10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2009; 5(5):458-465. doi:10.7150/ijbs.5.458 This issue Cite
Research Paper
Site-Dependent Differences in Clinical, Pathohistological, and Molecular Parameters in Metastatic Colon Cancer
Dept. of General Surgery, University of Freiburg, 79106 Freiburg, FRG
Received 2009-4-23; Accepted 2009-6-24; Published 2009-6-26
Abstract
The purpose was to develop a metastatic score specific to the hepatic and peritoneal site in colorectal cancer patients from clinical, pathohistological and molecular markers potentially reflecting oncogenic activation (OA) or epithelial-mesenchymal transition (EMT), where OA may reflect an activation and EMT the functional loss of certain genes. The primary tumour stage (OA, EMT), lymphonodal stage (OA), the presence of a lymphangiosis carcinomatosa (OA), histological grade (OA, EMT), and immunoblot extraction of E-cadherin (OA, EMT) were differentially rated with zero to one or two points due to their potential contribution to each process and the resulting scores were validated in 27 colorectal cancer patients (three patients with pre-malignant adenomas, 16 with primaries and two with local recurrencies, three of which were metastatic to the peritoneum, six metastatic to the liver and two metastatic to both, the liver and the peritoneum, and five with hepatic secondaries, one of which at histology was metastatic to the peritoneum too). As a single parameter only the N-stage significantly contributed to OA (p<0.05). Median OA and EMT scores, however, were 3.5 and 2 in the case of primaries without further spread, 5 and 4 in those nodal positive, 5 and 4 in the case of peritoneal implants, 6 and 2 in the case of liver metastases, and 6.5 and 3 in the case of a simultaneous hepatic and peritoneal spread, respectively. These differences were significant when scores from patients with and without liver metastases (OA, p<0.002) or with peritoneal implants and isolated hepatic spread (EMT, p<0.01) were compared. The results suggest a site-specific contribution of OA and EMT to tumour progression in human colon cancer.
Keywords: oncogenic activation, epithelial-mesenchymal transition, metastatic score, colon cancer
Introduction
Genetic signatures have been employed to determine the site and nature of cancer metastasis.1,2 Candidate genes, however, appear to be numerous, and frequently do not provide a phenotypic clue. Also, oncogenic activation and epithelial-mesenchymal transition have been recognised as particular pathways.3,4 In human colon cancer, for example, both an activation of the Ki-ras oncogene and neoexpression of N-cadherin have been frequently detected,5,6 the first being associated with lymphonodal spread and distant metastasis,5 while the other has been shown to balance the expression of epithelial E-cadherin and to confer EMT and a predisposition to serosal spread.6,7
Activated Ki-ras has been able to increase the expression of E-cadherin.8 EMT, on the other hand, is frequently associated with a functional loss of E-cadherin but may take place even in the presence of normal amounts of E-cadherin protein.6,7,9 OA and EMT may therefore refer to dual functions of E-cadherin as an oncogenic effector and a tumour suppressor molecule. Furthermore, a reduced therapeutic effectiveness of anti-thyrosine kinase receptor monoclonal antibodies in Ki-ras positive colon cancer may originate rather up- than downstream from Ki-ras, where Ki-ras controls the distribution of small GTPase rho proteins and focal adhesion.10 However, single factors or genes are rarely attributable to the whole process of tumour progression or cancer metastasis.
Scoring systems have been used to estimate the survival benefit of hepatic resection for colon cancer liver metastases.11,12 Scoring systems, however, focussing on the primary risk of liver metastasis, do not yet exist. And although OA and EMT parameters have been correlated with metastatic potential, a perpetual lack exists with respect to specific organ sites. In Gastrointestinal Stromal Tumours (GIST) we have been able to correlate the metastatic site with indicators of oncogenic activation. The principle was to use a set of clinical and pathohistological parameters representing the clinical phenotype.13 Here, we propose a scoring system to predict the metastatic outcome in human colon cancer by focussing on the contributions of OA and EMT to hepatic spread or peritoneal dissemination.
