10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2018; 14(14):1985-1992. doi:10.7150/ijbs.27378 This issue Cite
Research Paper
Androgen action augments ischemia-induced, bone marrow progenitor cell-mediated vasculogenesis
1. The Heart Research Institute, Newtown, Sydney NSW, 2042 Australia
2. Sydney Medical School, The University of Sydney, NSW 2006 Australia
3. ANZAC Research Institute, The University of Sydney, Concord Hospital NSW 2139 Australia
4. Royal Prince Alfred Hospital, Camperdown NSW Australia 2050
*Equal contribution
Received 2018-5-20; Accepted 2018-9-5; Published 2018-11-2
Abstract
Bone marrow-derived progenitor cell-mediated vasculogenesis is a key process for vascular repair and regeneration. However, the role of androgens in the mechanism of ischemia-induced vasculogenesis remains unclear. In this study, a gender-mismatch murine bone marrow transplant model was used to allow tissue tracking of transplanted cells. Bone marrow from 2-month-old male mice was transplanted into irradiated age-matched female recipients. Following the transplantation, ovariectomized female recipients were subjected to unilateral hindlimb ischemia and immediately implanted with either dihydrotestosterone (DHT) or placebo pellets. Laser Doppler perfusion imaging revealed that DHT significantly augmented blood flow recovery, with increased capillary density compared to placebo-treated female recipient controls. Flow cytometry analysis showed that DHT modulated vasculogenesis by increasing Sca1+/CXC4+ progenitor cell production in bone marrow and spleen and enhancing cell mobilization in circulating blood following hindlimb ischemia. Bone marrow cell homing was examined by detecting expression levels of male-specific SRY gene in the ischemic female tissues. DHT treatment promoted bone marrow cell homing to ischemic tissue shown by significantly higher SRY expression compared to placebo-treated females as well as reduced apoptotic features in DHT-treated females, including increased Bcl-2 expression, reduced Bax levels and decreased TUNEL staining. In conclusion, the gender-mismatched bone marrow transplant study shows that androgens directly enhance bone marrow cell-mediated vasculogenesis that contributes to ischemia-induced neovascularization.
Keywords: Androgen, ischemia, vasculogenesis, bone marrow cells, progenitors
Introduction
Androgens are critical in regulating vascular functions. Testosterone is involved in microvascular function, such as arterial stiffness and aortic elasticity [1, 2]. Increasing evidence indicates that androgen levels are inversely associated with cardiovascular risk factors and cardiovascular-related mortality [3]. Patients undergoing androgen deprivation therapy for prostate cancer are at increased risk of cardiovascular events, including myocardial infarction and cardiovascular mortality [4]. Low circulating androgens levels are predictors of progression of atherosclerosis [5], incident cerebrovascular events [6, 7] and cardiovascular mortality [8, 9]. Hence, androgen action has received much attention regarding its role in vascular biology.
We and others have previously demonstrated that androgens enhance post-ischemic vascular repair by promoting angiogenesis (the sprouting of new blood vessels from pre-existing vasculature) following ischemia [10, 11]. However, androgen effects on vasculogenesis, a key feature of postnatal neovascularization, are less well characterized. Upon ischemic injury, progenitor cells are produced in the bone marrow and mobilized into the circulation. Circulating progenitor cells are recruited and home to ischemic sites, where they subsequently facilitate vascular repair and regeneration by incorporation into newly formed blood vessels and/or by secreting pro-angiogenic cytokines [12, 13]. The circulating levels of progenitor cells are closely related to the pathogenesis of vascular diseases. Reduced numbers of circulating progenitor cells have been detected in many pathological conditions, such as myocardial infarction (MI), stroke, erectile dysfunction, peripheral artery disease and diabetes [14-16]. Furthermore, circulating endothelial progenitor cells (EPCs) are inversely associated with cardiovascular risk factors, such as diabetes, smoking, hypercholesterolemia and hypertension.
