10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2017; 13(6):723-734. doi:10.7150/ijbs.19642 This issue Cite
Research Paper
Exosomes from Melatonin Treated Hepatocellularcarcinoma Cells Alter the Immunosupression Status through STAT3 Pathway in Macrophages
1. Department of Oncology, the First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui, China;
2. Department of Pharmacy, the First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui, China;
3. Institute of Clinical Pharmacology, Anhui Medical University, Hefei 230032, Anhui, China;
4. Institute for Liver Diseases of Anhui Medical University, Hefei 230032, Anhui, China.
* These authors contributed equally to this work.
Received 2017-2-13; Accepted 2017-3-27; Published 2017-5-16
Abstract
Immunosuppression is a significant factor in the progression of tumor invasion and metastasis. Melatonin, a well-known hormone, has certain cytotoxic and immune regulatory effects to inhibit tumor function. Exosomes are small membrane vesicles released by many kinds of cells, which contain different macromolecules, such as mRNAs and microRNAs (miRNAs), and proteins that can mediate communications between cells. Tumor-derived exosomes may cause immunosuppression, however, it is unknown whether melatonin can attenuate an immunosuppressive status by altering the function of tumor-derived exosomes. In the present study, we evaluated the effects of hepatocellularcarcinoma-derived exosomes (Exo-con) and exosomes derived from hepatocellularcarcinoma cells treated with 0.1 mM melatonin (Exo-MT), on the expression of inflammatory factors and programmed death ligand 1(PD-L1) by co-culturing Exo-con and Exo-MT, respectively, with macrophages differentiated from THP-1 cells or RAW264.7 cells. Our in vitro results indicate that Exo-MT can downregulate the expression of PD-L1 on macrophages while Exo-con can upregulate the expression of PD-L1 through flow cytometry and immunofluorescence analysis. In addition, Exo-con upregulates the secretion of cytokines, such as IL-6, IL-10, IL-1β, and TNF-α in macrophages. Accordingly, Exo-MT could attenuate the high expression of these inflammatory cytokines. Furthermore, in vivo experiments confirmed the results found in vitro. PD-L1 expression and cytokine secretion were lower in the Exo-MT group compared with those in the Exo-con group. Working to identify a specific mechanism, our research shows that Exo-MT decreases STAT3 activation compared to the Exo-con group. In summary, we found exosomes from melatonin treated hepatocellularcarcinoma cells alters the immunosupression status through STAT3 pathway in macrophages. Our study may provide a new avenue to investigate the mechanisms of melatonin in regulating an immunosuppressive status.
Keywords: Hepatoma cell, melatonin, exosomes, PD-L1, cytokines, macrophages.
Introduction
Hepatocellular carcinoma (HCC), one of the malignant cancers which mortality ranks as the fifth in the world, continues to have a great extent of drug resistance and lack of effective treatment [1]. In the last several decades, many patients have succumbed to liver cancer despite extraordinary advances in the technologies related to diagnosis and therapeutic modalities [2]. The efficacy of traditional radiotherapy and chemotherapy methods for treating liver cancer has reached a plateau in recent decades. Therefore, there is an urgent need to develop new techniques in cancer therapy, which has become a prominent area in clinical research. Recently, immunotherapy was shown to improve therapeutic effects as an adjuvant therapy for cancer patients [3]. Immunotherapy can reduce the treatment side effects, increase the therapeutic effect and improve quality of life [4]. Thus, studies that address how to improve the immunotherapeutic effects in liver cancer patients has becomes more important and deserves further consideration.
The tumor microenvironment has been identified and comprises tumor cells, immune cells such as T cells, T regulatory cells (Tregs), dendritic cells, macrophages, cancer-associated fibroblasts or stromal cells, leading to cross activation or suppression of each cell type [5]. Each of these cellular components contributes to the treatment response and patient prognosis. In the HCC microenvironment, effector T cells, Tregs, and macrophages include the majority of immune cell components [6]. Researches have shown that there are different levels of immunosuppression in these immune cells that is related to the cross talk between tumor and immune cells. Whether there is a method that not only improves the function of immune cells in the microenvironment, but also can suppress tumor cells proliferation and relieve the immunosuppression in immune cells is unknown. Inhibiting the cross-talk between tumor cells and immune cells may be an ideal treatment strategy for liver cancer.
Melatonin (N-acetyl-5-methoxytryptamine) is an indolamine that is synthesized and secreted primarily by the pineal gland, and has important functions, such as activating antioxidant enzymes, enhancing anti-aging properties [7-9], and possessing anti-tumor activities [8, 10-14]. A large amount of evidence suggests that melatonin plays an important role in immunoregulation and anti-inflammatory responses [15]. Melatonin can inhibit the production of pro-inflammatory cytokines help to reduce inflammation, which is essential in promoting tumor invasion in the tumor microenvironment [16]. Our previous studies found melatonin can inhibit liver cancer cell proliferation, invasion and metastasis and has a synergistic effect with traditional chemotherapy drugs [17-19]. However, whether melatonin can block the cross-talk between tumor cells and immune cells in microenvironment and the mechanism involved in this process are unknown.
Exosomes, small membrane vehicles sized in 30-100 nm, contain a wide range of functional proteins, mRNAs, and miRNAs [20]. Recently, we have found exosomes play an important role in mediating cell-to-cell communication [21]. Exosomes secreted by tumor cells can transfer metastatic potential to the recipient cells by changing the immune status of the microenvironment to achieve immune escape of tumor cells [22]. Thus, exosomes have been identified as potent communicators between tumor cells and their microenvironment, thus contributing to an immunosuppressive status. If our results indicate that melatonin can exert anti-tumor function, as well as attenuate the immunosuppressive status in the microenvironment at the same time, these data will certainly deepen our understanding of the anti-tumor function of melatonin. This data may provide a more important theoretical basis for increasing melatonin in the treatment of liver cancer.
The main purpose of the current study was to examine the effect of exosomes derived from melatonin treated hepatocellularcarcinoma cells on macrophages function. We report here that Exo-MT can significantly decrease the expression of PD-L1 and inhibit cytokine secretion on macrophages while Exo-con had the opposite effect. Furthermore, we found Exo-con and Exo-MT regulate PD-L1 expression and cytokine secretion in macrophages through the STAT3 signaling pathway.