Patients and Methods
Clinical Data, Histology
Clinical data with respect to tumour extension, stage of the primary, grading, and serum CEA were retrieved from patient charts and routine pathohistological reports in a series of 27 colorectal cancer patients of the Surgical Department, University Hospital of Freiburg, Germany. Only patients without previous radio- and/or chemotherapy were included for at least one year.
The UICC TNM classification14 was used to determine the stage of the disease, and staging or grading data were retrieved from routine pathology reports.
Patient follow-up was accomplished over a period of 50-56 months by house doctor's interview in the form of a phone call or a written request.
Specimens
The use of human materials was approved by our institutional review committee. Informed consent to participate in the study was obtained from all patients prior to sample collection.
Tissue samples (Table 1) were collected from surgical specimens (three pre-malignant adenomas, 17 primary carcinomas, two local recurrencies, and seven liver metastases) for immunoblot quantitation of cellular E-cadherin protein by dissecting a representative anecrotic area from the tumour and from adjacent mucosa or liver parenchyma at a distance of at least 2 cm from the tumour. Samples were cleaned in phosphate-buffered saline (PBS), dissected into pieces of 0.1 to 0.2 g, and frozen in liquid nitrogen. Fifteen pathology specimens, two from an adenoma, 11 from primaries (two of which were metastatic to the liver and two to the peritoneal site), one from a local recurrence attributable to the peritoneal site, and one from a liver metastasis, were further processed for immunohistochemistry.
Sample origin for grading and immunoblot extraction of E-cadherin protein
| n | ||
|---|---|---|
| primaries | 19 | |
| adenomas | 3 | |
| carcinomas | 16 | |
| local recurrences | 2 | |
| liver metastases | 6 | |
n, number of patients
Immunoblot Analysis, Immunohistochemistry
For immunoblot analysis membrane-bound protein was extracted in the presence of 1% Triton X-100. An equivalent amount of protein was separated by 7.5% SDS PAGE electrophoresis and electrotransferred to 45 μm nitrocellulose membranes, incubated with the primary antibody at 1:1000 for 1.5 hours and developed using the Western Light Chemiluminescent Staining System (Tropix, Bredford, Ma., USA). A housekeeping positive control was run with every gel. The staining intensity of specific bands was quantified using an Imaging Densitometer GS 710 (BioRad, Göttingen, Germany) and the relative staining intensity was calculated relative to adjacent normal tissue.
For immunohistochemistry, formalin-fixed and paraffin-embedded tumour samples were cut at 4 μm and stained using the multilayer alkaline-phosphatase-antialkaline phosphatase (APAAP) method.15
In both procedures, the monoclonal antibody HECD-1 (Takara Biochemicals, Tokyo, Japan) was used for the detection of E-cadherin.
Development of a Prognostic Score from Clinical, Pathohistological and Molecular Data
A score for the estimation of oncogenic activation (OA) or epithelial-mesenchymal transition (EMT) was constructed by differential selection and weighing of clinical, pathohistological, and molecular parameters according to their potential contribution to the specific process. T-stage, N-stage, pathohistological grading, the presence of a lymphangiosis carcinomatosa, and immunoblot extraction of E-cadherin were assessed as parameters of OA, presumably reflecting growth stimulation, extravasation, lymphatic arrest, or an activation of differentiation pathways. For the examination of EMT, T-stage, pathohistological grading, and immunoblot expression of E-cadherin were assessed by different means, presumably reflecting loss of growth control or epithelial differentiation (Table 2). Data from immunohistochemistry were not included.