Androgens are associated with progenitor cell homeostasis. The basal levels of circulating progenitor cells are lower in hypogonadal men, in whom testosterone replacement therapy increases circulating progenitor cell levels [17]. Treatment of EPCs with a synthetic nonaromatizable androgen, methyltrienolone R1881, increases cell migration and proliferation in vitro [18]. However, the roles of androgens in modulating progenitor cells under ischemic conditions in vivo are not yet fully understood. Our group has previously shown that androgens promote progenitor cell production in the bone marrow following ischemia [10, 19], though, whether androgens enhance cell homing to the ischemic tissue has yet to be demonstrated. In this study, we sought to examine the effects of androgens on vasculogenesis, from the production of bone marrow-derived progenitor cells to cell mobilization into circulation, in particular cell homing to ischemic sites. Cell mobilization and homing are often considered to be stimulated by the secretion of pro-angiogenic cytokines at local ischemic site. The use of a gender mismatched bone marrow transplantation in mice allows us to examine the effects of androgens directly on male bone marrow-derived progenitor cells, independent of host tissue response.
Materials and Methods
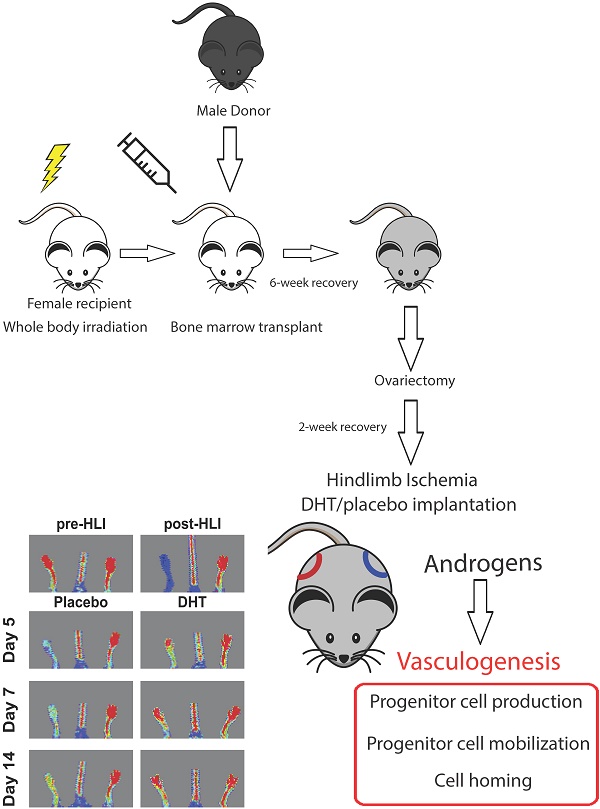
Experimental animals and hindlimb ischemia
All animal studies were conducted with ethical approval from the Sydney Local Health District Animal Ethics Committee (#2011-001). Two-month old female C57Bl/6J mice were irradiated with a single dose of 1000 Rads (Gammacell 40 Exactor, Centenary Institute) one day prior to bone marrow transplantation. Bone marrow cells (BMCs) from age-matched male C57Bl/6J mice were intravenously injected into the irradiated female mice (5×106 cells per injection). After 6-week recovery, female recipients were ovariectomized. After 2 weeks recovery, ovariectomized females were subjected to unilateral hindlimb ischemia (HLI) induction [20]. Immediately after the HLI surgery, mice underwent a subdermal placement of 1 cm silastic implants filled with crystalline dihydrotestosterone (DHT) or empty implants [21]. Laser Doppler perfusion imaging (LDPI) was performed prior to surgery (pre-HLI), post-HLI (day 0) and at indicated days after the surgery (moorLDI2-IR, Moor Instruments, UK). Blood flow was displayed as a heat map, whereby red represented full blood flow and blue represented minimal blood flow. Non-ischemic limb served as an internal control in each animal. Prior to HLI surgery, blood perfusion ratio was approximately 1.0 and dropped to close to zero after the surgery. Blood perfusion ratio is expressed as the ratio of blood flow in ischemic normalized to non-ischemic limb.
