10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2010; 6(5):443-453. doi:10.7150/ijbs.6.443 This issue Cite
Research Paper
Catalpol Increases Brain Angiogenesis and Up-Regulates VEGF and EPO in the Rat after Permanent Middle Cerebral Artery Occlusion
1. School of Pharmaceutical Sciences & School of Chinese Medicine, Southwest University, Chongqing 400715, China;
2. Department of Emergency Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China;
3. Department of Neurology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China;
4. Chongqing Medical And Pharmaceutical College;
5. Southwest University 2nd Hospital, Chongqing 400715, China
Received 2010-5-24; Accepted 2010-8-1; Published 2010-8-20
Abstract
To investigate the role and mechanism of catalpol in brain angiogenesis in a rat model of stroke, the effect of catalpol (5 mg/kg; i.p) or vehicle administered 24 hours after permanent middle cerebral artery occlusion (pMCAO) on behavior, angiogenesis, ultra-structural integrity of brain capillary endothelial cells, and expression of EPO and VEGF were assessed. Repeated treatments with Catalpol reduced neurological deficits and significantly improved angiogenesis, while significantly increasing brain levels of EPO and VEGF without worsening BBB edema. These results suggested that catalpol might contribute to infarcted-brain angiogenesis and ameliorate the edema of brain capillary endothelial cells (BCECs) by upregulating VEGF and EPO coordinately.
Keywords: Catalpol, VEGF, EPO, Permanent occlusion of middle cerebral artery, Angiogenesis
1. Introduction
Stroke has been emerging as one of the most common causes of mortality and morbidity in modern society. Although much progress has been made toward understanding the mechanistic basis of stroke, the effectiveness of drugs available for stroke patients is limited. Tissue plasminogen activator (TPA), which dissolves blood clots in the brain, is presently the only approved treatment for stroke; however, it is effective only in the first 3 h after the stroke and may lead to cerebral hemorrhage [1]. Many drugs focus on the ischemic penumbra and cascade of damage, including anti-N-methyl-D-aspartate receptor (Aptiganel and gavestinel), potassium channel agonists (MaxiPost), and GABA modulators (Zendra). However, the uses of these drugs for stroke have been abandoned because they are not effective and even harmful to stroke patients, despite their apparent effectiveness in animal models of brain ischemia [2]. Therefore, new drugs are in demand and need to be developed to treat stroke.
The neurovascular unit concept emphasizes not only the neuron but also the brain vascular structure [3-4]. Previous research on stroke has largely focused on neuroprotection, but neglected the ischemic vascular structure and the possible benefits of its functional reconstruction [1,4,5]. Brain vascular structures are coupled with brain neurons in structure and function [6]. Angiogenesis is associated with neurogenesis [7]. Mounting evidence has shown that vascular-remodeling occurs after stroke [8-9]. It has been shown that higher blood vessel counts correlate with longer survival in stroke patients [10]. The three-dimensional images of angiogenesis in the tissue surrounding focal brain infarcts are demonstrated by scanning electron microscopy with corrosion casting [11]. These studies suggest that targeting brain vascular-remodeling for drug discovery is necessary for stroke and even for ischemia disease.
Rehmannia Root is the main natural herbal medicine that plays an important role in treating stroke. Recently, catalpol, a main active component of Rehmannia Root, was determined to pose extensive ischemic neural protection, such as preventing the loss of hippocampal CA1 neurons and reducing working errors [12], modulating the expressions of Bcl-2 and Bax [13], attenuating apoptosis in the ischemic brain [13], and increasing hippocampal neuroplasticity by up-regulating PKC and BDNF in aged rats [14]. Catalpol improves Y-maze performance and the survival of neurons in the CA1 subfield after transient global ischemia in gerbils [15-16]. Our previous studies have demonstrated that catalpol at doses of 10 and 5 mg/kg can improve neurobehavioral outcome following permanent focal cerebral ischemia in Sprague Dawley rats, and upregulate the expression of growth-associated protein 43 (GAP-43) [17]. These findings suggest that catalpol contributes to neuroplasticity after stoke. However, whether catalpol can modulate brain angiogenesis after focal ischemia is unclear.
Erythropoietin (EPO) and Vascular endothelial growth factor (VEGF) have pleiotropic effects on brain function, including neuroprotection, and promotion of angiogenesis and neurogenesis [18]. Notably, EPO enhances angiogenesis, without aggravating brain edema, even used with VEGF [19].
In this study, we have investigated the effect of catalpol on angiogenesis following permanent middle cerebral artery occlusion (pMCAO) in rats. In order to explore the cellular and molecular mechanism by which catalpol may regulate the vascular plasticity of the brain, we examined the expression of VEGF and EPO by immunohistochemistry and western blotting.
2. Materials and Methods
2.1 Animals and diets
Healthy male Sprague-Dawley (SD) rats (220∼280 g) were obtained from the Experimental Animal Center, Chongqing University of Medicine, China. Animals were housed under conditions of natural illumination with food and water available ad libitum. These experiments were performed in accordance with China's guidelines for care and use of laboratory animals. Animals were divided into 3 groups randomly (1) the sham operated group (n = 24); (2) the vehicle group (n = 24); (3) the catalpol-treated group (n = 24).