Suggested factors involved are of genetic, intracellular, extracellular, or intercellular nature, where mutated Ki-ras may represent a genetic predisposition,8,10 snail transcriptional activation controlling growth control and apoptotic arrest,9 intergrins and cadherin effector molecules mediating cell-matrix and intercellular communication,16,17 and rac/rho-GTPases intracellular communicators of up- and downstream signals,18-20 Ki-ras and E-cadherin being predominantly involved in OA, intergrins and N-cadherin in EMT, and rac/rho constituting a molecular switch of both (see below). Tumour stage and grading as potential targets of apoptotic arrest and growth control were rated higher in the case of EMT and the contribution of E-cadherin, although of advertent nature, in the case of OA. The N-stage and the presence of a lymphangiosis carcinomatosa were not considered to be relevant consequences of EMT (Table 2).
Zero or one point were assigned to the pathohistological grading and the absence or presence of a lymphangiosis carcinomatosa as parameters of OA, as well as to immunoblot extraction of E-cadherin as parameter of EMT, and zero to two points were assigned to the T-stage, N-stage, and immunoblot extraction of E-cadherin as parameters of OA, and the grading and T-stage as those of EMT (Table 2). However, relative immunoblot extraction of E-cadherin from a right-sided primary of signet cell type histology was rated, although being 0.92 (Table 2), with one point for an immunostaining only at the cytoplasmic site, and immunoblot extraction of E-cadherin from a local recurrence was rated with two points relative to other tumour samples on the same blot in place of a missing colonic control.
In the case of liver metastasis, results were compared with Fong`s score, which has been proposed to indicate the risk of recurrence subsequent to a potential liver resection and which attributes one point to each, the time distance between primary and hepatic disease <12 months, largest liver nodule >5 cm, >1 liver nodule, lymphatic spread of the primary, and CEA >200 ng/ml, giving rise to a maximum of 5 points.11
Point attribution of single parameters to OA or EMT
| points | potential interactions | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | functional | molecular1 | ||
| OA | ||||||
| T-stage | adenoma | 1/2 | 3/4 | ↓growth control, apoptotic arrest | Ki-ras, rac, Trk-B | |
| N-stage | 0 | 1 | 2 | homing interactions | Ki-ras, specific T-cells | |
| lymphangiosis carcinomatosa | no | yes | / | ↑invasion, ↓adhesion, ↓anoikis | rac, Ki-ras, Trk-B | |
| grading | adenoma, 1 | 2/3 | / | cytoskeletal activation, ↓growth conrol, apoptotic arrest | rac, Ki-ras, cadherins, Trk-B | |
| immunoblot extraction of E-cadherin | T/N<0.1 | 0.1≤T/N< 0.33 | T/N≥0.33 | clustering, functional regulation | extracellular signals, rac, Ki-ras | |
| EMT | ||||||
| T-stage | adenoma, 1/2 | 3 | 4 | ↓growth control, apoptotic arrest | snail, twist integrins | |
| grading | adenoma, 1 | 2 | 3 | dedifferentiation, apoptotic arrest | N-cadherin, rho, twist integrins | |
| immunoblot extraction of E-cadherin | T/N≥0.33 | T/N<0,33 | - | repression of transcription factors | N-cadherin, rho, snail | |
T/N, ratio of E-cadherin protein extraction from tumour and adjacent normal tissue.1 Factors only mentioned in the text are listed.
Statistical analysis
The distribution of patient data and score values was determined as the median and range (parentheses). For the comparison of score values the two-sided U-Test according to Wilcoxon, Mann and Whitney was used. For correlation analysis of point-attributed parameters with patterns of clinical presentation (absence or presence of secondaries to the hepatic (OA) and/or peritoneal (EMT) site) the two-sided X2-homogeneity test was used. The relationship between OA and Fong´s score was determined by the use of Spearman´s rank correlation coefficient rs.
Results
Tumour and patient characteristics
In 27 patients, the median age was 59 (26-78) years, the ratio of men to women 16:11. Of 16 patients resected for a primary, five presented with synchronous hepatic spread. Follow-up in the remaining yielded the detection of metachronous hepatic disease in one and no evidence of recurrence in nine. One patient of these was not further accessible. Hepatic disease of patients admitted for resection was of a synchronous nature in two and metachronous in three. In two patients, a primary or a local recurrence were resected simultaneously with hepatic disease. Metachronous hepatic spread occurred at 21 (8-54) months from resection of the primary. Local recurrencies occurred two times at 13 and 31 months. Peritoneal spread was detected in 6 patients (1 x diffuse, 4 x local, and one local recurrence extending into the abdominal wall) and a lymphangiosis carcinomatosa in three. Further staging data and features of tumour progression are summarised in Tables 3 and 4.