Immunohistochemistry
Capillary and arteriolar densities were examined by immunofluorescence staining with rat monoclonal anti-laminin (ab11576, Abcam, Cambridge, UK), rat monoclonal anti-CD31 conjugated to phycoerythrin (ab25644, Abcam) and mouse monoclonal α-smooth muscle actin conjugated to fluorescein isothiocyanate (FITC) (F3777, Sigma, St. Louise, MO). Goat anti-rat IgG conjugated to Alexafluor 350 (A21093, Life Technologies, Grand Island, NY) was used as a secondary antibody. Capillary density is expressed as numbers of CD31+ cells per myocyte. Arteriolar density is expressed as numbers of vessels per myocyte. At least five micrograph images were quantified per mouse.
Mononuclear cell isolation
Mononuclear cells (MNCs) from the bone marrow, spleen and blood were isolated using Lympholytes®-M Cell Separation Media according to manufacturer's protocol (Cedarlane, Burlington, Ontario). MNCs were suspended in EGM-2 medium (Lonza, Basel, Switzerland) for flow cytometry analysis. Progenitor cell populations were identified by co-expression of the hematopoietic stem cell marker Sca1 and the SDF-1 receptor CXCR4, as previously described [10, 22]. MNCs were labelled with anti-mouse Sca1-V450 (560653, BD Bioscience, San Jose, CA) and anti-mouse CXCR4-APC (558644, BD Bioscience) for flow cytometric analysis (BDFACSVerse, Becton Dickinson, Franklin Lakes, NJ).
Quantification of male BMC homing
Gastrocnemius muscles were collected from ischemic and non-ischemic tissues of female recipients at day 3 post-HLI. Genomic DNA was isolated using Wizard Genomic DNA purification Kit (Promega, Madison, WI). Real-time quantitative PCR (qPCR) for male-specific SRY gene (Sex-determining Region Y) sequence was performed as described [23]. SRY forward GCTGGGATGCAGGTGGAAAA; reverse CCCTCCGATGAGGCTGATATT. Autosomal gene NME1 was used as internal control, forward ACAGCTCTGCATTCCTTACC; reverse AGAACAGAACACAGGTGATAGG (GeneWorks, SA, Australia). CFX384 TouchTM Real-time PCR detection system was used to perform qPCR (Bio-Rad). PCR conditions were as follows: 5 min at 95oC; 30 sec at 95oC, 30 sec at 56oC and 30 sec at 72oC (×40 cycles).
Real-time quantitative PCR
Muscle tissue was homogenized with TriReagent (Sigma). Extracted total RNA was reverse transcribed using iScriptTM cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). The expression levels of SDF-1, Bcl-2 and Bax were examined. SDF-1 forward AGACCCCCGAGGAAGGCTGAC; reverse CCAGTCAGTGCTGTCCCGCC. Bcl-2 forward CTCGTCGCTACCGTCGTGACTTCG; reverse CAGATGCCGGTTCAGGTACTCAGTC, Bax forward AAGCTGAGCGAGTGTCTCCGGCG; reverse GCCACAAAGATGGTCACTGTCTGCC (GeneWorks, SA, Australia). Mouse 36B4 was used as a housekeeping gene for normalization. 36B4 forward CAACGGCAGCATTTATAACCC; reverse CCCATTGATGATGGAGTGTGG. PCR conditions were as follows: 3 min at 95oC; 30 sec at 95oC, 30 sec at 60oC and 30 sec at 72oC (×40 cycles). Each run included negative reaction controls. Expression levels were calculated by the relative quantification method (∆∆Ct). RT-qPCR was performed in triplicate for each sample.
Immunofluorescence terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining
Apoptotic cells in ischemic tissues were determined by DeadEND Fluorometric TUNEL system (Promega), according to manufacturer's protocol. Briefly, OCT-embedded tissues were fixed with 4% paraformaldehyde and incubated in permeabilization solution. Samples were then incubated with TUNEL reaction mixture at 37oC in humid conditions for 1 hour and encapsulated by mounting medium Vectashield containing 4',6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA). TUNEL- and DAPI-positive cells were counted in ten randomly selected microscopic fields. The percentages of apoptotic cells were expressed as TUNEL+ cells per total number of DAPI+ cells.