Materials and Methods
Cell Culture
The human HCC cell lines HepG2 and Bel-7402 (Cell Bank of the Chinese Academy of Sciences, Shanghai, China) were cultured in high glucose Dulbecco's Modified Eagle's Medium (DMEM, Wisent, Canada) which was added with fetal bovine serum (10%), penicillin (100U/mL) and streptomycin (100mg/mL). Human monocytic THP-1 cells and the murine RAW264.7 macrophages cell line (Cell Bank of the Chinese Academy of Sciences, Shanghai, China) were cultured in Roswell Park Memorial Institute (RPMI 1640, Gibco, USA) culture medium containing 10% fetal bovine serum. THP-1 monocytes were differentiated into macrophages with 100 ng phorbol 12-myristate 13-acetate (PMA, Sigma, P8139) for 48 h. All cells were cultured at 37°C and with a humidified atmosphere of 5% CO2.
Exosome Isolation and Characterization
Exosomes were isolated from the cell supernatant of untreated cultured HCC cells (Exo-con) or HCC cells treated with melatonin (0.1 mM) (Exo-MT) using ExoQuick Precipitation Solution [23]. ExoQuick-TCTM exosome isolation kits and CD63 antibody were purchased from SBI (System Bioscience, USA). According to the instructions, we firstly collected the cell supernatants, and centrifuged (3000 g, 15 min) to remove cell debris. Then 10 ml of cell supernatant was mixed with 2 ml ExoQuick precipitation solution and incubated overnight at 4°C. Next, the mixture was centrifuged (1500 g, 30 min); the supernatant was discarded, and we centrifuged the sediment again (1500 g, 5 min) to remove excess fluid. Finally we resuspended the sediment with 160 μl PBS and subpackaged before being stored at -80°C. BCA protein assay kit (Beyotime Institute of Biotechnology, China) was used to quantify and standardize the protein concentrations in exosomes. The exosomes are designated as Exo-Con and Exo-MT for simplicity.
Transmission Electron Microscopy (TEM)
TEM was performed to reveal the exosome morphology as previously reported [24]. Briefly, the exosomes were prepared and diluted in PBS (100 μl), and then placed a 20 μl suspension liquid onto formvar carbon-coated copper grids for 1 minute at room temperature. The filter paper was used to removed excess suspension liquid. Next, the 2% phosphotungstic acid (PTA) was used to stain exosomes for 5 minutes at room temperature. Grids were then fixed with 2% glutaraldehyde for 5 minutes, followed by washing with PBS three times. Images were captured under 80 kV with a transmission electron microscope (JEM-1230; Jeol Ltd., Tokyo, Japan).
Immunofluorescence Assay
Immunofluorescence assay was performed to verify that the exosomes could be incorporated by macrophages. Exosomes (100 µl) were dissolved naturally after being removing from -80°C, and labeled with PKH67 (Sigma, Saint Louis, USA) following the manufacturer's procedure. The PKH67-labeled exosomes were co-cultured with macrophages for 12 h. Then the cells were fixed by 4% formaldehyde, and permeabilized with 0.1% Triton. After staining with DAPI, the cells were observed under a confocal microscopy (Leica, German).
Flow Cytometry
THP-1 macrophages were detached from the 12-well plates treated with either Exo-con or Exo-MT. After washing with cold PBS twice, the cells were collected and stained with PE-conjugated anti-human CD274 antibodies (BD Pharmingen). RAW264.7 cells and peritoneal macrophages were stained with FITC-conjugated anti-mouse F4/80 (clone BM8, Biolegend) and APC-conjugated anti-mouse CD274 antibodies (clone 10F.9G2, Biolegend) according to a standard flow cytometry staining protocol. The cells were analyzed by flow cytometry (BD Biosciences) according to the manufacturer's procedure. Flow cytometry data were analyzed using FlowJo (Tree Star) software.
A mouse cytokine bead array (CBA) kit was used to measure IL-10, IL-6, IFN-γ, monocyte chemoattractant protein-1 (MCP-1/CCL2), and TNF-α secretion in the supernatant of RAW264.7 and peritoneal macrophages. A human cytokine bead array (CBA) Kit was used to measure IL-6, IL-8, IL-10, IL-1β, and TNF-α in the supernatant of THP-1 macrophages. The data were acquired using a BD FACS Canto II (BD Biosciences, San Diego, CA) and analyzed using FCAP Array Soft Flow USA (BD Biosciences).
Immunocytochemistry Experiment
The immunocytochemistry experiment was performed using THP-1 derived macrophages co-cultured with either Exo-con or Exo-MT. Briefly, 4% paraformaldehyde was used to fix the cells, 0.1% Triton X-100 was used to permeabilize, and the cells were blocked with 10% normal goat serum for 30 min. The cells were then incubated with a monoclonal antibody E1L3N (Cell Signaling Technology, Cambridge, UK) at 4˚C overnight. Next, the cells were incubated with a HRP conjugated anti‑rabbit IgG at 37˚C for 1 h and then stained with DAB Detection Kit according to the manufacturer's instructions. Cells were counterstained with hematoxylin and sealed. Finally, the observed images were captured using an Olympus microscope (Olympus Corporation, Tokyo, Japan).
Animal Experiments
Six-week-old female BALB/c nude mice were purchased from Vital River Laboratory Animal Technology Company (Beijing, China). The animals were housed in a special animal facility where the temperature and humidity was controlled and maintained on a 12-h light/dark cycle. All of the animal experiment procedures were approved by Anhui Medical University Animal Care Committee, and performed in accordance with the guidelines from the National Institutes of Health for the care and use of Laboratory Animals. Nine mice were randomly divided into three groups; PBS group, Exo-con group, and Exo-MT group. They were injected with PBS (100 µl), Exo-con (100 µl), and Exo-MT (100 µl), respectively, from tail veins every other day 10 times. At the end of the experimental period, all mice were sacrificed, and peritoneal macrophages were extracted from the peritoneal irrigation fluid of the mice.