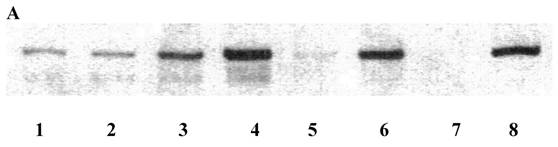
Relative immunoblot extraction of E-cadherin was below 0.33 in nine patients, above 0.33 and below 1.0 in ten, and above or equal 1.0 in eight (Table 5, Figure 1A). In the case of samples collected from both a primary or local recurrence and a liver metastasis, the respective higher relative immunoblot extraction of E-cadherin was utilised for the scoring process. However, relative immunoblot extraction of E-cadherin was comparable in primaries metastatic to or secondaries of the liver (data not shown). The size of the largest liver nodule was 5 (4-13) cm. Serum CEA was 41.7 (2.9-158) ng/ml in patients with hepatic disease only, compared to 3.95 (1.3-42.2) in patients with peritoneal implants (Table 6).
Clinical and pathohistological staging data of primaries
| n | ||
|---|---|---|
| T-stage | adenoma | 3 |
| T1 | - | |
| T2 | 1 | |
| T3 | 17 | |
| T4 | 6 | |
| N-stage | N0 | 7 |
| N1 | 9 | |
| N2 | 8 | |
| site | right hemicolon | 11 |
| transverse colon | 1 | |
| left hemicolon | 10 | |
| rectum | 5 | |
| grading | adenoma | 3 |
| 1 | - | |
| 2 | 19 | |
| 3 | 5 | |
n, number of patients
Clinical course of colorectal cancer patients
| n | |
|---|---|
| local recurrence | 2 |
| lymphangiosis carcinomatosa | 3 |
| peritoneal spread | 6 |
| hepatic spread | 13 |
n, number of patients
Immunoblot extraction of E-cadherin
| T/N | n |
|---|---|
| <0.1 | 2 |
| ≥0.1; <0.33 | 8 |
| ≥0.33 | 17* |
T/N, relative extraction of tumour and adjacent normal tissue; n, number of patients; * T/N≥1,0: 8 patients
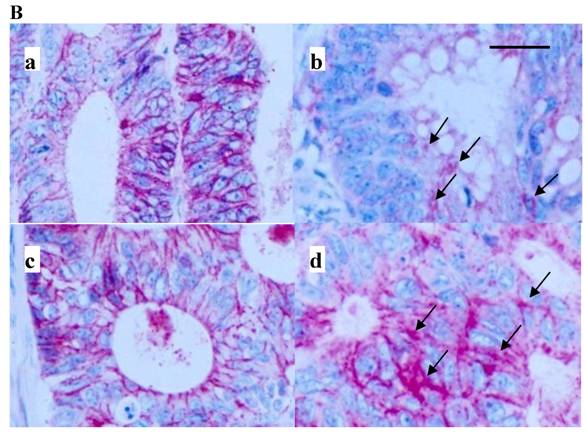
Immunostaining for E-cadherin was predominantly membranous (Figure 1B). Sometimes, however, and among two out of three patients with liver metastases investigated, a remarkable confocal staining was observed at the cellular surface (Figure 1B, b and d).
Both, immunoblot extraction and immunohistochemistry were also performed in identical samples for the detection of the N-terminus, exon-3 and C-terminus of β-catenin (antibodies from Nanotools, Teningen, Germany), providing dinstinct results with respect to intensity, distribution or subcellular localisation. Interestingly, in one patient with diffuse liver metastases an N-terminal deletion of β-catenin could be observed. However, no detailed correlations existed with respect to tumour progression and therefore these data are not further discussed.
