10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2009; 5(6):549-557. doi:10.7150/ijbs.5.549 This issue Cite
Research Paper
Protective Effects of Garlic and Silymarin on NDEA-Induced Rats Hepatotoxicity
1. Departments of Cancer Biology and Pathology, National Research Institute, Cairo University, Cairo, Egypt;
2. Department of Zoology, Faculty of Science, Helwan University, Cairo, Egypt;
3. Department of Surg-Oncology T, Tulane University Medical School, New Orleans, LA, USA;
4. Department of Physiology and Hypertension, Tulane University Medical School, New Orleans, LA, USA.
* Both authors are distributed equally to this work.
Received 2009-7-2; Accepted 2009-8-1; Published 2009-8-11
Abstract
Background — The present study was conducted to investigate the chemopreventive effects of garlic extract and silymarin on N-nitrosodiethylamine (NDEA) and carbon tetrachloride (CCl4)-induced hepatotoxicity in male albino rats. Methods and Results — Animals were pretreated with garlic, silymarin or both for one week prior to the injection of NDEA. Then animals received a single injection of NDEA followed by weekly subcutaneous injections of CCl4 for 6 weeks. Oral administration was then continued along with the injection of CCl4 for the duration of the experiment. Serum aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), hepatic lipid peroxidation (LPO), superoxide dismutase (SOD), reduced glutathione (GSH), glutathione-S-transferase (GST) and glutathione reductase (GSR) were measured. Injection of NDEA induced a significant elevation in serum AST, ALT and ALP. In the liver, NDEA increased oxidative stress through the increase in LPO and decrease in SOD, and GSH-dependent enzymes. Although administration of garlic or silymarin significantly reduced the liver toxicity, combined administration was more effective in preventing the development of hepatotoxicity. Conclusion — These novel findings suggest that silymarin and garlic have a synergistic effect, and could be used as hepatoprotective agents against hepatotoxicity.
Keywords: Hepatotoxicity, NDEA, Garlic, Silymarin, liver enzymes, oxidative stress, rats.
Introduction
Hepatocarcinoma is considered the fifth most common pathology worldwide and the most common type of liver cancer, representing up to 83% of all cases. Hepatitis viral infection, food additives, alcohol, fungal toxins (aflatoxins), toxic industrial chemicals, air and water pollutants are the major risk factors of liver toxicity (1), (2).
N-Nitrosodiethylamine (NDEA) is an N-nitroso alkyl compound described as an effective hepatotoxin and hepatotoxic in experimental animals, producing toxicity after repeated administration (3). NDEA is found in a wide variety of foods such as cheese, soybeans, smoked, salted and dried fish, cured meat and alcoholic beverages (4). Metabolism of certain therapeutic drugs is also reported to produce N-nitrosodiethylamine (5). NDEA became metabolically active by the action of cytochrome p450 enzymes to produce reactive electrophiles, which increase oxidative stress level leading to cytotoxicity, mutagenecity and carcinogenicity (6). Oxidative stress is considered as critical mechanism contributing to NDEA-induced hepatotoxicity, and the use of antioxidant agents reduced liver damage (7).
Garlic, Allium sativum, is a member of the lily family that has been cultivated by humans as a food plant for over 10,000 years. Ancient Egyptian records mentioned that use of garlic as a remedy for a variety of diseases (8). Recently, it has been found that the sulfur-containing compounds of garlic have anti-mutagenesis and anti-carcinogenesis effects. In vivo studies show that garlic and its associated sulfur components suppress the incidence of tumors in rodent models (9), (10), (11). Epidemiological findings also demonstrated an inverse relationship between garlic consumption and the incidence of stomach, colorectal and prostate cancer (12), (13), (14).
Silymarin, a standardized extract obtained from seeds of Silybum marianum, is widely used in treatment of liver diseases of varying origins (15). Seeds of S. marianum have been shown to treat liver and gall bladder disorders, including hepatitis, cirrhosis and jaundice and to protect the liver against poisoning from chemicals, environmental toxins, snake bites, insect stings, mushroom poisoning and alcohols (16). Due to its proven hepatoprotective and antioxidant properties, silymarin is being used in the current study as a standard agent for comparison with garlic on hepatoprotective effects (17). In the present study, we determine the mechanism of protective effect of garlic and silymarin in combination on hepatotoxicity induced by NDEA.