2.2 The pMCAO model
Strokes were induced by electrocoagulation of the right middle cerebral artery as described previously with minor modifications [20]. Briefly, rats were anesthetized and placed in a stereotaxic instrument (Shanghai Jiangwan) in the prone position. The scalp was opened and brain was exposed, held up lightly with a glass retractor, inferior cerebral vein and olfactory bundle were seen perspicuously and the right middle cerebral artery was along the brain surface verticality striding over inferior cerebral vein and olfactory bundle. The middle cerebral artery ventral to the olfactory tract was electrocoagulated (power 35W), resulting in infarction of the right dorsolateral cerebral cortex. Rats were prescreened to select those in line with the criteria described as Bederson [21].
2.3 Drug administration
Catalpol was dissolved in physiological saline, which was purchased from National Institute for the Control of Pharmaceutical and Biological Products (China) and its purity was more than 98%. Catalpol (5mg/kg, ip) were administered 24h after stroke and then daily for 7 days. Likewise, the sham-operation group and the vehicle group received equal volumes of physiological saline by ip injection. The dose of catalpol was based on our previous study [17] and Li's study [15-16].
2.4 Bederson's Score
After operation, the neurological function of all animals was evaluated daily with a 4-point scale as previously described [21]: (0) no apparent deficit, (1) contralateral forelimb flexion, (2) lowered resistance to lateral push without circling, and (3) circling to ipsilateral stroke if spontaneous activity.
2.5 Beam-Walking test
Beam walking test [22-23] was used to evaluate sensorimotor reflexes, motor strength and coordination. The testing apparatus was a 2.5 cm in diameter and 80 cm in length wooden beam elevated 100 cm above the floor with wooden supports as described by Stanley et al, and a 5cm thickness foam pillow placed under the beam avoid getting wound in a fall.
Rats were allowed to walk to a platform located at the end of the beam and their behaviors were recorded based on the following six criteria: (0) the rat traverses the beam without falling down; (1) the rat traverses the beam but footslips less than 50%; (2) the rat crosses the beam but footslips more than 50%; (3) the rat crosses the beam without the aid of the affected hindlimb; (4) the rat can't traverses the beam but can sit on the horizontal surface of the beam; (5) the rat will fall down when placed on the beam. Each trial consisted of five repetitions of this assay.
2.6 Examination of the healing Ischemic brain cortex and the surface vessels
Stroke model rats in each group were sacrificed 15 days after the operation. After removing the surface meninges, the surface vessels, distribution patterns, and the healing state of ischemic cortex were examined using a microscope attached to a digital camera as described previously [24].
2.7 Tissue preparation
Fifteen days after operation/stroke induction, the rats were deeply anesthetized with an overdose of Chloral Hydrate (35 mg/mL, i.p), and transcardially perfused with 0.9 % NaCl solution to rinse out the blood, followed by 250 mL of 4% formalin (4°C) to fix the brain tissue. After extraction from the skull, the brains were post-fixed in 4% formalin solution and subsequently cut into 30 µm coronal sections on a cryostat (Leica). For EPO analyses, imbedded brain tissue was used. Ipsilateral ischemic cortex (0.1 g per brain) in each group was also weighed for western blotting analyses.
2.8 Immunohistochemistry
Animal brain tissues were transcardially perfused and fixed with 4% paraformaldehyde, cryopreserved in 30% sucrose, and cut into three series of consecutive sections (30 μm) at a Cryostat. Each set of tissue sections was immunostained for PCNA, VWF or VEGF. For EPO analyses, imbedded brain tissue was used to cut as 5μm sections. For immunohistochemistry, tissue sections were incubated with rabbit polyclonal antibody against EPO (1:200, santa cruz, USA), VEGF(1:200, Wuhan Booster Biotech. Co., China). For double-fluorescence labeling, cross sections were incubated with the vessel marker antibody, rabbit polyclonal against VWF (1:200, Zhongshan Biotech. Co., China), together with the mouse monoclonal antibody against the proliferation marker PCNA (1:200, Wuhan Booster Biotech. Co., China). Immunohistochemistry for EPO was done with biotinylated goat anti-rabbit IgG (1:500; Vector Laboratories) and peroxidase-conjugated avidin-biotin complex (ABC kit; Wuhan Booster Biotech. Co., China), bound antibodies were visualized by addition of diaminobenzidine. VEGF sections then incubated with Cy3-labeled goat anti-rabbit IgG (Wuhan Boster Biotech. Co., China). PCNA and VWF then incubated with Cy3-labeled goat anti-rabbit IgG (Wuhan Boster Biotech. Co., China) and FITC-labeled goat anti-mouse IgG. After through washing, immunostained cells were observed under a Nikon microscope and were documented with a Nikon digital camera. Immunofluorescence staining for PCNA (green) and VWF (red) or VEGF (red) was visualized and documented with a confocal microscope (Leica). According to Acker [25] (Acker et al., 2001), the number of double-stained vessels and the intensity of staining were analyzed with Image Pro Plus Version 6.0. software for cerebral microvessels at the boundary zone of ischemia. Five fields of each slice were randomly selected for blinded scoring and analyses. Each experiment was performed three times.
