The comparable weights of periaortic lymph nodes in WT/TRAMP and SRC-1-/-/TRAMP mice. The weights of periaortic lymph nodes were recorded and the relative periaortic lymph node weight was calculated. The box-and-whisker plot presents the distribution of relative lymph node weights. Whiskers represent all data analyzed, excluding outliers (represented by black dots), black line indicates median, and box represents 25% of data greater than and less than median. WT/TRAMP mice: 8 weeks, n=6; 12 weeks, n=10; 18 weeks, n=10; 24 weeks, n=9; 30 weeks, n=7; SRC-1-/-/TRAMP mice: 8 weeks, n=3; 12 weeks, n=8; 18 weeks, n=10; 24 weeks, n=8; 30 weeks, n=11. B) Histological analysis of the periaortic lymph nodes from 24-week-old mice. Upper panel was H&E stained sections while the lower panel was immunostained with SV40 T antigen antibody to identify the cancer cells with a prostatic origin (brown color). LNC, lymph node cells; MTC, metastatic tumor cells. Images were taken at x200 magnification and the scale bars represent 50 μm in length.
Immunochemical analyses of SRC-1 and SRC-3 expression during prostate tumor initiation and progression. A) Immunohistochemistry for SRC-1 ,brown color, in the prostates of WT/TRAMP and SRC-1-/-/TRAMP (negative control) mice with indicated ages. B) Immunohistochemistry for SRC-3, brown color, in the prostates of WT/TRAMP and SRC-1-/-/TRAMP mice with indicated ages. A prostate sample of 24-week-old SRC-3-/-/TRAMP mouse was used as negative control. Images were taken at x200 magnification and the scale bars represent 50 μm in length. C) Western blot analyses of SRC-1 and SRC-3 in the prostates and tumors of WT/TRAMP and SRC-1-/-/TRAMP mice ages as indicated. Tubulin was used as a loading control. D) Western blot analyses of SRC-1 and SRC-3 in the prostates of WT (SRC-1+/+, non-TRAMP) and SRC-1-/- mice. β-actin was used as a loading control. The stronger bands in panel D compared with those in panel C were because of a longer exposure time to the X-ray films in an independent experiment.
Evaluation of SV40 T antigen and SRC-3 expression in the prostate tissue of SRC-1-/-/TRAMP and WT/TRAMP mice
Immunohistochemistry demonstrates the presence of SV40 T antigen in SRC-1-/-/TRAMP and WT/TRAMP mouse prostate. SV40 T antigen immunostaining was uniform throughout the luminal epithelium of the prostate tissue. Prostate stromal cells, on the contrary, exhibited only scattered SV40 T antigen expression (Fig. 2B). Therefore, SRC-1 deficiency did not alter SV40 transgene expression.
Protein levels of SRC-3 in SRC-1-/-/TRAMP and WT/TRAMP mouse prostates were examined by both immunohistochemistry and immunoblotting. In both WT/TRAMP and SRC-1-/-/TRAMP mice, SRC-3 immunoactivity was relatively low at 18 weeks of age in both normal and hyperplastic luminal epithelial cells, while at 24 weeks, SRC-3 immunoactivity was significantly increased in the prostate tumor cells (Fig. 4B). SRC-3 immunostaining specificity was validated by using SRC-3-null prostate as a negative control (Fig. 4B).
Unlike SRC-1, SRC-3 protein level increased progressively during prostate carcinogenesis in WT/TRAMP mice as assayed by immunoblotting (Fig. 4C). Interestingly, at 12 weeks of age SRC-3 in the prostates of SRC-1-/-/TRAMP mice was significantly higher than that in the prostates of WT/TRAMP mice, and this high level was maintained throughout the older age time points (Fig. 4C). These results demonstrate that SRC-3 is overexpressed during prostate carcinogenesis in the prostates of both WT/TRAMP and SRC-1-/-/TRAMP mice and SRC-3 overexpression in SRC-1-/-/TRAMP mice comes earlier compared with WT/TRAMP mice.
We also performed immunoblotting analysis to examine SRC-3 protein levels in the prostates of WT and SRC-1-/- mice. We found no difference in SRC-3 levels between WT and SRC-1-/- prostates (Fig. 4D). These results suggest that SRC-1 ablation does not alter SRC-3 protein level in normal prostate and thus, SRC-3 overexpression is an event linked with prostate tumorigenesis.