Materials and Methods
Chemicals
N-nitrosodiethylamine (NDEA), Elman's reagent [5,5`-dithiobis-(2-nitrobenzoic acid],1-chloro-2,4-dinitrobenzene, Oxidized glutathione (GSSG), NADPH, nitroblue tetrazolium and phenazine methosulpha were purchased from Sigma-Aldrich. Silymarin was supplied by CID Co. (Cairo, Egypt). The colorimetric aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) nitric oxide assay kits were provided by Biodiagnostic Co. (Cairo, Egypt).
Preparation of Garlic Extract
Garlic extract was prepared by homogenizing the required amount of dehusked cloves of garlic in an appropriate volume of distilled water to prepare a concentration of 20 mg/ml (18). The homogenate was centrifuged at 3000 x g for 10 minutes to remove particulate matter and the supernatant fraction was used for the experiment. Garlic extract is composed of different beneficial molecules, especially antioxidants and disulfide.
Experimental design and biochemical analyses
One hundred and sixty male rats weighing 100-150 g (10 weeks old) were obtained from the Animal House Colony of the National Cancer Institute, Cairo University, Egypt. The animals were maintained on standard laboratory diet and water ad libitum. All animals received professional humane care in compliance with the guidelines of the Ethical Committee of Medical Research of National Cancer Institute, Cairo University, Egypt. After one week, animals were divided into eight groups with 20 rats each. Group 1 (control) was orally administered 1ml corn oil/rat for 3 months. Group 2 received 20 mg/Kg/day of garlic for 3 months by gavage according to Samaranayake et al (19). Group 3 received 50 mg/Kg/day Silymarin in corn oil (20) for 3 months. Group 4 received garlic and silymarin in combination using the same previous doses for 3 months. Group 5 received a single i.p injection of N-nitrosodiethylamine (NDEA; 200 mg/kg b.wt) followed by a weekly subcutaneous injection of CCl4 for 6 weeks as reported by Sundaresan and Subramanian (21). Group 6 was pretreated with the same dose of garlic, group 7 was pretreated with silymarin, and group 8 with both garlic and silymarin in combination, for one week prior to NDEA injection. Oral administration continued for all three pretreated groups, along with concomitant injection of CCl4 for the duration of the experiment,
Rats were then sacrificed at the end of treatment and blood samples were collected. Serum was then separated and stored at -70ºC. Liver was removed and divided into 2 pieces; one piece for biochemical studies and the second for histology and IHC. Serum AST and ALT were measured according to Reitman and Frankel (22). ALP was determined according to Belfield and Goldberg (23). Nitric oxide level (NO) in serum was measured according to the method described by Berkels et al (24). Lipid peroxidation (LPO), SOD, GSH, glutathione reductase (GSR) and glutathione-S-transferase (GST) were determined in the liver homogenate according to Esterbauer and Cheeseman (25), Niskikimi et al (26), Ellman (27), Zanetti (28) and Habig et al (29), respectively and protein concentration in the liver homogenate was determined according to Lowry et al (30).
Histology and Immunohistochemistry
Fixed liver tissues were then embedded in paraffin and sectioned to 5 µm thick. Ordinary Haematoxylin and eosin staining were used according to standard protocols. Sections were cut onto positive-charged slides and incubated with monoclonal anti-survivin antibody (1: 500) for 2 hours at 4ºC. The secondary antibody and the avidin-biotin complex were applied to sections. DAB was used as a chromogen and sections were counterstained with Mayer's Hematoxylin. Sections with <5% positive cells were scored as negative (31).
Flow cytometry
Tissue samples were prepared by manual disaggregation procedure. Briefly, a few drops of RPMI were added to tissue and then minced until complete tissue disaggregation was achieved. Suspended cells were filtered using a 50 µm pore size mesh and then centrifuged at 1000 rpm for 10 min. Cells were resuspended in PBS, counted and washed by calcium buffer then centrifuged at 1500 rpm for 5 min. Some cells were used to assess cell viability by trypan blue method. The pellet was resuspended and then cells were counted. Annexin-PI apoptotic assay was carried out using IQP-120F Kit (IQ Products,Groningen, Netherlands). FAC scan Becton-Dickinson (BD) flow-cytometer was used and data were analyzed using cell Quest software. Peripheral human blood lymphocytes were used as a control for this study.
Statistical analysis
Data were presented as mean ± standard error (SE). The difference between groups was assessed using F-test (one way analysis of variance ANOVA) by MSTAT-C program, Version 4, USA.