Western Blot Analysis
Western blot analysis was performed as previously described [25]. The STAT3 and p-STAT3, Calnexin antibodies (Cell Signaling Technology, USA) were used as primary antibodies for western blot analysis. S3I-201, an inhibitor of STAT3, was purchased from the Selleck Company, USA. And β-actin antibody was purchased from Zhongshan Biotechnology, Beijing, China. Briefly, the cells and exosomal lysates were centrifuged (12,000 g, 10 minutes) at 4°C, and the supernatants were then collected. Each sample protein was loaded on a 10% gel and separated by SDS-PAGE and subsequently transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). Proteins were detected by incubation with a specific antibody at 4°C overnight. The enhanced chemiluminescence reagent (Thermo Fisher, USA) was used to visualize the immunoreactive bands and signals were obtained from an Image Quant™ LAS-4000 Mini Imager (Fuji, Japan). The protein density of each band was determined using Scion Image Software (version 4.0.3.2.) for semi-quantitative analysis.
Statistical Analysis
We used SPSS 16.0 software (SPSS Inc., Chicago, USA) to analyze data. Two-tailed Student's t-test was used to evaluate the difference between the two groups, and three or more comparisons were compared by one-way ANOVA. We independently carried out all the experiments at least three times, and the results are presented as mean ± standard deviation (SD). A value of p < 0.05 was considered statistically significant.
Results
Identification of exosomes with transmission electron microscope and western blot
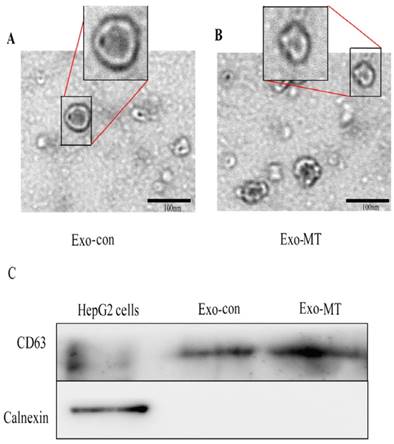
To study the effects of melatonin on the function of exosomes delivered from tumor cells, we first took measures to verify the isolated pellets we obtained from the supernatant by ExoQuick extracting solution were true exosomes. Each isolated pellet was captured under a transmission electron microscope (TEM) and analyzed by western blotting. Representative TEM images of exosomes obtained from the supernatant of HepG2 cells under different conditions (untreated control, treated with 0.1 mM melatonin) are shown in Fig 1A and Fig 1B, respectively. Homogeneous populations of small cup-shaped circular vesicles (30-100 nm in diameter) were observed. Cellular markers of exosomes were abundant, such as Alix, TSG101, CD63, CD81, CD9, and Hsc70. Among them, CD63 is an evolutionarily conserved protein in exosomes and a widely used biomarker for testing exosomes. Calnexin, is a integral protein of the endoplasmic reticulum (ER) that exists in the cell, which should not appear in exosomes. Thus, we used western blot to further confirm that the isolated pellets were exosomes by detecting CD63 and not Calnexin in all the samples derived from HepG2 cells (Fig. 1C). These results support the conclusion that the isolated pellets retrieved were true exosomes. Furthermore, we used the BCA protein assay kit to quantify the protein concentration in the Exo-con (60.04 ±1.45 µg/ml) and Exo-MT (57.32 ±4.14 µg/ml). This result suggests that melatonin has no effect on the amount of exosomes secreted by tumor cells.
Characterization of exosomes isolated from supernatant of samples. Transmission electron microscope (TEM) images of exosomes derived from supernatant samples of HepG2 cells from the control-cultured group (A), 0.1 mM MT group (B), Bar, 100 nm. (C) CD63 and Calnexin expression in HepG2 cells and exosomes isolated from supernatant of samples were assessed by western blot analysis.
Exosomes can be taken up by macrophages
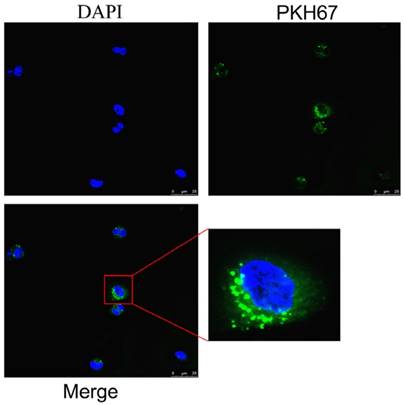
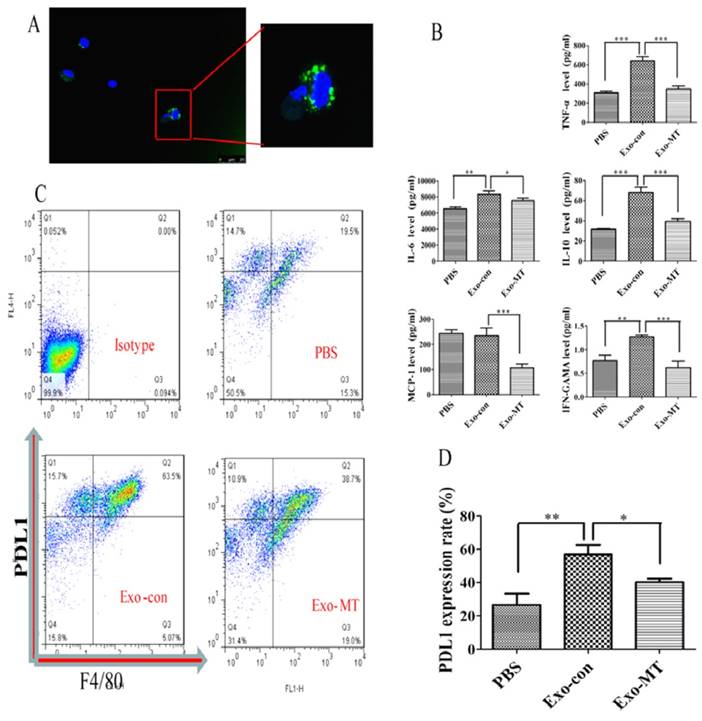
To determine the effects of exosomes on the function of macrophages, we examined whether exosomes could enter macrophages. An immunofluorescence assay was performed by using exosomes labeled with PKH67, a green fluorescence dye. As shown in Fig. 2, green fluorescence was clearly observed in macrophages around nuclei using the confocal microscope, which supported the conclusion that the extracellular exosomes could be taken up by macrophages.
Immunofluorescence assay verified exosomes can be taken in by macrophages. Green fluorescence representative PKH67-labeled exosomes, blue are cell nuclei. Exosomes can be taken in by macrophages as shown in the images and the location of exosomes was found in the cytoplasm around the nucleus.