DISCUSSION
Certain steroid receptor coactivators such as SRC-3 are putative oncogenes in multiple cancers. Our laboratory demonstrated inactivation of SRC-3 could arrest prostate cancer at well-differentiated stages in TRAMP mice. Tumors in these mice neither progressed to poorly-differentiated stages nor metastasized. In the current study, we show that SRC-1 knockout does not arrest prostate cancer progression in TRAMP mice. Contrary to SRC-3-/-/TRAMP mice, SRC-1-/-/TRAMP mice develop prostate cancer at a similar rate and extent to WT/TRAMP mice. Both groups have similar tumor morphology and pattern of metastases.
The failure of SRC-1 deletion to alter prostate cancer progression in the TRAMP model is at odds with clinical data correlating SRC-1 expression level and increased metastatic potential (18). One potential explanation for the contradiction is a requirement of SRC-1 overexpression for induction of more severe tumor phenotypes. Recently, we demonstrated that SRC-1 was upregulated in mammary tumors in MMTV-polyoma middle T transgenic mice, and that the overexpressed SRC-1 promoted tumor cell metastasis through increasing HER2 and colony-stimulating factor 1 (CSF-1) protein levels (21). In the present study, SRC-1 was not overexpressed and accordingly, its loss-of-function did not affect prostate tumor cell metastasis. These results support the notion that overexpressed SRC-1 may be still a dangerous factor in hormonally promoted cancers such as breast and prostate cancers.
Further aiming to determine why our in vivo data in TRAMP mice did not correspond with cell line findings, we measured SRC-3 protein levels in advanced prostate tumors in SRC-1-/-/TRAMP mice. Interestingly, SRC-3 levels were significantly elevated in both SRC-1-/-/TRAMP and WT/TRAMP mice. In contrast, SRC-1 was expressed at relatively constant level in tumor cells throughout progression. These results suggest either a compensatory role for SRC-3 in SRC-1 mediated functions or a primacy of SRC-3 in mediating prostate tumorigenesis in the TRAMP model. The notion of partial redundancy between SRC-1 and SRC-3 in prostate carcinogenesis is consistent with multiple lines of evidence demonstrated in previous studies. First, both SRC-1 and SRC-3 interacted with multiple nuclear receptors and expression of either SRC-1 or SRC-3 could promote the receptor-mediated gene transcription. Second, although SRC-1 null mice had nearly normal development and growth and most SRC-3 null mice were viable and had normal life span, most of SRC-1 and SRC-3 double knockout mice were lethal before birth (reviewed in ref. 23).
SRC-3 may act as a prostate cancer oncogene via several mechanisms. In the simplest case, it may directly coactivate AR-responsive transcriptional profiles in luminal epithelial cells. Alternatively, it may act outside of the luminal epithelial cell to stimulate the secretion of a diffusible growth factor. Such a mediator may be either a paracrine signal or an endocrine hormone, such as IGF-1, a molecule associated with prostate cancer progression. SRC-3 has been shown to maintain IGF-1 in circulation by regulating IGFBP-3 mRNA levels in multiple tissues. Global SRC-3 deletion decreases systemic IGF-1 levels and retards somatic growth (22). SRC-3-deficient mammary tumors also expressed lower IGF-1 mRNA (12). Finally, SRC-3 may be relevant in tumor cells not derived form the luminal epithelium. Precursor cell populations, capable of differentiation to multiple lineages within a given tumor have been identified in multiple cancers. SRC-3 may be relevant for the expansion or progression of a precursor population in the prostate that gives rise to tumor cells histologically resembling luminal epithelial cells. Identifying the role of SRC-3 in prostate cancer will require gene deletion targeted to luminal epithelial cells as well as other prostatic cell types.
In conclusion, we demonstrate here that SRC-1 expression during mouse prostate carcinogenesis is relatively consistent and that SRC-1 deletion does not alter prostate tumor initiation, growth or progression in TRAMP mice. In contrast, SRC-3 is overexpressed during prostate carcinogenesis, indicating differential regulatory mechanisms for SRC-1 and SRC-3 promoters in the prostate tumor cells. The SRC-3 may play a dominant role versus SRC-1 in promoting prostate cancer progression in TRAMP mice. However, questions as to a role for increased SRC-1 in aggressive patient tumors indicate this coactivator warrant future assessment.