2.9 Electron microscopy observation of brain vascular endothelial cells
Rats in each group were anesthetized with 3.5% chloral hydrate (35 mg/mL, i.p) and perfused for 2-3 min through the ascending aorta with 0.9% normal saline, followed by ice-cold fixative (4% paraformaldehyde in 0.1 M PBS for 30 min). The brains were removed for immunocytochemical electron microscopic studies and fixed in 4% paraformaldehyde in 0.1 M PBS pH 7.4 for 3∼4 h at 4°C. The brain was then rinsed in PBS for 30 min, treated with 1% OsO4 for 30 min, dehydrated in sequential ethanol gradients, and embedded in Epon 618. Ultrathin sections were processed according to the postembedding procedure. Briefly, ultramicrocut was made by Leica ultramicrotome to collect 60nm-thick sections. The sections were mounted on formvar-coated copper grids, incubated in uranyl acetate- acetate lead double electron stain, and observed for brain vascular endothelial cells.
2.10 Western blotting
According to K.N. Nam [26] and Cao Huang [27], brain cortex in peri-ischemic were lysed on ice in lysis buffer [50 mm Tris-HCl (pH 8.2), 0.5 M saccharose, 10 mMHEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 1 mM EDTA, 10% (v/v) glycerine, 1 mM DTT, 1 mM PMSF, 10μg/mL Aprotinin, and 5μg/mL Leupeptin]. After centrifugation at 16,000 ×g for 10 minutes. Protein content in cleared lysate was determined by Bradford Assay. Lysate samples containing 40μg of protein were fractionated by SDS-10% polyacrylamide gel electrophoresis and then electroblotted onto PCVF membranes. The membranes were probed with primary antibodies as EPO, VEGF (1:250, 1:300, Satcruze Co., USA), and β-actin(1:200, Booster Biotech. Co., Wuhan, China), then incubated with the horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG (1:2500; Booster Biotech. Co., Wuhan, China), the PVDF membrane was put into DAB fluid for coloration. Immunoreactivity was digitally scanned by ScanMaker E6 system and quantified using Quantity one 4.5.1 (Bio-Rad) software. β-actin was used as an internal control for all Western blotting.
2.11 Image and data analysis
After capturing images with a digital camera, quantification of the results from immunohistochemistry, immunofluorescence, western blotting was performed with Image Pro Plus Version 6.0. software. EPO or VEGF-positive cells were counted at five different fields in the inner border of the peri-ischemic cortex in five sections per rat, the total number of EPO or VEGF-positive cells per image (cells/cm 2, objective × 20) was calculated by an observer blind to the experimental treatment. In each section, five peri-ischemia cortical areas outside labeled neurons were chosen randomly to obtain an average value for the subtraction of background by an observer blind to the experimental treatment.
2.12 Statistical analyses
Data were expressed as mean ± S.E.M. All data were analyzed by one-way analysis of variance (ANOVA) using SPSS 11.0 software. A value of p < 0.05 was considered statistically significant.
3. Results
3.1 Catalpol improve sensorimotor performance in stroke rats
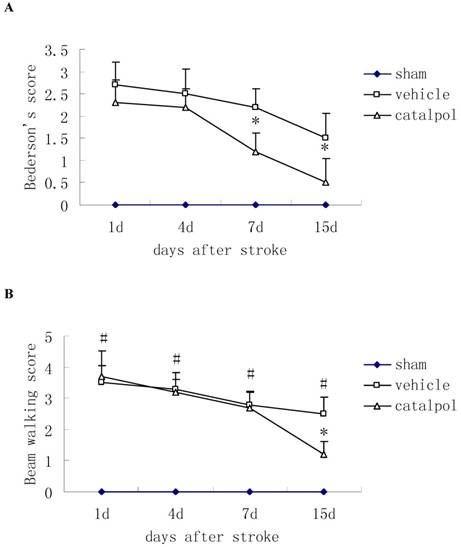
Post-stroke administration of catapol reduced Bederson's score in rats, indicating improved motor function relative to vehicle control, catapol significantly reduced Bederson's scores in rats at 7 and 15 days after stroke (Fig. 1A).
The Beam walking test was used to measure sensorimotor function. During the course of treatment, beam walking scores were reduced; by day 15 following stroke, score reductions in catapol-treated versus vehicle-treated animals reached statistical significance (P<0.05) (Fig. 1B).
Effects of intraperitoneal injection with catalpol on sensorimotor performance in post-surgical rats at days 1, 4, 7 and 15. (A) Post-stroke treatment with catalpol reduced Bederson's score in stroke rats and (B) decreased beam working score in stroke rats. The data are presented as mean ± SE. * p<0.05 compared with vehicle group, #p<0.01 compared with sham operation group.