Exosomes delivered from hepatocellularcarcinoma cells with or without melatonin treatment regulated the expression of PD-L1 and cytokine secretion in macrophages
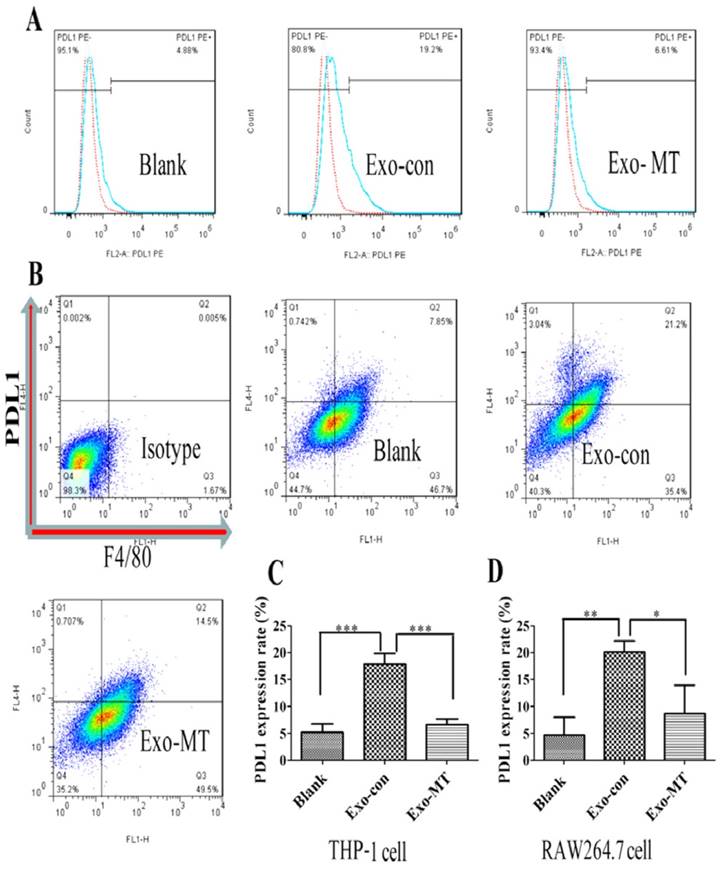
To further test the impact of exosomes on the immune function of macrophages, exosomes delivered from liver cancer cells (Exo-con) and exosomes delivered from melatonin treated liver cancer cells (Exo-MT) were incubated with THP-1 macrophages. Exo-con increased the expression of PD-L1 on the PMA-induced THP-1 differentiated macrophages, approxiamtely four times more than that in the control group as determined by flow cytometry (Fig. 3A). These results suggest Exo-con may suppress the immune status in tumor microenvironment by up-regulating PD-L1 expression on macrophages. Surprisingly, Exo-MT could significantly reverse this effect, reducing PD-L1 expression on macrophages. This phenomenon was further confirmed by using exosomes derived from another HCC cell line Bel-7402 cells (data not shown) and in mouse RAW264.7 macrophages (Fig. 3B).
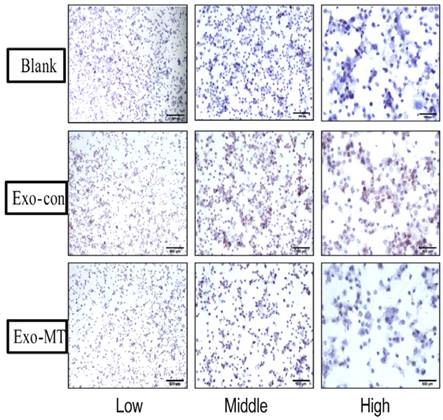
To further confirm these results in vitro, an immunocytochemistry experiment using THP-1 derived macrophages to demonstrate PD-L1 expression was performed. As shown in Fig. 4 the control group had virtually no expression of PD-L1 (no brown color), while the Exo-con group (much more brown color) induced PD-L1 positive expression, which indicated that Exo-con indeed upregulated PD-L1 expression on macrophages. Furthermore, Exo-MT could reverse the upregulation of PD-L1 expression presenting less brown color compared with the Exo-con group regardless of magnification.
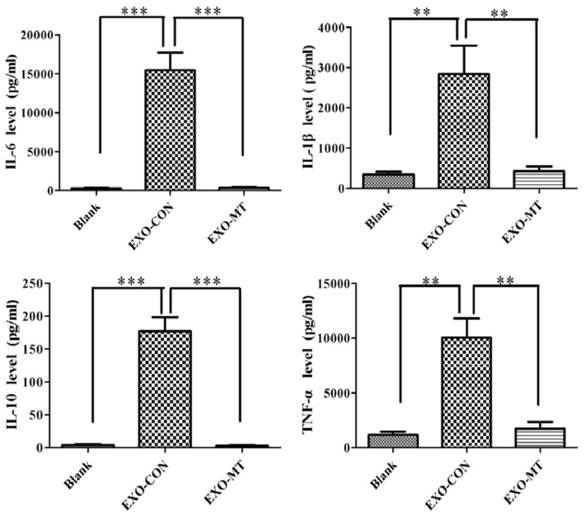
To further understand the impact of exosomes, obtained from tumor cells under different treatments, on the function of macrophages, we quantified the amount of cytokines secreted by THP-1 differentiated macrophages that were treated with either Exo-con or Exo-MT by the cytokine bead array (CBA) inflammatory cytokines kit. As shown in Fig. 5, IL-6, IL-1β, IL-10, and TNF-α were significantly up-regulated in the Exo-con group compared to the control group, while these cytokines were significantly decreased in the Exo-MT group compared to Exo-con group. These finding suggest that exosomes secreted by tumor cells under different conditions can influence the cytokines secretion profile of macrophages.