Immunoblot extraction (A) of and in-situ staining (B) for E-cadherin of colon cancer (A, lane 1, 3, 5, 7) and corresponding mucosal (A, lane 2, 4, 6, 8) samples. Lane 1 and 2, A, and a, B, are from a primary of the descending colon of a 66-year-old male, pT4pN0M0G2, T/N 0.97, lane 3 and 4, A, and b, B, are from a cecal primary of a 58-year-old male, pT3pN1M1G2 and diffuse liver metastases, T/N 0.5, lane 5 and 6, A, and c, B, are from a sigmoid primary of a 73-year-old female, pT4pN1M0G2, T/N 0.1, and lane 7 and 8, A, are from a transverse primary of a 24-year-old male, pT3pN0pM0G3, T/N 0.015. d, B is from a synchronous liver metastasis of a 59-year-old male with a left-sided primary, pT3pN1G3. Note that in B, dark membranous staining is exclusively due to labelling for E-cadherin. Also, note confocal labelling for E-cadherin in b and d, B (arrows). E-cadherin protein in A was run at 120 kD. Bar in B: 40 µm. Samples in B were prestained by H&E. A coloured version of B is available in the electronically published journal. T/N, relative extraction of tumour and adjacent normal tissue.
OA, EMT, and Fong scores of patients metastatic to the hepatic or peritoneal site
| sex, age | TNM-classification of primary | grading | metastatic site | synchronous / metachronous | time interval to incidence of metastases [months] | number of liver nodules | number of peritoneal nodules | size of largest liver nodule [cm] | CEA at initial presentation [ng/ml] | E-cad. T/N | Fong score9 | metastatic score, OA/EMT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| w, 59 | pT4pN0M1 | 2 | peritoneum | synchronous | 0 | local, few | 4 | 1,0 | 4 / 5 | |||
| w, 66 | pT4pN1M0 | 3 | peritoneum | metachronous | 13 | solid tumour | 4 | >1,0 | 5 / 4 | |||
| w, 68 | pT3pN2M1 | 2 | peritoneum | synchronous | 0 | diffuse | 1.3 | 0 | 5 / 4 | |||
| m, 66 | pT3pN0M0 | 2 | liver | metachronous | 25 | multiple | / | / | 0,3 | / | 4 / 3 | |
| m, 58 | pT3pN0M1 | 2 | liver | synchronous | 0 | single | 4 | 2.9 | 1,05 | 1 | 5 / 2 | |
| m, 64 | pT3pN1M0 | 2 | liver | metachronous | 16 | few | / | 4.0 | 1,52† | 2 | 6 / 2 | |
| m, 58 | pT3pN1M1 | 2 | liver | synchronous | 0 | diffuse | - | 20 | 0,5 | 4 | 6 / 2 | |
| m, 59 | pT3pN1M1 | 2 | liver | synchronous | 0 | few | 6.5 | 41.7 | 3,83† | 3 | 6 / 2 | |
| m, 62 | pT3pN1M0 | 2 | liver | metachronous | 53 | multiple | 13 | 158 | 0,75† | 3 | 6 / 2 | |
| m, 58 | pT3pN1M1 | 2 | liver | synchronous | 0 | single | 7 | 27.4 | 0,37† | 3 | 6 / 2 | |
| w, 45 | pT2pN2M1 | 2 | liver | synchronous | 0 | single, satellites | 8 | 130 | 0,3/1,0† | 4 | 6 / 2 | |
| m, 56 | pT4pN2M1 | 2 | liver | synchronous | 0 | single, micrometastases | >5 | 36.2 | 0,81 | 4 | 7 / 3 | |
| w, 77 | pT3pN2M1 | 2 | liver | synchronous | 0 | multiple | >5 | 56.5 | 1,4 | 4 | 8 / 2 | |
| m, 53 | pT3pN2M0 | 3 | liver, peritoneum | metachronous | 8 | single | local, few | 4.5 | 11.4 | 0,17† | 1 | 6 / 4 |
| w, 59 | pT3pN2M0 | 2 | liver, peritoneum | metachronous | 21 | single | local, diffuse | 4 | 42.2 | 0,26/ 0,96† | 1 | 7 / 2 |