Data Analysis
All animal experiments consisted of n=6 per group. Data are expressed as mean ± SEM. Statistical analysis between two groups was performed using unpaired Student's t-test. Statistical analysis between more than two groups was performed using one-way or two-way ANOVA with Bonferroni adjustment post-test as indicated. p<0.05 was considered statistically significant.
Results and Discussion
DHT enhances ischemia-induced neovascularization in female recipients of male bone marrow
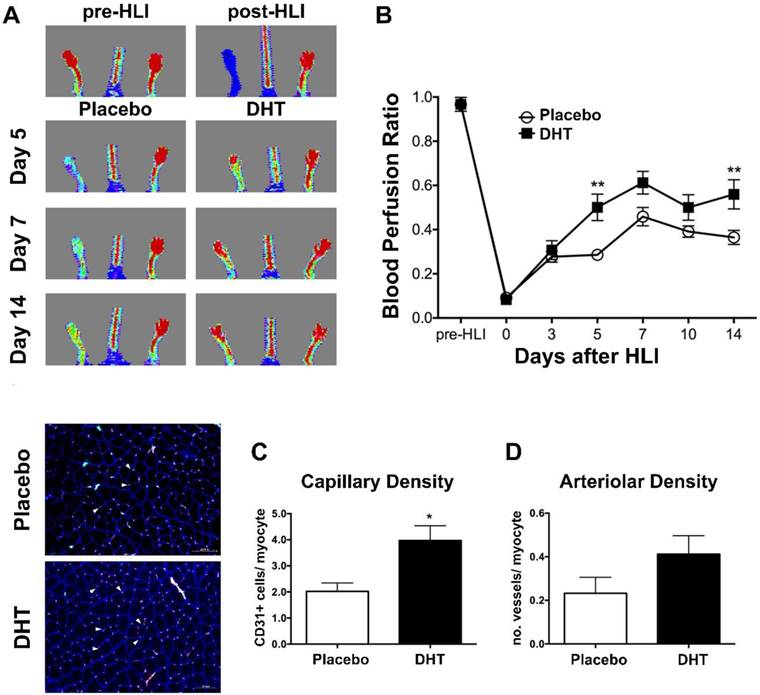
Clinical studies have demonstrated an inverse correlation between endogenous androgen levels and circulating progenitor cell levels [17]. However, the basal levels of circulating progenitor cells in the peripheral blood of men do not fully reflect the actions of androgens on vasculogenesis during ischemic injury. To investigate the role of androgens in modulating bone marrow-derived progenitor cell response in the presence of ischemia, female recipients transplanted with male bone marrow received a subdermal implantation of silastic implant filled with dihydrotestosterone (DHT) or empty implant immediately after hindlimb ischemia. Following induction of hindlimb ischemia (HLI), DHT significantly enhanced blood flow recovery in recipient mice compared to placebo controls at day 5 post-HLI (DHT: 0.501 ± 0.06 vs. placebo: 0.286 ± 0.02, P=0.001) (Figure 1A and 1B). Consistently, DHT significantly increased the number of CD31+ in ischemic tissues of bone marrow recipients compared to placebo controls at day 14 post-HLI (Capillary density, DHT: 3.969 ± 0.570 vs. placebo: 2.019 ± 0.3223, P=0.018) (Figure 1C). A modest increase in the number of vessels (arteriolar density) was also observed in DHT-treated bone marrow recipients, albeit not significantly (P=0.147), compared to placebo controls at day 14 post-HLI (Figure 1D).