To further confirm the results we obtained in vitro, we performed experiments in vivo by injecting PBS (100 µl), Exo-con (50 µg/ml, 100 µl), or Exo-MT (50 µg/ml, 100 µl) through the tail vein into mice once every other day 10 times. Peritoneal macrophages were then isolated, and exosomes injected into mice from the tail vein could also enter into peritoneal macrophages (Fig. 6A). Our results indicated that the Exo-con group upregulated PD-L1 expression on macrophages, while the Exo-MT group lower the expression of PD-L1 (Fig. 6B). Moreover, cytokines such as IL-6, TNF-α, IFN-γ, and IL-10, were all up-regulated in the Exo-con group, while Exo-MT group induced less expression of these cytokines compared with Exo-con group. Furthermore, Exo-con did not upregulate MCP-1 levels, while Exo-MT downregulated the MCP-1 level. (Fig. 6C)
PD-L1 expression on THP-1 differentiated macrophages and RAW264.7 macrophages regulated by Exo-con and Exo-MT. THP-1 derived macrophages (Blank Group) to slightly express PD-L1, but when treated with Exo-con, (A) PD-L1 expression was upregulated almost four times over compared with the Blank Group while Exo-MT could reverse the upregulation of PD-L1 expression. In RAW264.7 macrophages, we found similar results, (B) Exo-con upregulated PD-L1 expression, while Exo-MT attenuated the effect. We used SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) for statistical analysis of the flow cytometry analysis results in THP-1 derived macrophages (C) and RAW264.7 macrophages (D) . Data are presented as mean ± SD (error bar) of at least three independent experiments. * and ** represent P < 0.05 and P < 0.01, respectively. Red dotted line represents isotype control; blue dotted line, respectively, represents Exo-con and Exo-MT (A).
Exo-con and Exo-MT regulate PD-L1 and cytokine expression through the STAT3 signal pathway
To further understand the detailed mechanism underlying the alterations on macrophages with exosomes, we examined the STAT3 pathway, which might be involved in the regulation of PD-L1 expression and cytokine secretion through western blot analysis [26, 27]. As shown in Fig. 7A, the active STAT3 transcription factor, phosphorylated STAT3 (p-STAT3), was upregulated in the Exo-con group, while the active form was significantly downregulated in the Exo-MT group compared to the Exo-con group. To establish this mechanism more clearly, we verified that the STAT3 signal pathway was indeed active during exosome incubation by using the STAT3 inhibitor S3I-201 (100 µM) (Fig. 7C). With the addition of the inhibitor PD-L1 expression on macrophages treated by Exo-con was reduced (Fig. 7E), which provided further support that the STAT3 pathway participates in the regulation of PD-L1 expression by exosomes.
Discussion
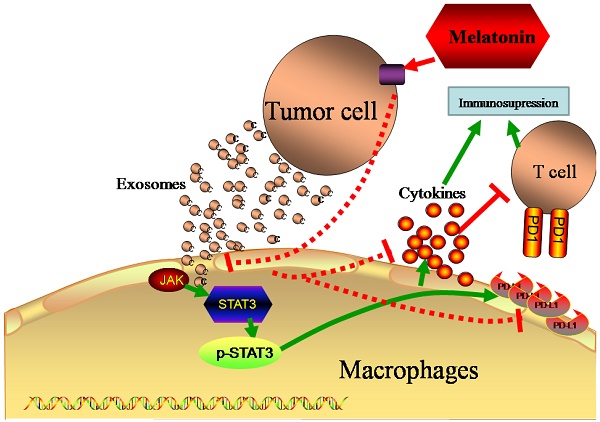
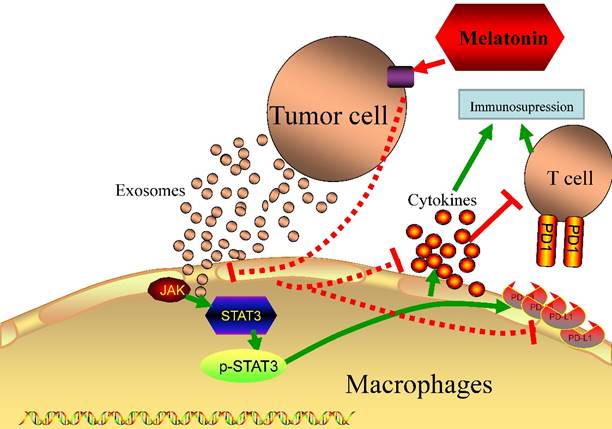
Since an immunosuppressive state is one of the most important factors in tumor progression, it has recently received increased attention in clinical tumor therapy [3], especially in HCC, which lacks efficient therapeutic methods in clinical treatment. In addition, it has been widely reported that tumor cells can cause an immunosuppressive state in the tumor microenvironment via unknown mechanisms [28]. Thus, it is useful to begin to reveal the possible mechanisms that result in an immunosuppression state. In this study, we have observed that tumor cell-delivered exosomes can upregulate PD-L1 expression and the secretion of cytokines, which may contribute to immunosuppressive status in macrophages. The most important finding of the present study, however, is that exosomes derived from melatonin treated hepatocellularcarcinoma cells could attenuate the immunosuppressive status on macrophages both in vitro and in vivo (Fig 8).
Melatonin has been widely reported to regulate the immune system, both within the innate immune response and the adaptive immune response [29]. Melatonin can have an impact on many immune cells to achieve the function of immune regulation. In an innate immune response, melatonin can modulate the main cellular components, such as macrophages, NK cells and monocytes, by increasing cellularity or promoting cell activity [30]. It has been reported that melatonin has anti-inflammatory effects in LPS-stimulated RAW264.7 macrophages [31]. However, no information exists on whether melatonin can alter tumor cells to attenuate an immunosuppressive state. The present results indicate that exosomes, derived from hepatocellularcarcinoma cells treated with melatonin 0.1 mM, significantly decreased PD-L1 and cytokine secretion from macrophages. When hepatocellularcarcinoma cells were treated with melatonin, melatonin not only functioned as a cytotoxic drug but also modulates the exosomic properties secreted by tumor cells to attenuate the immunosuppressive state of macrophages. Hence, we conclude that melatonin indeed has effects on immune regulation and can even relieve the immunosuppressive state caused by tumor cells through exosomes. Our results suggest that melatonin may be a potential and efficient drug that has the potential to be used as a future liver cancer immunotherapy, which will certainly widen the application of melatonin in clinic treatments.
Immunocytochemistry was performed using THP-1 derived macrophage cells climbing glass slides showing PD-L1 expression. Illustration of PD-L1 positive expression in THP-1 differentiated macrophages treated with Exo-con and Exo-MT; the difference is clearly observed under microscope with low, middle, and high power lens. Blue for hematoxylin staining cell nucleus, Brown for PD-L1 positive expression.
Cytokines were quantified with a cytokine bead array (CBA) kit. The supernatant of THP-1 differentiated macrophages were quantified after being treated with either Exo-con or Exo-MT containing different levels of cytokines as the figure shows. Data are presented as the mean ± SD (error bar) of at least three independent experiments. * and ** represent P < 0.05 and P < 0.01, respectively.