| w, 75 | pT4pN2M1 | 3 | liver, peritoneum | synchronous | 0 | liver diffuse | single | - | 3.9 | 2,0 | 4 | 8 / 4 |
-, no data because of diffuse hepatic spread; /, no data available; E-cad., immunoblot extraction of E-cadherin; T/N, ratio of tumour to adjacent normal tissue; †, tissue harvest from a hepatic nodule
Scoring Results
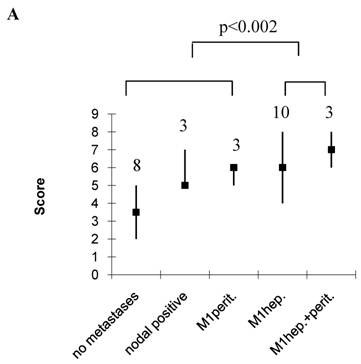
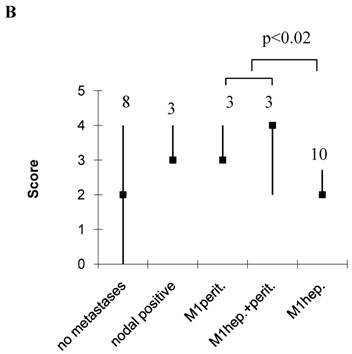
As a single parameter, only the N-stage significantly correlated with hepatic disease (OA, p<0.05, Table 7). However, immunoblot extraction of E-cadherin contributed nearly significant to hepatic (OA) or tumour size and grade to peritoneal disease (EMT) (Table 7). OA scores were significantly higher in patients with hepatic spread compared to those without, being 6 (4-8) in patients with hepatic spread only, or 7 (6-8) in those with hepatic and peritoneal, compared to 3.5 (2-5) without tumour spread, 5 (5-7) with lymphonodal, and 6 (5-6) with peritioneal spread only (p<0.002, Figure 2A). EMT scores on the other hand were significantly higher in patients with peritoneal implants compared to those with hepatic only, being 3 (3-4) in those with peritoneal implants only and 4 (3-4) in those with hepatic and peritoneal implants, compared to 2 (2-3) with hepatic implants only (p<0.02, Figure 2B). Interestingly, in the case of a hepatic and peritoneal disease, OA and EMT scores were higher compared to those with an isolated hepatic or peritoneal disease, suggesting a contribution of both pathways to this kind of tumour distribution. Also, OA and EMT scores were higher in patients with an isolated lymphonodal compared to those without any tumour spread, being 5 (5-7) compared to 3.5 (2-5) in the case of OA, and 3 (3-4) compared to 2 (0-4) in the case of EMT (Figure 2). We also calculated the ratio of OA and EMT scores, which was elevated particulary in the case of an isolated hepatic spread, but, beyond this, did not provide additional information (data not shown).
Fong´s scores, being 3 (1-4) in liver-metastatic patients, correlated with those of OA in the case of hepatic disease only (rs=0.830, p<0.005, data available from 9 patients) or if additional peritoneal disease was included (rs=0.622, p<0.025, 12 patients) (Table 6).
Contribution of single parameters of OA and EMT to liver metastasis or peritoneal spread, respectively
| OA | EMT | |||
|---|---|---|---|---|
| X2 | FG | X2 | FG | |
| T-stage | 1.07 | 1 | 3.88 | 2 |
| N-stage | 6,93 | 2 | / | / |
| grading | - | - | 5.44 | 2 |
| lymphangiosis carcinomatosa | - | - | / | / |
| Immunoblot extraction of E-cadherin | 2,45 | 1 | 0,39 | 1 |
Critical p-values (p<0.05), FG = 1: 3.84, FG = 2: 5.99; FG, freedom grade; -, no calculation available for the reason of parameter distribution; /, not determined
Score values in patients without, and with lymphonodal, peritoneal, hepatic, or both peritoneal and hepatic spread. Median, range, number of patients. A, OA; B, EMT.