Blood flow recovery of DHT- and placebo-treated female recipients transplanted with male bone marrow following ischemia. Irradiated female C57Bl/6J mice were transplanted with bone marrow from age-matched male C57Bl/6J mice. Mice were implanted with DHT or placebo pellet following hindlimb ischemia (HLI). (A) Representative images of LDPA following ischemia. (B) Quantification of blood perfusion ratio (ischemic limb index/non-ischemia limb index) of DHT- and placebo-treated female recipients. Data are presented as mean ± SEM. n=6. ** p<0.01 vs. placebo-treated recipient mice. Two-way ANOVA with Bonferroni adjustment post-test. (C) Capillary density, numbers of CD31+ per myocyte, and (D) arteriolar density, number of vessels per myocyte, of DHT- and placebo-treated female recipients post-HLI. Left, representative image of immunohistochemical staining for CD31+ (red, arrowed) and α-smooth muscle actin (green). n=6. Data are presented as mean ± SEM. * P <0.05 vs. placebo-treated females. Unpaired Student's t-test.
DHT augments ischemia-induced production and mobilization of Sca1+/CXCR4+ progenitor cells
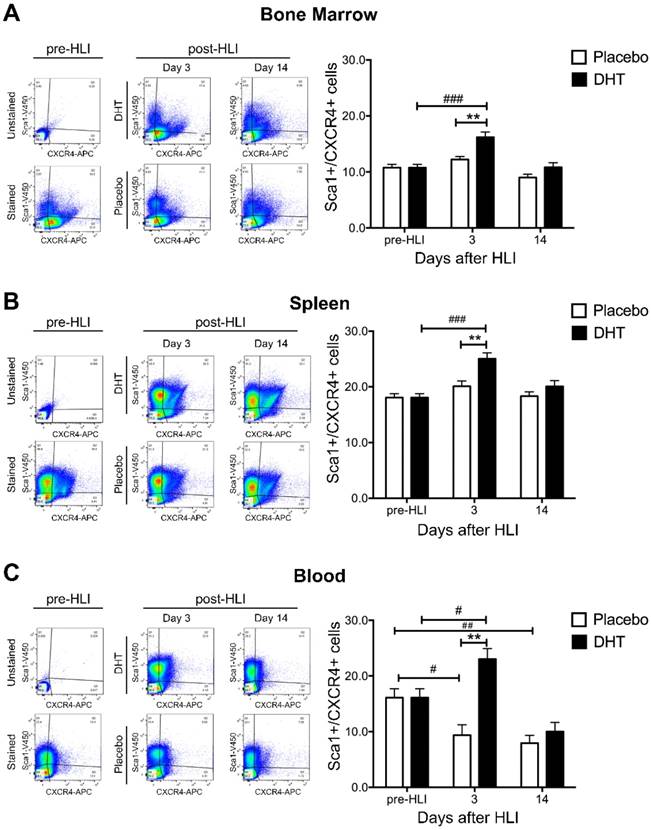
To determine the effects of androgens on vasculogenesis, the production and mobilization of Sca1+/CXCR4+ progenitor cells was examined in bone marrow, spleen and circulating blood of DHT- or placebo-treated female bone marrow recipients following ischemia. In the absence of DHT, ischemia did not increase Sca1+/CXCR4+ progenitor cell production in the bone marrow and spleen (Figure 2A and 2B). Numbers of circulating Sca1+/CXCR4+ progenitor cells gradually declined in the blood of placebo-treated female recipients post-HLI (day 3: 9.36 ± 1.87 vs. pre-HLI: 16.10 ± 1.60, P=0.026; day 14 7.92 ± 1.40 vs. pre-HLI: 16.10 ± 1.60, P=0.08) (Figure 2C). Depletion of sex hormones is likely to negatively affect progenitor cell homeostasis, where ischemia-induced progenitor cells production was attenuated in ovariectomized, placebo-treated female recipients. Subsequently, there was a gradual decline in circulating blood progenitor cell levels due to cell exhaustion in the absence of exogenous androgen supplementation.