Results
Protective effects of garlic extract and silymarin on NDEA-induced hepatotoxicity
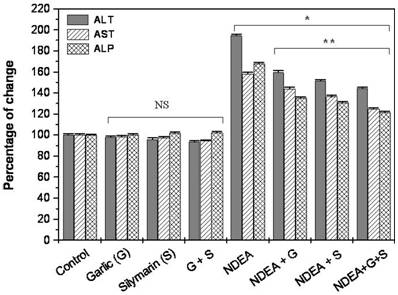
Our data revealed that the administration of NDEA induced a significant increase (P<0.01) in ALT, AST and ALP activity in serum as compared to control. Treatment with garlic or silymarin showed a significant decrease in serum ALT, AST and ALP activity (P<0.01; Figure 1) induced by NDEA. The combination effect between garlic and silymarin was higher compared to garlic or silymarin alone (Figure 1).
Effect of NDAE after garlic and silymarin treatments on serum ALT, AST and ALP activity in male rats. Data are represented as percentage of change ± percentage of standard error. Significance change is calculated as *p<0.01 compared to untreated control and **p<0.01 compared to NDEA-treated rats. NS: non-significant results.
Effect of garlic and silymarin on NDEA-induced liver oxidative stress
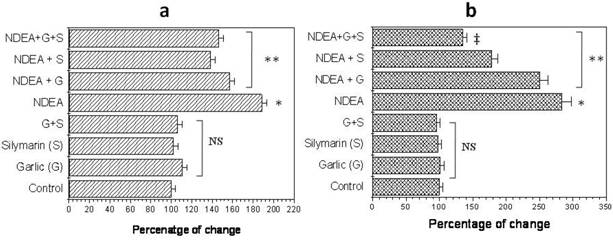
NDEA injection showed a significant increase (P<0.01) in hepatic lipid peroxidation level (LPO) and serum nitric oxide (NO) compared to control group (Figure 2a & b). Rats that received garlic, silymarin or both showed a significant decrease (P<0.01) in serum NO induced by NDEA (Figure 2a). Hepatic LPO significantly decreased (P<0.01) in NDEA-administrated rats treated with garlic or silymarin (Figure 2b). The effect of silymarin was greater than garlic, however the administration of both agents together showed a greatest improvement of LPO (Figure 2b).
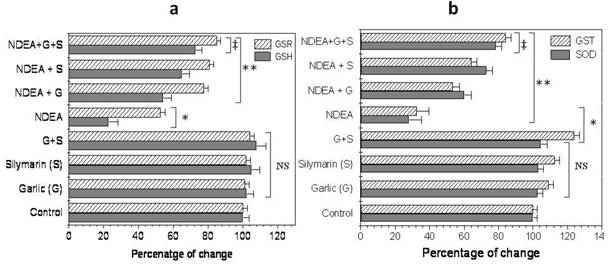
Furthermore, the injection of NDEA caused a significant decrease (P<0.01) in hepatic glutathione level (GSH), glutathione reductase (GSR), superoxide dismutase (SOD) and glutathione-S-transferase (GST) activity, compared to the control (Figure 3a & b). Garlic or silymarin showed a significant increase (P<0.01) in hepatic GSH level and GSR, GST and SOD activity (Figure 3a & b). Moreover, the combination of garlic and silymarin was more significant compared to garlic or silymarin alone (Figure 3a & b).
Effect of NDAE injection with garlic and silymarin treatments on serum nitric oxide (a) and liver lipid peroxidation (b). Data are represented as percentage of change ± percentage of standard error. Significance change is calculated as *p<0.01 compared to untreated control, **p<0.01 compared to NDEA-treated rats and ‡p<0.01 compared to NDEA with garlic or silymarin alone. NS: non-significant results.
Effect of NDAE injection with garlic and silymarin treatments on hepatic glutathione (GSH) content and glutathione reductase (GSR) activity (a), glutathione-S-transferase (GST) and superoxide dismutase (SOD) activity (b). Data are represented as percentage of change ± percentage of standard error. Significance change is calculated as *p<0.01 compared to untreated control, **p<0.01 compared to NDEA-treated rats and ‡p<0.01 compared to NDEA with garlic or silymarin alone. NS: non-significant results.