Discussion
In human colorectal cancer, the metastatic outcome can be easily studied because of its almost exclusive portal-venous drainage into the liver sinusoid. The liver represents an organ of entodermal origin too and this may account for a preferred homing of epithelially derived cancer cells. Dissemination towards the serosal site, however, may simply take place by detaching cancer cells.
Our results suggest a differential contribution of clinical, pathohistological and molecular parameters to hepatic or peritoneal spread in colorectal cancer. OA scores were significantly higher in patients with liver metastases compared to those without and EMT scores were significantly higher in patients with peritoneal implants compared to those with an isolated hepatic spread. A simultaneous employment of OA and EMT was suggested in patients with lymphonodal, or a combined peritoneal and hepatic spread. Finally, the existence of a lymphangiosis carcinomatosa may reveal a particular feature of cellular activation with respect to vascular invasion, adhesion and dissemination.
On a molecular level, the flip sides of OA and EMT may well be demonstrated by the antagonistic functions of rac and rho.18,19 Rac controls rho and focal adhesion in a dominant fashion.18 This, in turn, constitutes a direct link towards organ metastasis, which has been shown to be of a dominant nature too.21 Activation of Rac induces focal accumulation of E-cadherin protein at the membranous site and contributes to cadherin-mediated crosstalk of cells,18,20 adhesive plasticity and eventually to a successful homing within the liver parenchyma. Activation of rho, however, has rather been attributed to a loss of cellular adhesion and EMT.
In experimental mouse tumour systems, molecular markers of OA and MT have been implicated in the metastatic process. Thus, an activation of the Thyrosinkinase TrkB3 (OA) or of the transcription factor twist4 (EMT) were able to induce liver and lung metastasis, in support of our finding that activation of both pathways may contribute to the metastatic process at different organ sites. Also, TrkB, Ki-ras, and twist did all contribute to an interruption of apoptotic pathways,3,4,8 suggesting thereby an interaction with tumour size and grade.
Lymphonodal spread of the primary and serum CEA have been identified as predictors for the outcome from resection of hepatic metastases.11,12 Indeed, the concentrations of CEA and those of soluble serum E-cadherin correlated in patients with colorectal liver metastases.22 A significant correlation between OA´ and Fong´s scores, the latter of predicting the risk of recurrence subsequent to hepatic resection, further demonstrates the robustness of such scores incorporating easily accessible clinical results. However, the exact molecular pathways of CEA shedding are not yet understood and we therefore decided to not include CEA into the scoring process.
Recently, Landemaine et al. identified genes coding for β8-integrin and P-cadherin as part of a signature to contribute to breast cancer lung metastasis, thereby stressing the environmental question for a successful homing of tumour cells.1 However, clinical, pathohistological, and cellular markers are also likely to represent the metastatic significance of varying genes.
While hepatic organ metastasis may require the activation of differentiation pathways, metastasis to the peritoneal surface may rather be associated with patterns of epithelial-mesenchymal transition. Finally, the lymphonodal environment may be additionally determined by organ-specific lymphocytes. Although statistical correlations of single genes are informative, we also suggest linking site-specificity to patterns of clinical and phenotypic presentation in order to meet the homing requirements of a specific organ site.
