ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2016; 12(9):1150-1154. doi:10.7150/ijbs.15747 This issue Cite
Review
Epithelial Sodium Channels in Pulmonary Epithelial Progenitor and Stem Cells
1. Institute of Lung and Molecular Therapy, Xinxiang Medical University, Xinxiang, Henan 453003, China;
2. School of Public Health, Xinxiang Medical University, Xinxiang, Henan 453003, China.
3. Department of Cellular and Molecular Biology, University of Texas Health Science Center at Tyler, Tyler, Texas 75708, USA;
4. Texas Lung Injury Institute, University of Texas Health Science Center at Tyler, Tyler, Texas 75708, USA.
Abstract
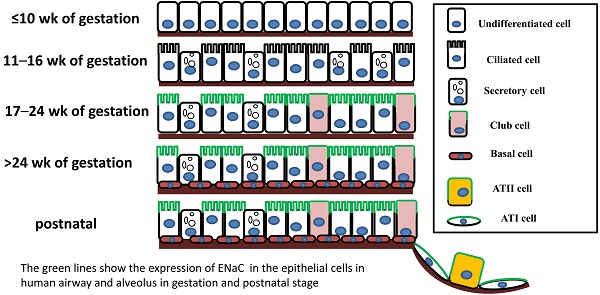
Regeneration of the epithelium of mammalian lungs is essential for restoring normal function following injury, and various cells and mechanisms contribute to this regeneration and repair. Club cells, bronchioalveolar stem cells (BASCs), and alveolar type II epithelial cells (ATII) are dominant stem/progenitor cells for maintaining epithelial turnover and repair. Epithelial Na+ channels (ENaC), a critical pathway for transapical salt and fluid transport, are expressed in lung epithelial progenitors, including club and ATII cells. Since ENaC activity and expression are development- and differentiation-dependent, apically located ENaC activity has therefore been used as a functional biomarker of lung injury repair. ENaC activity may be involved in the migration and differentiation of local and circulating stem/progenitor cells with diverse functions, eventually benefiting stem cells spreading to re-epithelialize injured lungs. This review summarizes the potential roles of ENaC expressed in native progenitor and stem cells in the development and regeneration of the respiratory epithelium.
Keywords: amiloride-inhibitable sodium channels, mesenchymal stem cells, proliferation, differentiation, pluripotent stem cells.

 Global reach, higher impact
Global reach, higher impact