Effect of garlic and silymarin on survivin expression and liver histology in NDEA-induced hepatotoxicity
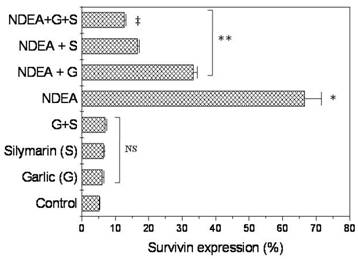
There was no significant change of survivn expression between rats treated with garlic extract or silymarin or both compared to unretaed control rats (Figure 4). However, Survivin positive cells were detected in (66.6%) of NDEA-treated rats, and then dramatically decreased in rats treated with garlic + NDEA (33.3%) or silymarin +NDEA (16.6%), respectively (Figure 4).
Effect of NDAE injection with garlic and silymarin treatments on survivin expression (Upper panel) and immunostaining (Lowe panel) in hepatic cells in rats. Data are represented as percentage of change ± percentage of standard error. Significance change is calculated as *p<0.01 compared to untreated control, **p<0.01 compared to NDEA-treated rats and ‡p<0.01 compared to NDEA with garlic or silymarin alone. NS: non-significant results.
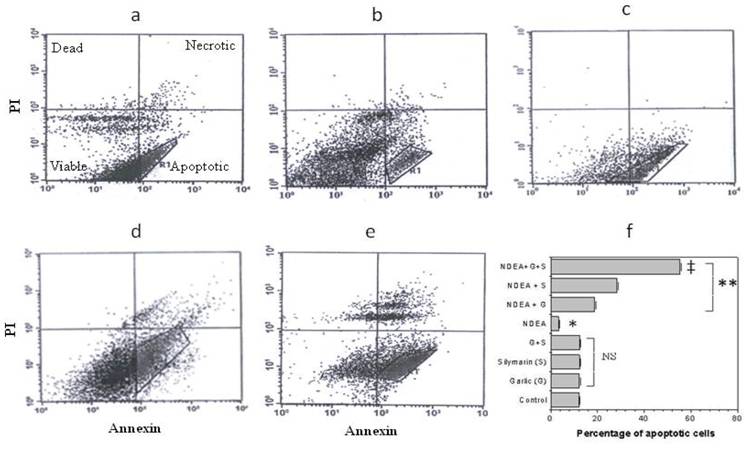
Effect of garlic and silymarin on NDEA-induced cell apoptosis
Flow cytometric analysis was performed to investigate the anti-apoptotic effects of garlic and silymarin on liver apoptosis cells induced by NDEA. Cells were dual-stained with Annexin V-FITC and propidium iodide (PI) (Figure 5a-e). The percentage of apoptotic cells in the liver of control, treated with garlic and/or silymarin was 12.41, 12.50, 12.54 and 12.58 % respectively. In animals treated with NDEA, the percentage of apoptotic cells in the liver was decreased to 3.04% (Figure 5f) due to the increased resistance to apoptosis related to the switch of cell from normal to cancer cells (Figure 5f). Interestingly, apoptosis was significantly increased in rats treated with garlic+NDEA, silymarin+NDEA and garlic+silymarin+NDEA (19.03%, 28.05%, and 56.42% respectively (Figure 5f).
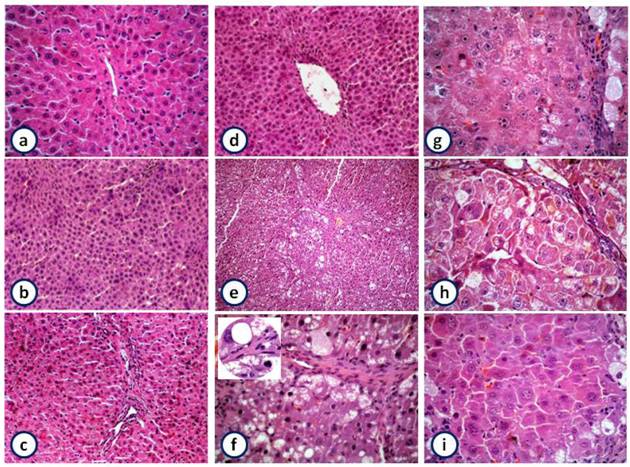
Histological studies reveal that liver sections from control rats showed normal architecture, characterized by polyhedral shaped hepatocytes and cytoplasm granulated with small uniform nuclei. Hepatocytes were arranged in well-organized hepatic cords and separated by narrow blood sinusoids. Animals treated with garlic and/or silymarin exhibited normal architecture, indicating the non-toxic effect of garlic and silymarin (Figure 6a to d). In contrast, the liver sections of rat injected with NDEA showed loss of lobular architecture, fibrosis and fatty infiltration (Figure 6e). Also, the nuclei of many hepatocytes appeared malignant or have features of degenerating and dividing process (Figure 6f).