Acknowledgements
We thank Dr. Norman Greenberg for providing TRAMP mice and Arthur Chung for initiating this project. This work is partially supported by National Health Institutes grants DK058242, CA112403 and CA119689.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Cooperberg MR, Moul JW, Carroll PR. The changing face of prostate cancer. J Clin Oncol. 2005;23:8146-51
2. Damber JE, Aus G. Prostate cancer. Lancet. 2008;371:1710-21
3. Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550-5
4. McKenna NJ, Xu J, Nawaz Z, Tsai SY, Tsai MJ, O'Malley BW. Nuclear receptor coactivators: multiple enzymes, multiple complexes, multiple functions. J Steroid Biochem Mol Biol. 1999;69:3-12
5. Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O'Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922-5
6. Gehin M, Mark M, Dennefeld C, Dierich A, Gronemeyer H, Chambon P. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol Cell Biol. 2002;22:5923-37
7. Mark M, Yoshida-Komiya H, Gehin M. et al. Partially redundant functions of SRC-1 and TIF2 in postnatal survival and male reproduction. Proc Natl Acad Sci U S A. 2004;101:4453-8
8. Wang Z, Rose DW, Hermanson O. et al. Regulation of somatic growth by the p160 coactivator p/CIP. Proc Natl Acad Sci U S A. 2000;97:13549-54
9. Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O'Malley BW. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci U S A. 2000;97:6379-84
10. Zhou HJ, Yan J, Luo W. et al. SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res. 2005;65:7976-83
11. Kuang SQ, Liao L, Wang S, Medina D, O'Malley BW, Xu J. Mice lacking the amplified in breast cancer 1/steroid receptor coactivator-3 are resistant to chemical carcinogen-induced mammary tumorigenesis. Cancer Res. 2005;65:7993-8002
12. Kuang SQ, Liao L, Zhang H, Lee AV, O'Malley BW, Xu J. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res. 2004;64:1875-85
13. Chung AC, Zhou S, Liao L, Tien JC, Greenberg NM, Xu J. Genetic ablation of the amplified-in-breast cancer 1 inhibits spontaneous prostate cancer progression in mice. Cancer Res. 2007;67:5965-75
14. Yan J, Yu CT, Ozen M, Ittmann M, Tsai SY, Tsai MJ. Steroid receptor coactivator-3 and activator protein-1 coordinately regulate the transcription of components of the insulin-like growth factor/AKT signaling pathway. Cancer Res. 2006;66:11039-46
15. Yan J, Erdem H, Li R. et al. Steroid receptor coactivator-3/AIB1 promotes cell migration and invasiveness through focal adhesion turnover and matrix metalloproteinase expression. Cancer Res. 2008;68:5460-8
16. Qin L, Liao L, Redmond A. et al. The AIB1 oncogene promotes breast cancer metastasis by activation of PEA3-mediated matrix metalloproteinase 2 (MMP2) and MMP9 expression. Mol Cell Biol. 2008;28:5937-50
17. Gregory CW, He B, Johnson RT. et al. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61:4315-9
18. Agoulnik IU, Vaid A, Bingman WE3rd. et al. Role of SRC-1 in the promotion of prostate cancer cell growth and tumor progression. Cancer Res. 2005;65:7959-67
19. Gingrich JR, Barrios RJ, Morton RA. et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096-102
20. Shappell SB, Thomas GV, Roberts RL. et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270-305
21. Wang S, Yuan Y, Liao L. et al. Disruption of the SRC-1 gene in mice suppresses breast cancer metastasis without affecting primary tumor formation. Proc Natl Acad Sci U S A. 2009;106:151-6
22. Liao L, Chen X, Wang S, Parlow AF, Xu J. Steroid receptor coactivator 3 maintains circulating insulin-like growth factor I (IGF-I) by controlling IGF-binding protein 3 expression. Mol Cell Biol. 2008;28:2460-9
23. Xu J, Li Q. Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol. 2003;17:1681-92
Author contact
![]() Correspondence to: Jianming Xu, Department of Molecular and Cellular Biology, Baylor College of Medicine, One Baylor Plaza, Houston, TX 77030. E-mail: jxutmc.edu
Correspondence to: Jianming Xu, Department of Molecular and Cellular Biology, Baylor College of Medicine, One Baylor Plaza, Houston, TX 77030. E-mail: jxutmc.edu

 Global reach, higher impact
Global reach, higher impact