Acknowledgements
We thank Anette Schmitt-Gräff, Dept. of Pathology of the University of Freiburg, for generous support in producing the immunohistology images.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Landemaine T, Jackson A, Bellahcène A. et al. A six-gene signature predicting breast cancer lung metastasis. Cancer Res. 2008;68:6092-9
2. Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nature Gen. 2003;33:49-54
3. Douma S, van Laar T, Zevenhoven J, Meuwissen R, van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034-40
4. Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927-39
5. Finkelstein SD, Sayegh R, Christensen S, Swalsky PA. Genotypic classification of colorectal adenocarcinoma. Biologic behavior correlates with K-ras-2 mutation type. Cancer. 1993;71:3827-38
6. Rosivatz E, Becker I, Bamba M, Schott C, Diebold J, Mayr D, Höfler H, Becker K-F. Neoexpression of N-cadherin in E-cadherin positive colon cancers. Int J Cancer. 2004;111:711-9
7. Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12:1-9
8. Guerrero S, Casanova I, Farré L, Mazo A, Capellà G, Mangues R. K-ras codon 12 mutation induces higher level of resistance to apoptosis and predisposition to anchorage-independent growth than codon 13 mutation or proto-oncogene overexpression. Cancer Res. 2000;60:6750-6
9. Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Höfler H, Becker K-F. Differential expression of the epithelial-mesenchymal transition regulators snail, sip1, and twist in gastric cancer. Am J Pathol. 2002;161:1881-91
10. Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type Kras is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626-34
11. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-21
12. Nordlinger B, Guiguet M, Vaillant J-C, Balladur P, Boudjeme K, Bachellier P, Jaeck D, Association Francaise de Chirurgie. Surgical resection of colorectal carcinoma metastases to the liver. Cancer. 1996;77:1254-62
13. Wilmanns C, Eggstein S, Ruf G. Score prediction of metastatic risk in Gastrointestinal Stromal Tumours (GIST). Zentralbl Chir. 2007;132:509-14
14. Sobin LH, Wittekind C. TNM classification of malignant tumours, 6th ed. New York: Wiley-Liss. 2002
15. Schmitt-Gräff A, Ertelt V, Allgaier HP, Koelble K, Olschewski M, Nitschke R, Bochaton-Piallat ML, Gabbiani G, Blum HE. Cellular retinol-binding protein-1 in hepatocellular carcinoma correlates with β-catenin, Ki-67 index, and patient survival. Hepatology. 2003;38:470-80
16. Safina AF, Varga AE, Bianchi A, Zheng Q, Kunnev D, Liang P, Bakin AV. Ras alters epithelial-mesenchymal transition in response to TGFbeta by reducing actin fibers and cell-matrix adhesion. Cell Cycle. 2009;8:284-98
17. Klymkovsky MW, Parr B. The body language of cells: the intimate connection between cell adhesion and behaviour. Cell. 1995;83:5-8
18. Jou T-S, Nelson WJ. Effects of regulated expression of mutant rhoA rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J Cell Biol. 1998;142:85-100
19. Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190rhoGAP regulate cell-cell adhesion by coordinating antagonism between rac and rho. Cell. 2006;127:1027-39
20. Niessen CM, Yap AS. Another job for the talented p120-catenin. Cell. 2006;127:875-7
21. Staroselsky AH, Pathak S, Chernajovsky Y, Tucker SL, Fidler IJ. Predominance of the metastatic phenotype in somatic cell hybrids of the K-1735 murine melanoma. Cancer Res. 1991;51:6292-8
22. Wilmanns C, Grossmann J, Steinhauer S, Manthey G, Weinhold B, Schmitt-Gräff A, von Specht B-U. Soluble serum E-cadherin as a marker of tumour progression in colorectal cancer patients. Clin Exp Metastasis. 2004;21:75-8
Author contact
![]() Correspondence to: Dr. C. Wilmanns, Verbundkrankenhaus Bernkastel/Wittlich, Dept. of Vascular Surgery, Koblenzer Str. 91, 54516 Wittlich, Germany. Tel.: 49-6571-151161; Fax: 49-6571-151162; e-mail: c.wilmannsde
Correspondence to: Dr. C. Wilmanns, Verbundkrankenhaus Bernkastel/Wittlich, Dept. of Vascular Surgery, Koblenzer Str. 91, 54516 Wittlich, Germany. Tel.: 49-6571-151161; Fax: 49-6571-151162; e-mail: c.wilmannsde

 Global reach, higher impact
Global reach, higher impact