The pretreatment of rats receiving NDEA with garlic or silymarin showed extended portal tract infiltrated with mononuclear inflammatory cells, less fibrosis, less disarrangement and degeneration of hepatocytes (Figure 6g & h). Furthermore, the combination of garlic and silymarin showed minimal pleomorphism, vaculation and fibrosis compared to NDEA induced animals (Figure 6i).
Flow cytometry shows the effect of garlic (c) and silymarin (d) or combinations (e) as hepatoprotective agents with NDEA treated (b) rats in comparison to control (a). Accumulative data (f) shows the percentage of apoptosis for NDEA-induced hepatotoxicity in rats and pretreated with garlic and silymarin. Significance change is calculated as *p<0.01 compared to untreated control, **p<0.01 compared to NDEA-treated rats and ‡p<0.01 compared to NDEA with garlic or silymarin alone. NS: non-significant results.
Histopathology of liver showing the normal architecture and cells with granulated cytoplasm and small uniform nuclei of control, garlic, silymarin or both of them, respectively liver (a-d) (HE X100). NDEA-treated rats show loss of architecture, fibrosis and fatty infiltration (e; X100 and f; X400) and magnified part shows malignant nuclei (X1000). Rats pretreated with garlic (g) or silymarin (h) or both (i) before injection of NDEA showing minimal pleomorphism, vaculation, fibrosis, less disarrangement and degeneration of hepatocytes (HE X 400).
Discussion
N-nitrosodiethylamine (NDEA) is a major environmental carcinogen suggested to increase the generation of reactive oxygen species (ROS) resulting in oxidative stress and cellular injury (32). Since liver is the main site of NDEA metabolism, the production of ROS in the liver may be responsible for its carcinogenic effects (33).
In this study, we demonstrated that the injection of NDEA to rats lead to a marked elevation in the levels of serum AST, ALT and ALP which is indicative of hepatocellular damage, as previously reported (33). This elevation could potentially be attributed to the release of these enzymes from the cytoplasm into the blood circulation after rupture of the plasma membrane and cellular damage. Serum AST, ALT and ALP are biomarkers in the diagnosis of hepatic damage because they are released into the circulation after cellular damage (34). Administration of garlic or silymarin, alone or in combination significantly reduced the activity of liver enzymes in NDEA induced rats as previously reported (35). Due to its ability to reduce free radical-induced oxidative damage in the liver(36), garlic extract has been shown to decrease liver enzymes in serum and prevent liver damage of rats with liver fibrosis (37). Silymarin prevents liver damage by maintaining the integrity of the plasma membrane, thereby suppressing the leakage of enzymes (35).
NDEA is well known to generate free radicals, disturbing the antioxidant status and ultimately leading to oxidative stress and carcinogenesis (38). Lipid peroxidation plays an important role in carcinogenesis (39) and may lead to the formation of several toxic products, such as malondialdehyde (MDE) and 4-hydroxynonenal. These products can attack cellular targets including DNA, thereby inducing mutagenecity and carcinogenicity (40). Recently, the increase in lipid peroxidation was reported during NDEA-induced hepatocarcinogenesis (41). In line with this finding there was a significant increase in the level of lipid peroxidation in the liver of rats treated with NDEA. However, groups treated with garlic, silymarin or both displayed a significant reduction in lipid peroxidation when compared to animals treated with NDEA alone. The observed reduction in the level of lipid perioxidation in garlic treated animals was presumably due to its ability to scavenge the hydroxyl and peroxyl radicals (42). Also, it has been shown that aged garlic extract protects vascular endothelial cells from H2O2-induced oxidative damage by inhibiting lipid peroxidation (43). Restoration in the levels of lipid peroxidation in groups treated with silymarin could be related to its ability to scavenge reactive oxygen species (44).