3.2 Effect of catalpol on the vascular pattern of the cerebral cortex surface
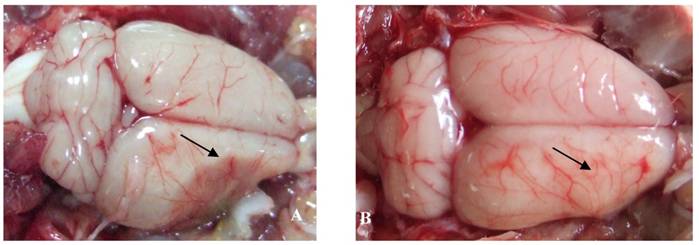
The effect of catalpol on the vascular pattern of the rat cerebral cortex surface was examined following pMCAO. In vehicle-treated animals, liquefactive necrosis of the brain was observed 15 days after pMCAO. Brains from vehicle-treated animals exhibited a few deranged microvessels (Figure 2A). By contrast, the focus of cerebral ischemia was near normal in catalpol-treated animals; the brains of these animals exhibited more branched vessels crossing and gathering radially to the surface of cerebral ischemia. All these vessels arborized to form a continuous network of small blood vessels (Figure 2B).
The vascular pattern in cerebral cortical surface in rats 15 days after pMCAO. (A) In the vehicle-treated group, the pale brain surface had few vessel branch points, infarct areas were characterized by liquefactive necrosis, cortical surface vessels were scarce and rearranged, several discontinued vascular structures were observed, and the radial patterns were lost. (B) In the catalpol-treated group, brain surface vessel branch points increased obviously, vascular structures continued, focus on the infarct area was present, and the vessel radial patterns and brain tissue infarct area were close to normal. Arrow points to vascular structures around the ischemia area.
3.3 Catalpol enhanced brain angiogenesis in the peri-infarcted area of the cortex
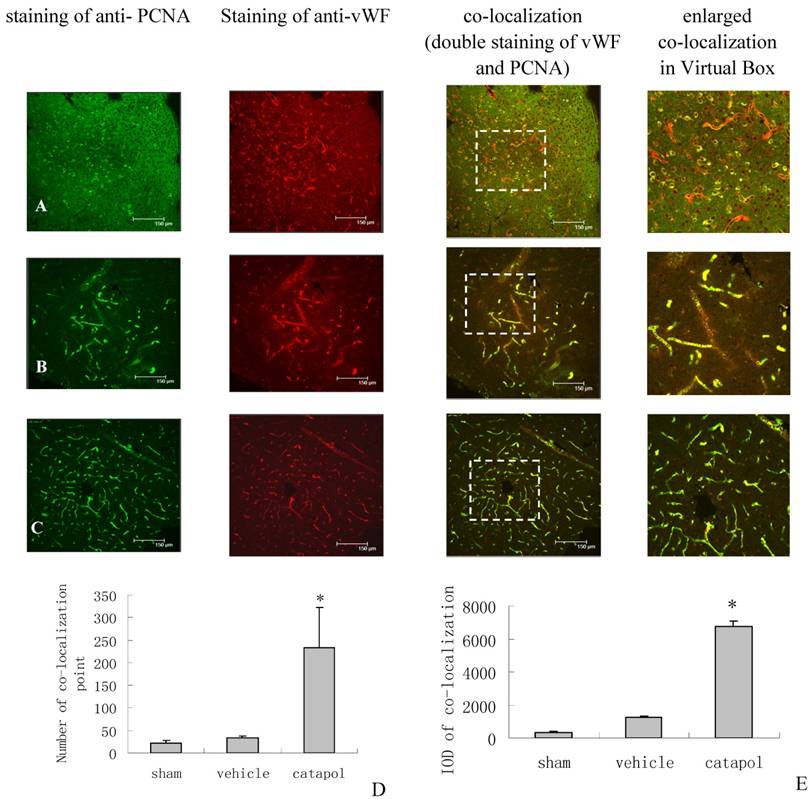
The effect of catalpol on angiogenesis was then examined by immunostaining of brain sections for von Willebrand Factor (vWF), a marker of endothelial cells, and for proliferating cell nuclear antigen (PCNA), a marker of cell proliferation. VWF and PCNA co-localization points in the sham operation group were scarcly observed (Fig 3A).Compared with the vehicle-treated group (34 ± 3.25) (Fig 3B), the number of VWF and PCNA co-localization points in the catalpol-treated group (Fig 3C and D) increased significantly (p < 0.05). The number of vWF and PCNA co-localization points in the catalpol-treated group (Fig 3C and D) was 233.67 ± 89.51, nearly 6 times that in the vehicle group (p < 0.01) (Fig 3B and D). These results also agreed with results obtained from integral optical density (IOD) analyses in the vWF-PCNA co-localization area (Fig 3E). These data demonstrate that catalpol plays an important role in cerebral ischemia angiogenesis.
3.4 Effects of catalpol on brain capillary endothelial cells (BCECs)
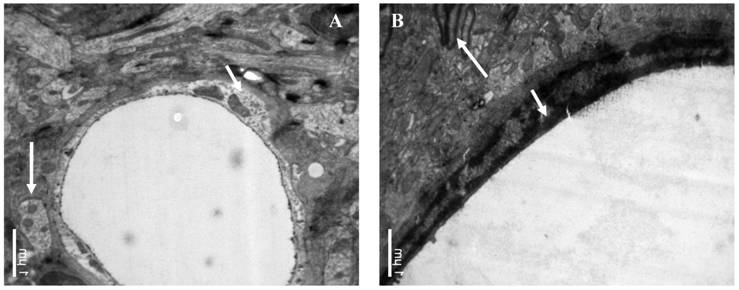
The influence of catapol treatment on BCEC microstructure following pMCAO were examined by transmission electron microscopy. Compared to vehicle cotntrol (Fig 4A), catalpol significantly reduced BCEC edema (Fig 4B). The number of chondriosomes in the catalpol-treated group was higher than that in the vehicle group and close to normal levels.