PD-L1 (B7-H1, CD274) is a ligand for PD-1, which decreases anti-tumor immunity by inducing T cell apoptosis, energy exhaustion, or IL-10 production when combined with PD-1 [32]. The PD-1 and PD-L1 pathway blockade has become an advanced and active research area that elicits durable anti-tumor responses and long time remissions in a certain numbers of patients with different types of cancers [33]. Thus, how to further improve and widen the clinical effect of anti-PD therapy is a crucial topic in the field of cancer immunology and immunotherapy. PD-L1 usually exists in tumor cells, but high levels of PD-L1 protein expression has been observed in APCs (dendritic cells, macrophages), and fibroblasts [32]. It has been reported that PDL1+ macrophages were enriched predominantly in the peritumoral stroma of HCC [34]. Moreoever, the expression of PD-L1 on macrophages have an immunosuppressive function, such as suppressing tumor-specific T cell immunity, and contribute to the growth of human tumors [35]. Viral infection can increase the PDL1 expression on macrophages,[36] while we found both Exo-con and Exo-MT could change the PD-L1 expression on macrophages. Thus, we tentatively postulate that melatonin may decrease the PD-L1 expression on macrophages through regulating the function of tumor exosomes, which would be the most important currently known mechanism of melatonin in anti-tumor function.
Cytokine secretion is an important mechanism by which macrophages exert immune regulatory functions. Cytokines secreted by macrophages play a crucial role both in the inflammation progress and the immune state, which may be a potential target or indicator reflecting the microenvironment state [37]. Tumor-derived exosomes can stimulate secretion of pro-inflammatory cytokines in recipient cells, including IL-6, TNF, TGF-β and can promote suppression of T cell function and their proliferation [38]. Similarly, we found both Exo-con and Exo-MT could change cytokines secretion in macrophages. Interleukin-6 (IL-6), one of the major cytokines in the tumor microenvironment, is an important factor that is highly expressed in most cancers as reported. It can regulate almost all hallmarks of cancer and has multiple signaling pathways, including apoptosis, survival, proliferation, invasiveness and metastasis, to promote tumorigenesis [39]. Our studies indicated that IL-6 secreted by macrophages was highly up-regulated in the Exo-con group compared to that in the control and Exo-MT group, which is consistent with the change in PD-L1 expression. Interleukin-10 (IL-10), a negative factor in regulating the immune system was found to be up-regulated in the Exo-con group, which would play a crucial role in suppressing the immune system for the tumor microenvironment [40]. However, Exo-MT significantly decreased IL-10 level, which may help to restore the immune function in the tumor microenvironment. IL-1b, one of the inflammatory cytokines, is associated with the pathogenesis and progression in many diseases [41]. Induction of IL-1β could promote breast cancer cells invasion and metastasis development [42]. Our results indicated IL-1β levels were significantly increased in Exo-con group, which would be beneficial for tumor progression, while Exo-MT could reverse IL-1β up-regulation. Another important cytokine we studied is tumor necrosis factor-α (TNFα), a key multifunctional cytokine with pleiotropic actions that regulate immune responses [43]. Furthermore, TNFαcould regulate the epithelial mesenchymal transition (EMT) process which is associated with tumor progression, metastasis and drug resistance [44]. In present study, TNFα levels were significantly up-regulated in the Exo-con group while decreased in Exo-MT group, which would be highly related to the immune status in the tumor microenvironment. Thus, the results above indicated that the exosomes derived from melatonin treated hepatocellularcarcinoma cells could regulate the immune status through cytokines secretion in macrophages.
Cytokines assessed by the FCAP array kit and PD-L1 expression assessed by FCM in vivo. As shown (A), extraneous exosomes could be taken up by the macrophages. Cytokines secreted at different levels by peritoneal macrophages extracted from nude mice treated with PBS, Exo-con, and Exo-MT (B). PD-L1 expression results (C) are similar with those in vitro. Data are presented as mean ± SD (error bar) of at least three independent experiments. (D) *and ** represent P < 0.05 and P < 0.01, respectively.
Western blot analysis of STAT3 signal pathway key-protein level. (A) p-STAT3 protein level from Exo-con treated THP-1 differentiated macrophages were obviously increased compared with the Blank Group, while the Exo-MT treated group showed reduced p-STAT3 expression. Specific STAT3-inhibitor S3I-201 indeed inhibited the upregulation of p-STAT3 expression in the Exo-con Group (C). When macrophages were treated with Exo-con combined with S3I-201, PD-L1 expression decreased (E). Data are presented as mean ± SD (error bar) of at least three independent experiments. *and ** represent P < 0.05 and P < 0.01, respectively.
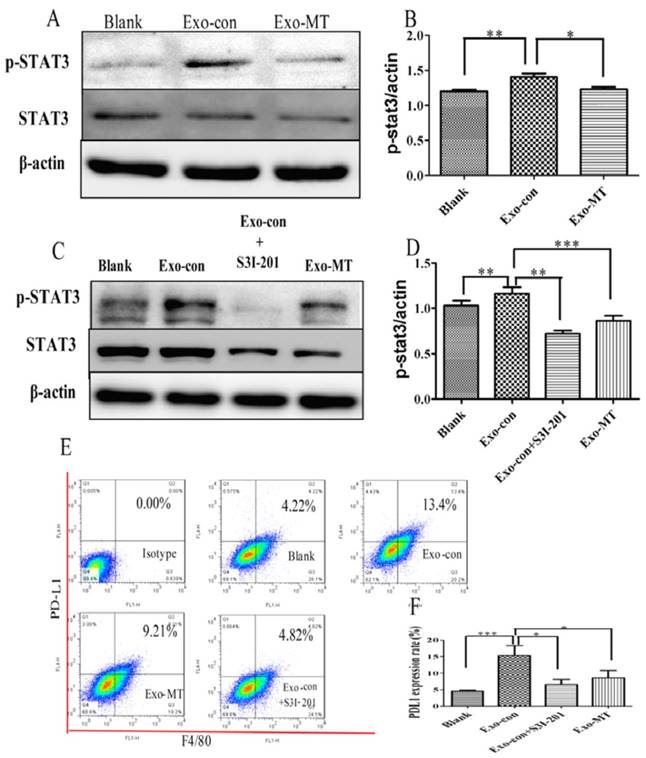
STAT3, key active regulators of immunity and inflammation, has been shown to regulate PD-L1 and cytokine secretion [45]. Thus, we studied the changes of STAT3 signal pathway in macrophages separately co-cultured with Exo-con and Exo-MT, and found the levels of STAT3 activation indeed changed which were in consistent with the PD-L1 expression as well as cytokine secretion. We confirmed this by using STAT3 inhibitory S3I-201, p-STAT3 was significantly reduced while PD-L1 expression decreased.