Free radical scavenging enzymes such as superoxide dismutase (SOD) protect the biological systems from oxidative stress. The current study showed a significant decrease in SOD activity in rats treated with NDEA. On the other hand, there was a significant increase in SOD activities in groups treated with garlic, silymarin, and combination. It has been reported that administration of garlic and silymarin significantly decreased lipid peroxidation and increased endogenous antioxidants, such as SOD, CAT, and GSH (45). Oxidative stress-induced tissue damage can be prevented or ameliorated by favoring the balance towards a lower oxidative stress status. The present data show that injection of NDEA caused depletion of GSH, which may be responsible for the increased lipid peroxidation (33), (35). Treatment with garlic, silymarin, or both increased the GSH content in the liver compared to animals treated with NDEA alone. Arivazhaga et al (46) reported that rats treated with N-methyl-Ń-nitro-N-nitrosoguanidine as garlic extract increased GSH level. Since administration of aqueous garlic extract prevented the hepatic GSH depletion, it appears that the protective effect of garlic extract involves the maintenance of antioxidant capacity in protecting the hepatic tissue against oxidative stress. Moreover, silymarin has been reported to maintain the GSH homeostasis in the system (20). This might be the reason for elevated glutathione levels observed during silymarin treatment.
A significant decrease in the activity of GSH dependent enzymes, GST and GSR was observed in NDEA treated rats. This may be due to the decreased expression of these antioxidants during hepatocellular damage as previously described by Kweon et al (47). Administration of garlic and silymarin, alone or in combination, to NDEA induced rats significantly increased the activity of GSR. This may be due to the reduction in DNA adduct formation by Se-enriched garlic (48) and to the inhibition of carcinogen-induced nuclear damage by sulfides in garlic (49). Our study shows that treatment with silymarin improved the activities of GSR and GST. This improvement may have resulted from changing the tissue redox system by scavenging the free radicals and improving the antioxidant status in the liver during NDEA hepatotoxicity (35).
The present study shows that serum NO level significantly increased in NDEA treated animals. It has been reported that elevated levels of lipid peroxidation stimulates host cells, mainly monocytes/macrophages, to produce and release NO by induction of inducible nitric oxide synthase (iNOS) protein, resulting in cytotoxicity and DNA damage (50). In the view of the current data, garlic has been found to decrease serum NO level in rats injected with NDEA. The inhibitory effect of garlic and silymarin on NO is mainly due to the inhibition of the induction of iNOS protein/enzyme (51), (52).
The results obtained from flow cytometry show a significant decrease in the percent of apoptotic cells in NDEA-induced hepatocellular carcinoma, which was reversed with garlic, silymarin, or their combination treatment. Combined administration of tomato and garlic significantly inhibited the development of hamster buccal pouch carcinomas and induced apoptosis (53). This was evidenced by down-regulation of Bcl-2 and up-regulation of Bax, Bim, p53 and caspases 8 & 3. Also, lower expression of survivin was observed in rats pretreated with garlic, silymarin, or their combination when compared with the NDEA-treated group, as indicated also by several authors (53). Treatment with NDEA showed distinct alterations from untreated rats, such as loss of lobular architecture, fibrosis, fatty infiltration, and malignant nuclei as reported by Ramakrishnan et al (44). However, pretreatment with garlic, silymarin, or both with NDEA showed improvement in the histopathological features.