Angiogenesis surrounding ischemic cortical area as demonstrated by immunocytochemistry and laser scanning confocal microscopy. The effects of Catalpol on angiogenesis were indicated by double-staining for VWF, a marker of endothelial cells and for proliferating cell nuclear antigen (PCNA), a marker of cell proliferation. Co-labeling of PCNA (green) and VWF (red) demonstrates angiogenesis, i.e., endothelial proliferation in the capillaries, in the peri-infarcted area at 15 days after pMCAO. Co-localization of PCNA and VWF is yellow. (A) Sham operation group, (B) Vehicle-treated group, (C) Catalpol-treated group. Bars = 150 μm in A, B, and C. This analysis demonstrated that few vessels were double-stained by VWF and PCNA in sham-operated rats (A), but significant remodeling of the microvessel network occurred in the infarcted hemisphere and the number of vessels with small diameter and short segment increased at 15 days after the stroke. More vessels were double-stained for VWF and PCNA in catalpol-treated group (C). Statistical analyses are shown in the graph of the number of vessels co- labeled for PCNA and VWF (D). The results above agreed with the results of IOD analyses in the co-localization area (E). (*P < 0.01).
Ultrastructural observations of brain capillary endothelial cells (BCECs). In the vehicle-treated group (A), BCEC edema, chromatin rarefaction (short arrow), and chondriosome swelling (long arrow) were observed. (B) In the catalpol group, BCECs, pykno-chromatin (short arrow), and chondriosome number and shape (long arrow) were close to normal or near normal. Bars = 1μm.
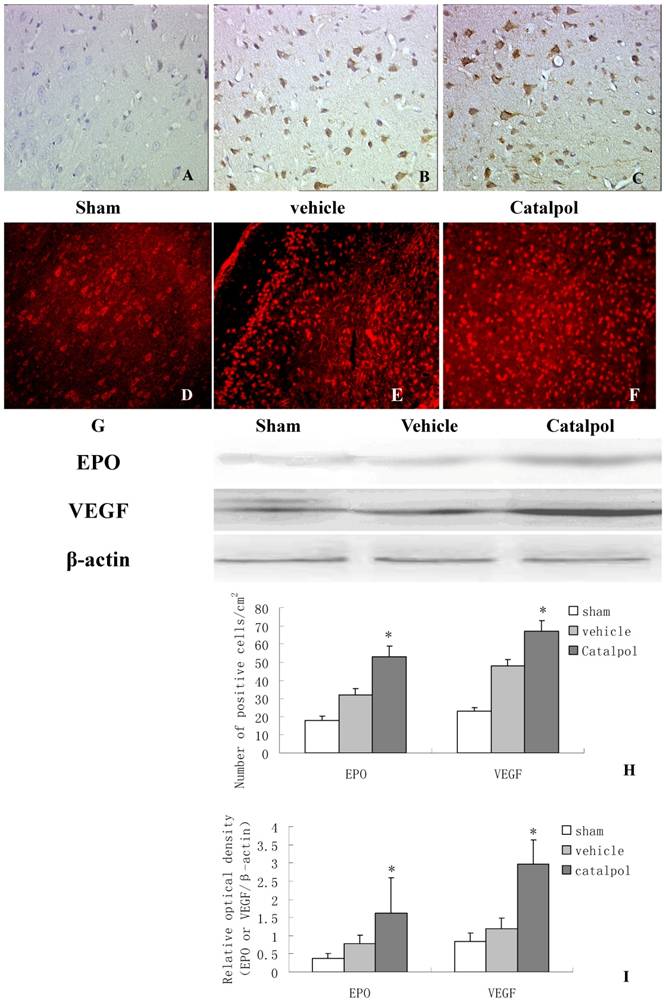
3.5 Catalpol upregulated EPO and VEGF expression in rat brain following pMCAO
To monitor the influence of catapol on EPO and VEGF expression, we performed immunohistochemical analyses of brain sections derived from rats following pMCAO. EPO and VEGF- positive cells were detected in the cell membrane and cytoplasm. Few EPO positive cells were observed in brain sections derived from sham-treated animals (Fig 5A). Compared to vehicle-treated animals (Fig 5B), brain sections derived from catapol-treated animals exhibited significantly increased EPO expression (Fig 5C). Statistical analyses revealed 7.2 ± 1.40 positive cell/cm2 vs 15.3 ± 2 positive cell/cm2 in vehicle-treated versus catapol-treated animals (p< 0.05). Similar results were obtained by western blot analyses (Fig 5G and I); As for VEGF, immunofluorescence analyses demonstrated that catalpol at the dose of 5mg/ kg significantly upregulated VEGF expression compared with the vehicle group; the number of positive cell/cm2 vehicle-treated versus catapol-treated animals was 8± 1.6 vs 17 ± 2.5; (p < 0.01) (Fig. 5E,F,H). Once again, similar results were obtained by western blot analyses (Fig 5G and I). Statistical analyses were shown in Fig 5I.