In conclusion, we have found that liver cancer cell-delivered exosomes can up-regulate PD-L1 expression and cytokine secretion in macrophages. However, the exosomes secreted by liver cancer cells after treatment by melatonin did not cause the same effects, which means that melatonin could change the function of tumor exosomes to attenuate immunosuppression in macrophages. The possible mechanism was associated with regulating the STAT3 signal pathway. Our study and previous studies provide evidence to support melatonin is functioned not only as a cytotoxic drug but also can change the function of tumor exosomes to attenuate immunosuppressive status in tumor microenvironment. Thus, the use of melatonin to treat primary liver cancers may be of clinical value in the future.
Schematic diagram of different exosomes regulate the function of macrophages. Tumor delivered exosomes can increase the PD-L1 expression and cytokines secretion in macrophages to cause immunosupressive status through the STAT3 signal pathway, while exosomes derived from melatonin treated tumor cells could attenuate the immunosuppressive status.
Abbreviations
miRNAs: microRNAs; HCC: hepatoma carcinoma cell; PD-L1: programmed death ligand 1; MT: melatonin; Exo-con: hepatocellularcarcinoma-derived exosomes; Exo-MT: exosomes derived from hepatocellularcarcinoma cells treated with 0.1 mM melatonin; mRNA: messenger RNA; SD: standard error; TEM: transmission electron microscopy; FCM: flow cytometry; CBA: cytokine bead array; PTA: phosphotungstic acid.
Acknowledgements
We thank the members of the Institute of Clinical Pharmacology of Anhui Medical University for technical assistance.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81272739, 81572430 to G Sun, Grant Nos. 81372577, 81522009 to H Wang and Grant Nos. 81602115 to F Wang).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Huang Y, Liu J, Fan L, Wang F, Yu H, Wei W. et al. miR-663 overexpression induced by endoplasmic reticulum stress modulates hepatocellular carcinoma cell apoptosis via transforming growth factor beta 1. Onco Targets Ther. 2016;9:1623-1633
2. Facciorusso A, Serviddio G, Muscatiello N. Local ablative treatments for hepatocellular carcinoma: An updated review. World J Gastrointest Pharmacol Ther. 2016;7:477-489
3. Pusztai L, Karn T, Safonov A, Abu-Khalaf MM, Bianchini G. New Strategies in Breast Cancer: Immunotherapy. Clin Cancer Res. 2016;22:2105-2110
4. Garfall AL, Stadtmauer EA. Cellular and vaccine immunotherapy for multiple myeloma. Hematology Am Soc Hematol Educ Program. 2016;2016:521-527
5. Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, Huse JT. et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352:aad3018
6. Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G. et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393-1404
7. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61:253-278
8. Xin Z, Jiang S, Jiang P, Yan X, Fan C, Di S. et al. Melatonin as a treatment for gastrointestinal cancer: a review. J Pineal Res. 2015;58:375-387
9. Paterniti I, Cordaro M, Esposito E, Cuzzocrea S. The antioxidative property of melatonin against brain ischemia. Expert Rev Neurother. 2016;16:841-848
10. Kim CH, Kim KH, Yoo YM. Melatonin protects against apoptotic and autophagic cell death in C2C12 murine myoblast cells. J Pineal Res. 2011;50:241-249
11. Uguz AC, Cig B, Espino J, Bejarano I, Naziroglu M, Rodriguez AB. et al. Melatonin potentiates chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic tumor cells. J Pineal Res. 2012;53:91-98
12. Wang J, Xiao X, Zhang Y, Shi D, Chen W, Fu L. et al. Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J Pineal Res. 2012;53:77-90
13. Sanchez-Hidalgo M, Lee M, de la Lastra CA, Guerrero JM, Packham G. Melatonin inhibits cell proliferation and induces caspase activation and apoptosis in human malignant lymphoid cell lines. J Pineal Res. 2012;53:366-373
14. Wei JY, Li WM, Zhou LL, Lu QN, He W. Melatonin induces apoptosis of colorectal cancer cells through HDAC4 nuclear import mediated by CaMKII inactivation. J Pineal Res. 2015;58:429-338
15. Mauriz JL, Collado PS, Veneroso C, Reiter RJ, Gonzalez-Gallego J. A review of the molecular aspects of melatonin's anti-inflammatory actions: recent insights and new perspectives. J Pineal Res. 2013;54:1-14
16. Xia MZ, Liang YL, Wang H, Chen X, Huang YY, Zhang ZH. et al. Melatonin modulates TLR4-mediated inflammatory genes through MyD88- and TRIF-dependent signaling pathways in lipopolysaccharide-stimulated RAW264.7 cells. J Pineal Res. 2012;53:325-334
17. Fan L, Sun G, Ma T, Zhong F, Lei Y, Li X. et al. Melatonin reverses tunicamycin-induced endoplasmic reticulum stress in human hepatocellular carcinoma cells and improves cytotoxic response to doxorubicin by increasing CHOP and decreasing survivin. J Pineal Res. 2013;55:184-194
18. Fan L, Sun G, Ma T, Zhong F, Wei W. Melatonin overcomes apoptosis resistance in human hepatocellular carcinoma by targeting survivin and XIAP. J Pineal Res. 2013;55:174-183
19. Zha L, Fan L, Sun G, Wang H, Ma T, Zhong F. et al. Melatonin sensitizes human hepatoma cells to endoplasmic reticulum stress-induced apoptosis. J Pineal Res. 2012;52:322-331
20. Mincheva-Nilsson L, Baranov V. Cancer exosomes and NKG2D receptor-ligand interactions: impairing NKG2D-mediated cytotoxicity and anti-tumour immune surveillance. Semin Cancer Biol. 2014;28:24-30