From these observations it can be concluded that the combination of garlic and silymarin may suppress the formation of NDEA induced hepatotoxicity in rats by alleviating lipid peroxidation through scavenging of free radicals, or by enhancing the activity of antioxidants.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674-87
2. Jemal A, Siegel R, Ward E. et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66
3. Jose K, Kuttan R, Bhattacharya K. Effect of Emblica officinalis extract on hepatocarcinogenesis and carcinogen metabolism. Journal of clinical biochemistry and nutrition. 1998;25:31-39
4. Liao DJ, Blanck A, Eneroth P. et al. Diethylnitrosamine causes pituitary damage, disturbs hormone levels, and reduces sexual dimorphism of certain liver functions in the rat. Environ Health Perspect. 2001;109:943-7
5. Akintonwa DA. The derivation of nitrosamines from some therapeutic amines in the human environment. Ecotoxicol Environ Saf. 1985;9:64-70
6. Archer MC. Mechanisms of action of N-nitroso compounds. Cancer Surv. 1989;8:241-50
7. Vitaglione P, Morisco F, Caporaso N. et al. Dietary antioxidant compounds and liver health. Crit Rev Food Sci Nutr. 2004;44:575-86
8. Block E. The chemistry of garlic and onions. Sci Am. 1985;252:114-9
9. Liu J, Lin RI, Milner JA. Inhibition of 7,12-dimethylbenz[a]anthracene-induced mammary tumors and DNA adducts by garlic powder. Carcinogenesis. 1992;13:1847-51
10. Amagase H, Milner JA. Impact of various sources of garlic and their constituents on 7,12-dimethylbenz[a]anthracene binding to mammary cell DNA. Carcinogenesis. 1993;14:1627-31
11. Song K, Milner JA. Heating garlic inhibits its ability to suppress 7, 12-dimethylbenz(a)anthracene-induced DNA adduct formation in rat mammary tissue. J Nutr. 1999;129:657-61
12. You WC, Blot WJ, Chang YS. et al. Allium vegetables and reduced risk of stomach cancer. J Natl Cancer Inst. 1989;81:162-4
13. Dorant E, van den Brandt PA, Goldbohm RA. A prospective cohort study on the relationship between onion and leek consumption, garlic supplement use and the risk of colorectal carcinoma in The Netherlands. Carcinogenesis. 1996;17:477-84
14. Hsing AW, Chokkalingam AP, Gao YT. et al. Allium vegetables and risk of prostate cancer: a population-based study. J Natl Cancer Inst. 2002;94:1648-51
15. El-Samaligy MS, Afifi NN, Mahmoud EA. Evaluation of hybrid liposomes-encapsulated silymarin regarding physical stability and in vivo performance. Int J Pharm. 2006;319:121-9
16. Kren V, Walterova D. Silybin and silymarin-new effects and applications. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149:29-41
17. Dhiman RK, Chawla YK. Herbal medicines for liver diseases. Dig Dis Sci. 2005;50:1807-12
18. Balasenthil S, Arivazhagan S, Ramachandran CR. et al. Effects of garlic on 7,12-Dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Cancer Detect Prev. 1999;23:534-8
19. Samaranayake MD, Wickramasinghe SM, Angunawela P. et al. Inhibition of chemically induced liver carcinogenesis in Wistar rats by garlic (Allium sativum). Phytother Res. 2000;14:564-7
20. Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clin Drug Investig. 2002;22:51-65
21. Sundaresan S, Subramanian P. S-allylcysteine inhibits circulatory lipid peroxidation and promotes antioxidants in N-nitrosodiethylamine-induced carcinogenesis. Pol J Pharmacol. 2003;55:37-42
22. Retiman S, Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56-63
23. Belfield A, Goldberg DM. Revised assay for serum phenyl phosphatase activity using 4-amino-antipyrine. Enzyme. 1971;12:561-73
24. Berkels R, Purol-Schnabel S, Roesen R. Measurement of nitric oxide by reconversion of nitrate/nitrite to NO. Methods Mol Biol. 2004;279:1-8
25. Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407-21
26. Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849-54
27. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70-7
28. Zanetti G. Rabbit liver glutathione reductase. Purification and properties. Arch Biochem Biophys. 1979;198:241-6
29. Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130-9
30. Lowry OH, Rosebrough NJ, Farr AL. et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-75
31. Ramchurren N, Cooper K, Summerhayes IC. Molecular events underlying schistosomiasis-related bladder cancer. Int J Cancer. 1995;62:237-44
32. Bartsch H, Hietanen E, Malaveille C. Carcinogenic nitrosamines: free radical aspects of their action. Free Radic Biol Med. 1989;7:637-44
33. Bansal AK, Bansal M, Soni G. et al. Protective role of Vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem Biol Interact. 2005;156:101-11