4. Discussion
There are three principal findings emerged from the present study. Firstly, catalpol treatment improved neurofunction after stroke, as evidenced by enhanced scores in the beam walking test designed to evaluate sensorimotor reflexes, motor strength and coordination. Secondly, our results show that catapol enhances brain angiogenesis following stroke without worsening stroke brain edema. Finally, we demonstrate that the ameliorative effects of catalpol on stroke brain are mediated by enhanced expression of EPO and VEGF. Our findings thus provide new insights into the likely regulatory mechanisms of catapol.
Ischemic stroke is a serious disease caused by a thrombus (blood clot), which can result in permanent neurological damage, complications, and even death [26].The standard method of treatment is to dissolve the clot and restore blood flow in the blocked vessel. The drug TPA is approved for this use; however, TPA is used only 3-6 hours after stroke, and the more rapidly blood flow is restored to the brain, the fewer brain cells die [27]. Recent research has suggested that an alternative approach to restore blood flow is to promote angiogenesis in regions surrounding the ischemic brain.
As one of the most potentangiogenic factors, VEGF is up-regulated by focal cerebral ischemia not only in animal models but also in human patients [10-11] as an angiogenic, neurotrophic, and neuroprotective factor [28-30]. VEGF also plays a vital role during neural [29,31] and vascular remodeling [32-33] after stroke. Our results showed that ischemia induced VEGF expression, which was not enough for vascular remodeling, but catalpol treatment increased VEGF expression together with increased microvessel formation, healed the ischemic brain, and improved neurobehavioral score. These data suggested that catalpol stimulated brain angiogenesis after stroke by increasing the secretion of endogenous VEGF.
Catalpol upregulated EPO and VEGF expression in pMCAO rat brains. Immunohistochemistry results showing neurons with EPO (A, B, C, 200×) and VEGF (D, E, F, 200×) in the peri-infarcted area of a pMCAO rat, a sham-operated rat (A & D), a vehicle-treated rat (B & E), and a catalpol-treated rat (C & F). EPO and VEGF expression detected by western blot showed in (G). The internal control was β-actin. Vs vehicle group *p < 0.05. The experiments repeated three times and 6 rats used in each group. Statistical bars shown as H and I respectively.
Moreover, VEGF, known as vascular permeability factor, is associated with angiogenesis, vascular permeability and neuroprotection. VEGF enhances angiogenesis, but worsens rather than improves cerebral haemodynamics after stroke [34-35]. Low-dose VEGF aggravates hemorrhagic transformation [36]. Inhibition of endogenous VEGF by topical application of anti-VEGF antibody in the ischemic cortex decreased the blood-brain barrier (BBB) disruption [37]. VEGF is in part responsible for the BBB disruption during the early stage of focal cerebral ischemia [37]. These adverse effects suggested that caution should be heeded when considering the use of only VEGF for stroke patients [38].
EPO is a pleiotropic factor [39]. EPO and EPO receptor (EPO-R) are expressed in neurons, astrocytes, and endothelial cells after focal permanent ischemia in mice. EPO is a neuroprotective factor [40-41], which improves functional recovery and reduces neuronal apoptosis [42] and inflammation [43]. EPO has a mitogenic and positive chemotactic effect on endothelial cells and endothelial progenitor cells [44-45]. It stimulates angiogenesis in vitro and in vivo [18,40]. Therefore, the EPO/EPO-R system is implicated in the process of neuroprotection [46] and restructuring (such as angiogenesis) after ischemia [18,46]. In this study, catalpol increased EPO expression in neurons and endothelial-like cells surrounding the vessels. Therefore, catalpol may improve neurofunction and neural and vascular remodeling after stroke by activating EPO.
In this study, we observed that catalpol increased VEGF expression but did not increase vascular permeability (Fig 3b), which may be related to the simultaneous increase of EPO expression in the brain. EPO reduces the side effects of VEGF, which protects the BBB against VEGF-induced permeability in vitro [19]. EPO and VEGF promote neural outgrowth (Bocker-Meffert et al., 2002) and exhibit equal angiogenic potential [18]. Furthermore VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis [47], and EPO-R promotes VEGF expression and angiogenesis in peripheral ischemia in mice [48]. Therefore, the advantageous reciprocal interactions between EPO and VEGF on angiogenesis, which can be induced by catapol may be an effective way to treat stroke patients. Brain injury helps EPO to cross the BBB [49-50], which may then produce a synergistic effect with VEGF. Catalpol is the effective component of Rehmannia glutinosa, which can increase blood level of EPO (data not shown). Moreover, catapol can cross the BBB, as detected by HPLC even in normal rats [51].