21. Li L, Li C, Wang S, Wang Z, Jiang J, Wang W. et al. Exosomes Derived from Hypoxic Oral Squamous Cell Carcinoma Cells Deliver miR-21 to Normoxic Cells to Elicit a Prometastatic Phenotype. Cancer Res. 2016;76:1770-1780
22. Iorgulescu JB, Ivan ME, Safaee M, Parsa AT. The limited capacity of malignant glioma-derived exosomes to suppress peripheral immune effectors. J Neuroimmunol. 2016;290:103-108
23. T. Umezu, K. Ohyashiki, M. Kuroda, J.H. Ohyashiki, Leukemia cell to endothelial cell communication via exosomal miRNAs, Oncogene. 2013;32:2747-2755
24. Mrizak D, Martin N, Barjon C, Jimenez-Pailhes AS, Mustapha R, Niki T. et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J Natl Cancer Inst. 2015;107:363-370
25. Liu JT, Li WC, Gao S, Wang F, Li XQ, Yu HQ. et al. Autophagy Inhibition Overcomes the Antagonistic Effect Between Gefitinib and Cisplatin in Epidermal Growth Factor Receptor Mutant Non-Small-Cell Lung Cancer Cells. Clin Lung Cancer. 2015;16:e55-66
26. Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27:409-416
27. Lang R. Tuning of macrophage responses by Stat3-inducing cytokines: molecular mechanisms and consequences in infection. Immunobiology. 2005;210:63-76
28. Jiang T, Zhou C. The past, present and future of immunotherapy against tumor. Translational lung Cancer Res. 2015;4:253-264
29. Carrillo-Vico A, Lardone PJ, Alvarez-Sanchez N, Rodriguez-Rodriguez A, Guerrero JM. Melatonin: buffering the immune system. Int J Mol Sci. 2013;14:8638-8683
30. Calvo JR, Gonzalez-Yanes C, Maldonado MD. The role of melatonin in the cells of the innate immunity: a review. J Pineal Res. 2013;55:103-120
31. Phiphatwatcharaded C, Topark-Ngarm A, Puthongking P, Mahakunakorn P. Anti-inflammatory activities of melatonin derivatives in lipopolysaccharide-stimulated RAW 264.7 cells and antinociceptive effects in mice. Drug Dev Res. 2014;75:235-245
32. Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4
33. Takada K, Toyokawa G, Okamoto T, Akamine T, Takamori S, Katsura M. et al. An Immunohistochemical Analysis of PD-L1 Protein Expression in Surgically Resected Small Cell Lung Cancer Using Different Antibodies and Criteria. Anticancer Res. 2016;36:3409-3412
34. Zhao Q, Xiao X, Wu Y, Wei Y, Zhu LY, Zhou J. et al. Interleukin-17-educated monocytes suppress cytotoxic T-cell function through B7-H1 in hepatocellular carcinoma patients. Eur J Immunol. 2011;41:2314-2322
35. Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C. et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327-1337
36. Staples KJ, Nicholas B, McKendry RT, Spalluto CM, Wallington JC, Bragg CW. et al. Viral infection of human lung macrophages increases PDL1 expression via IFNbeta. PloS one. 2015;10:e0121527
37. Ilangumaran S, Ferbeyre G. Editorial: Cytokines in inflammation, aging, cancer and obesity. Cytokine. 2016;82:1-3
38. Atretkhany KN, Drutskaya MS, Nedospasov SA, Grivennikov SI, Kuprash DV. Chemokines, cytokines and exosomes help tumors to shape inflammatory microenvironment. Pharmacol Ther. 2016;168:98-112
39. Choi EY, Jin JY, Lee JY, Choi JI, Choi IS, Kim SJ. Melatonin inhibits Prevotella intermedia lipopolysaccharide-induced production of nitric oxide and interleukin-6 in murine macrophages by suppressing NF-kappaB and STAT1 activity. J Pineal Res. 2011;50:197-206
40. Liu F, Dai W, Li C, Lu X, Chen Y, Weng D. et al. Role of IL-10-producing regulatory B cells in modulating T-helper cell immune responses during silica-induced lung inflammation and fibrosis. Sci Rep. 2016;6:28911
41. Gomes FI, Aragao MG, Barbosa FC, Bezerra MM, de Paulo Teixeira Pinto V, Chaves HV. Inflammatory Cytokines Interleukin-1beta and Tumour Necrosis Factor-alpha - Novel Biomarkers for the Detection of Periodontal Diseases: a Literature Review. J Oral Maxillofac Res. 2016;7:e2
42. Bouchard G, Therriault H, Bujold R, Saucier C, Paquette B. Induction of interleukin-1beta by mouse mammary tumor irradiation promotes triple negative breast cancer cells invasion and metastasis development. Int J Radiat Biol. 2016:1-38
43. Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222-225
44. Wang H, Fang R, Wang XF, Zhang F, Chen DY, Zhou B. et al. Stabilization of Snail through AKT/GSK-3beta signaling pathway is required for TNF-alpha-induced epithelial-mesenchymal transition in prostate cancer PC3 cells. Eur J Pharmacol. 2013;714:48-55
45. Cortez MA, Ivan C, Valdecanas D, Wang X, Peltier HJ, Ye Y. et al. PDL1 Regulation by p53 via miR-34. J Natl Cancer Inst. 2016;108:303-313
Author contact
![]() Corresponding authors: Dr. Hua Wang, Department of Oncology, the First Affiliated Hospital of Anhui Medical University; Institute for Liver Diseases of Anhui Medical University, 218 Jixi Road, Hefei 230032, Anhui, China. Email: wanghuaedu.cn Dr. Guo-Ping Sun, Department of Oncology, the First Affiliated Hospital of Anhui Medical University, 218 Jixi Road, Hefei 230022, Anhui, China. Email: sungpedu.cn Tel. +86055162923509, Fax +8605512923509
Corresponding authors: Dr. Hua Wang, Department of Oncology, the First Affiliated Hospital of Anhui Medical University; Institute for Liver Diseases of Anhui Medical University, 218 Jixi Road, Hefei 230032, Anhui, China. Email: wanghuaedu.cn Dr. Guo-Ping Sun, Department of Oncology, the First Affiliated Hospital of Anhui Medical University, 218 Jixi Road, Hefei 230022, Anhui, China. Email: sungpedu.cn Tel. +86055162923509, Fax +8605512923509

 Global reach, higher impact
Global reach, higher impact