34. Naik SR, Panda VS. Antioxidant and hepatoprotective effects of Ginkgo biloba phytosomes in carbon tetrachloride-induced liver injury in rodents. Liver Int. 2007;27:393-9
35. Pradeep K, Mohan CV, Gobianand K. et al. Silymarin modulates the oxidant-antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur J Pharmacol. 2007;560:110-6
36. Gedik N, Kabasakal L, Sehirli O. et al. Long-term administration of aqueous garlic extract (AGE) alleviates liver fibrosis and oxidative damage induced by biliary obstruction in rats. Life Sci. 2005;76:2593-606
37. Nakagawat S, Kasug S, Matsuura H. Prevention of liver damage by aged garlic extract and its components in mice. Phytotherapy Research. 1989;3:50- 53
38. Gey KF. Prospects for the prevention of free radical disease, regarding cancer and cardiovascular disease. Br Med Bull. 1993;49:679-99
39. Banakar MC, Paramasivan SK, Chattopadhyay MB. et al. 1alpha, 25-dihydroxyvitamin D3 prevents DNA damage and restores antioxidant enzymes in rat hepatocarcinogenesis induced by diethylnitrosamine and promoted by phenobarbital. World J Gastroenterol. 2004;10:1268-75
40. de Zwart LL, Meerman JH, Commandeur JN. et al. Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic Biol Med. 1999;26:202-26
41. Jeyabal PV, Syed MB, Venkataraman M. et al. Apigenin inhibits oxidative stress-induced macromolecular damage in N-nitrosodiethylamine (NDEA)-induced hepatocellular carcinogenesis in Wistar albino rats. Mol Carcinog. 2005;44:11-20
42. Horie T, Awazu S, Itakura Y. et al. Identified diallyl polysulfides from an aged garlic extract which protects the membranes from lipid peroxidation. Planta Med. 1992;58:468-9
43. Yamasaki T, Lau BH. [Garlic compounds protect vascular endothelial cells from oxidant injury]. Nippon Yakurigaku Zasshi. 1997;110(Suppl 1):138P-141P
44. Ramakrishnan G, Raghavendran HR, Vinodhkumar R. et al. Suppression of N-nitrosodiethylamine induced hepatocarcinogenesis by silymarin in rats. Chem Biol Interact. 2006;161:104-14
45. Kiruthiga PV, Shafreen RB, Pandian SK. et al. Silymarin protection against major reactive oxygen species released by environmental toxins: exogenous H2O2 exposure in erythrocytes. Basic Clin Pharmacol Toxicol. 2007;100:414-9
46. Arivazhagan S, Balasenthil S, Nagini S. Modulatory effects of garlic and neem leaf extracts on N-methyl-N'-nitro-N-nitrosoguanidine (MNNG)-induced oxidative stress in Wistar rats. Cell Biochem Funct. 2000;18:17-21
47. Kweon S, Park KA, Choi H. Chemopreventive effect of garlic powder diet in diethylnitrosamine-induced rat hepatocarcinogenesis. Life Sci. 2003;73:2515-26
48. Ip C, Lisk DJ. Modulation of phase I and phase II xenobiotic-metabolizing enzymes by selenium-enriched garlic in rats. Nutr Cancer. 1997;28:184-8
49. Wargovich MJ, Goldberg MT. Diallyl sulfide. A naturally occurring thioether that inhibits carcinogen-induced nuclear damage to colon epithelial cells in vivo. Mutat Res. 1985;143:127-9
50. Raso GM, Meli R, Di Carlo G. et al. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001;68:921-31
51. Gu G, Du Y, Hu H. et al. Synthesis of 2-chloro-4-nitrophenyl alpha-L-fucopyranoside: a substrate for alpha-L-fucosidase (AFU). Carbohydr Res. 2003;338:1603-7
52. Chang HP, Chen YH. Differential effects of organosulfur compounds from garlic oil on nitric oxide and prostaglandin E2 in stimulated macrophages. Nutrition. 2005;21:530-6
53. Bhuvaneswari V, Rao KS, Nagini S. Altered expression of anti and proapoptotic proteins during chemoprevention of hamster buccal pouch carcinogenesis by tomato and garlic combination. Clin Chim Acta. 2004;350:65-72
Author contact
![]() Correspondence to: Khalid Matrougui, Ph.D. Department of Physiology, Hypertension and Renal Center of Excellence, Tulane Cancer Center, Tulane University, 1430 Tulane Ave, New Orleans LA-70112. Phone-(504)-889-2588, Fax-(504)-988-2675, email: kmatrougedu; Emad Kandil, MD. Department of Surg-Oncology, Tulane University, 1430 Tulane Ave, New Orleans LA-70112, Phone-(504)-988-7485, email: ekandiledu
Correspondence to: Khalid Matrougui, Ph.D. Department of Physiology, Hypertension and Renal Center of Excellence, Tulane Cancer Center, Tulane University, 1430 Tulane Ave, New Orleans LA-70112. Phone-(504)-889-2588, Fax-(504)-988-2675, email: kmatrougedu; Emad Kandil, MD. Department of Surg-Oncology, Tulane University, 1430 Tulane Ave, New Orleans LA-70112, Phone-(504)-988-7485, email: ekandiledu

 Global reach, higher impact
Global reach, higher impact