In conclusion, our data suggested that catalpol modulated angiogenesis through increased EPO and VEGF after stroke, which may be the mechanism by which catalpol reduced ischemic neuronal damage and enhanced functional recovery. Taken together, these data suggested that catalpol may improve collateral circulation and provide impact on stroke patients through new blood vessel formation. Future research may elucidate the specific signaling pathways through which catalpol increases angiogenesis.
Acknowledgements
This work was supported by grants from the Fundamental Research Funds for the Central Universities (No.XDJK2009C081) and Southwest University Dr. Foundation (No.104290-20710906) and NSFC General Projects (No.81073084).
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Beenken S.W, Bland K.I. Biomarkers for breast cancer. Minerva Chir. 2002;57:437-448
2. Albers G.W, Goldstein L.B, Hall D. et al. Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. Jama. 2001;286:2673-2682
3. Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328-335
4. Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347-360
5. Krupinski J, Kaluza J, Kumar P. et al. Some remarks on the growth-rate and angiogenesis of microvessels in ischemic stroke. Morphometric and immunocytochemical studies. Patol Pol. 1993;44:203-209
6. Craven R. Go with the flow. Nature review. 2002;3:585
7. Morris DC, Yeich T, Khalighi MM. et al. Microvascular structure after embolic focal cerebral ischemia in the rat. Brain Res. 2003;972:31-37
8. Hawkins B.T, Davis T.P. The blood-brain barrier/ neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173-185
9. Lo E.H, Broderick J.P, Moskowitz M.A. tPA and proteolysis in the neurovascular unit. Stroke. 2004;35:354-356
10. Krupinski J, Kaluza J, Kumar P. et al. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794-1798
11. Krupinski J, Stroemer P, Slevin M. et al. Three-dimensional struc-ture and survival of newly formed blood vessels after focal cerebral ischemia. Neuroreport. 2003;14:1171-1176
12. Li D.Q, Li Y, Liu Y. et al. catalpol prevents the loss of CA1 hippocampal neurons and reduces working errors in gerbils after ischemia-reperfusion injury. Toxicon. 2005;46:845-851
13. Li D.Q, Bao Y.M, Li Y. et al. catalpol modulates the expressions of Bcl-2 and Bax and attenuates apoptosis in gerbils after ischemic injury. Brain Res. 2006;1115:179-185
14. Liu J H.Q, Zou W. et al. catalpol increases hippocampal neurop-lasticity and up-regulates PKC and BDNF in the aged rats. Brain Res. 2006;23:68-79
15. Li D.Q, Bao Y.M, Zhao J.J. et al. Neuroprotective properties of catalpol in transient global cerebral ischemia in gerbils: dose-response, therapeutic time-window and long-term effi-cacy. Brain Res. 2004;1029:179-185
16. Li D.Q, Duan Y.L, Bao Y.M. et al. Neuroprotection of catalpol in transient global ischemia in gerbils. Neurosci Res. 2004;50:169-177
17. H.F Zhu, Dong Wan, Yong Luo. et al. catalpol up-regulated GAP-43 protein expression and improved behavior outcome in focal cerebral ischemia rats. Chinese Pharmacological Bulletin. 2007;23:1231-1236
18. Jaquet K, Krause K, Tawakol-Khodai M. et al. Erythropoietin and VEGF exhibit equal angiogenic potential. Microvasc Res. 2002;64:326-333
19. Martinez-Estrada O.M, Rodriguez-Millan E, Gonzalez-De Vicente E. et al. Erythropoietin protects the in vitro blood-brain barrier against VEGF-induced permeability. Eur J Neurosci. 2003;18:2538-2544
20. Chen P, Goldberg DE, Kolb B L.M. et al. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci U S A. 2002;99:9031-9036
21. Bederson L.H. Pitts, and Daves RL. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472-476
22. G. Stanley K. Harvey V. Slivova, et al, Ganoderma lucidum sup-presses angiogenesis through the inhibition of secretion of VEGF and TGF-beta1 from prostate cancer cells. Biochem Biophys Res Commun. 2005;330:46-52
23. Takata K, Yamauchi H, Tatsuno H. et al. Is the ipsilateral cortex surrounding the lesion or the non-injured contralateral cortex important for motor recovery in rats with photochemically induced cortical lesions? Eur Neurol. 2006;56:106-112
24. Zhang ZG Z.L, Tsang W, Soltanian-Zadeh H. et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379-392
25. Acker T, Beck H, Plate K.H. Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and -2 suggests an important role of astrocytes in cerebellar vascu-larization. Mech Dev. 2001;108:45-57
26. K.N. Nam et al. Genipin inhibits the inflammatory response of rat brain microglial cells. International Immunopharmacology. 2010;10:493-499
27. Cao Huang, Pedro Yuxing Xia, Hongxia Zhou. Sustained Expres-sion of TDP-43 and FUS in Motor Neurons in Rodent's Life-time. Int J Biol Sci. 2010;6:396-406
28. Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochimica et Biophysica Acta. 2009. 1802(1):80-91
29. The European Cooperative Acute Stroke Study (ECASS). Throm-bolysis with Alteplase 3 to 4.5 Hours after Acute Ischemic Stroke. New England Journal of Medicine. 2008;359(13):1317-1329
30. Chen Y.H, Wu H.L, Chen C.K. et al. Angiostatin antagonizes the action of VEGF-A in human endothelial cells via two distinct pathways. Biochem Biophys Res Commun. 2003;310:804-810
31. Jin K, Mao X.O, Greenberg D.A. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobio. 2006;66:236-242
32. Yasuhara T, Shingo T, Kobayashi K. et al. Neuroprotective effects of vascular endothelial growth factor (VEGF) upon dopaminergic neurons in a rat model of Parkinson's disease. Eur J Neurosci. 2004;19:1494-1504
33. Wang Y, Jin K, Mao X.O. et al. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neu-romigration. J Neurosci Res. 2007;85:740-747
34. Kanno S, Oda N, Abe M. et al. Roles of two VEGF receptors, Flt-1 and KDR, in the signal transduction of VEGF effects in human vascular endothelial cells. Oncogene. 2000;19:2138-2146
35. Louissaint AJr, Rao S, Leventhal C. et al. Coordinated inte-raction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945-960
36. Wang Y, Kilic E, Kilic U. et al. VEGF overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain. 2005;28:52-63
37. Zhang Z.G, Zhang L, Jiang Q. et al. VEGF enhances angioge-nesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829-838
38. Abumiya T, Yokota C, Kuge Y. et al. Aggravation of hemorrhagic transformation by early intraarterial infusion of low-dose vascular endothelial growth factor after transient focal cerebral ischemia in rats. Brain Res. 2005;1049:95-103
39. Chi O.Z, Hunter C, Liu X. et al. Effects of anti-VEGF antibody on blood-brain barrier disruption in focal cerebral ischemia. Exp Neurol. 2007;204:283-287
40. Manoonkitiwongsa PS S.R, McCreery DB, Whitter EF. et al. Neu-roprotection of ischemic brain by vascular endothelial growth factor is critically dependent on proper dosage and may be compromised by angiogenesis. J Cereb Blood Flow Metab. 2004;24:693-702
41. Kitadai Y, Sasaki A, Ito M. et al. Helicobacter pylori infection influences expression of genes related to angiogenesis and invasion in human gastric carcinoma cells. Biochem Biophys Res Commun. 2003;311:809-814
42. Marti H.H. Erythropoietin and the hypoxic brain. J Exp Biol. 2004;207:3233-3242
43. Sola A, Rogido M, Lee B.H. et al. Erythropoietin after Focal Cerebral Ischemia Activates the Janus Kinase-Signal Trans-ducer and Activator of Transcription Signaling Pathway and Improves Brain Injury in Postnatal Day 7 Rats. Pediatr Res. 2005;57:481-487
44. Kretz A, Happold C.J, Marticke J.K. et al. Erythropoietin promotes regeneration of adult CNS neurons via Jak2/Stat3 and PI3K/AKT pathway activation. Mol Cell Neurosci. 2005;29:569-579
45. Zhang J, Li Y, Cui Y. et al. Erythropoietin treatment improves neurological functional recovery in EAE mice. Brain Res. 2005;1034:34-39
46. Anagnostou A, Lee E.S, Kessimian N. et al. Erythropoietin has a Mitogenic and Positive Chemotactic Effect on Endothelial Cells. PNAS. 1990;87:5978-5982
47. Burbelo P.D, Ching K.H, Mattson T.L. et al. Rapid antibody quantification and generation of whole proteome antibody response profiles using LIPS (luciferase immunoprecipitation systems). Biochem Biophys Res Commun. 2007;352:889-895
48. Bernaudin M, Marti H.H, Roussel S. et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643-651
49. Tam B.Y, Wei K, Rudge J.S. et al. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nat Med. 2006;12:793-800
50. Nakano M, Satoh K, Fukumoto Y. et al. Important role of eryt-hropoietin receptor to promote VEGF expression and angi-ogenesis in peripheral ischemia in mice. Circ Res. 2007;100:662-669
51. Brines M.L, Ghezzi P, Keenan S. et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain in-jury. Proc Natl Acad Sci U S A. 2000;97:10526-10531
52. Statler P.A, McPherson R.J, Bauer L.A. et al. Pharmacokinetics of High-Dose Recombinant Erythropoietin in Plasma and Brain of Neonatal Rats. Pediatr Res. 2007;61:671-675
53. He Yao, Zhu Huifeng, Li Wanyu. et al. HPLC determination of catapol in cerebrospinal fluid of rat. China Journal of Chinese Materia Medica. 2009;34(13):1717-171
Author contact
![]() Corresponding author: Professor Xiao-Yu Xu, Laboratory of Molecular Pharmacology, School of Pharmaceutical Science & School of Chinese Medicine, Southwest University, Chongqing 400715, China. Tel: +86-23-6825-0761; Fax: +86-23-6825-1225; E-mail: zhfbscicn; xxy0618 com
Corresponding author: Professor Xiao-Yu Xu, Laboratory of Molecular Pharmacology, School of Pharmaceutical Science & School of Chinese Medicine, Southwest University, Chongqing 400715, China. Tel: +86-23-6825-0761; Fax: +86-23-6825-1225; E-mail: zhfbscicn; xxy0618 com

 Global reach, higher impact
Global reach, higher impact