At day 3 post-HLI, DHT significantly increased Sca1+/CXCR4+ progenitor cell production in the bone marrow and spleen compared to pre-HLI (Bone marrow: 16.20 ± 0.949 vs. 10.75 ± 0.600, P<0.0001; Spleen: 25.05 ± 1.054 vs. 18.06 ± 0.706, p<0.0001) (Figure 2A and 2B). In parallel, DHT also enhanced Sca1+/CXCR4+ progenitor cell mobilization in circulating blood at day 3 post-HLI compared to pre-HLI (23.04 ± 1.886 vs. 16.10 ± 1.604, P=0.029) (Figure 2C). Comparing to placebo-treated controls, DHT-treated female recipients exhibited higher levels of progenitor cell production in the bone marrow and spleen, as well as cell mobilization in the circulating blood at day 3 post-HLI (Table 1), indicating that androgen augmentation of vasculogenesis is associated with increased progenitor production and mobilization.
The levels of Sca1+/CXCR4+ progenitor cells in female recipients at day 3 post-HLI.
| DHT | Placebo | P-value | |
|---|---|---|---|
| Bone marrow | 16.20 ± 0.949 | 12.23 ± 0.516 | P=0.001 |
| Spleen | 25.05 ± 1.054 | 20.11 ± 0.932 | P=0.001 |
| Blood | 23.04 ± 1.886 | 9.36 ± 1.867 | P<0.0001 |
The levels of Sca1+/CXCR4+ progenitor cells in female recipients with male bone marrow post-ischemia. Flow cytometry analysis of the percentage of Sca1+/CXCR4+ progenitor cells in mononuclear cell population from (A) bone marrow, (B) spleen and (C) circulating blood isolated from DHT- and placebo-treated female recipients with male bone marrow pre-HLI and post-HLI. Data are presented as mean ± SEM. n=6. # p<0.05, ## p<0.01, ### p<0.001 vs. pre-HLI. ** p<0.01 vs. placebo-treated recipient mice. Two-way ANOVA with Bonferroni adjustment post-test.
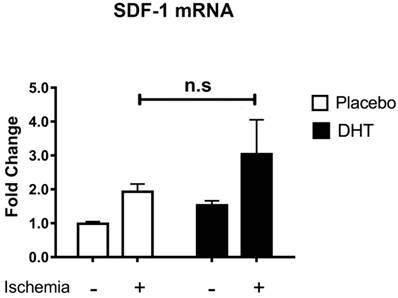
Stromal cell-derived factor 1 (SDF-1) is the ligand for CXCR4 that facilitates progenitor cell mobilization [24]. Our group has previously shown that DHT augments SDF-1 levels in local ischemic muscle tissues, which associates with increased levels of bone marrow Sca1+/CXCR4+ progenitor cells [10]. In addition, Chen et al. showed that testosterone increases SDF-1 expression with enhanced mobilization and homing of CD34+ progenitor cells in the ischemic myocardium after myocardial infarction [11]. Both studies suggest that androgens enhance progenitor cell mobilization in association with increased production of pro-angiogenic SDF-1 levels at ischemic sites. Given that DHT is a non-aromatizable androgen and does not induce any estrogen receptor-mediated effects [21], host tissues were comparable between placebo and DHT treatment. SDF-1 expression level was not significantly different (P=0.769) in ischemic tissues of placebo- and DHT-treated female recipients (Figure 3). This indicated that androgens modulate progenitor cell response in recipients of male bone marrow cells without altering SDF-1 levels in female recipient.
One limitation of the study is that progenitor cell populations are diverse, each with distinctive cell surface markers [25]. Sca1+/CXCR4+ cells may not fully represent the majority of progenitor cell populations. However, we consider the current markers are suitable for the study given that CXCR4 is the receptor for SDF-1, which is a major cytokine for progenitor cell recruitment [24]. Secondly, in addition to hindlimb ischemia model, Sca1+/CXCR4+ progenitor cells have been demonstrated to promote revascularization in burn wounding, supporting that Sca1+/CXCR4+ progenitors play a critical role in neovascularization [22, 26]. Further studies are required to investigate the role of androgens in modulating the dynamics of different progenitor cell populations in response to ischemia.
DHT augments bone marrow cell (BMC) homing and improves tissue salvage following ischemia
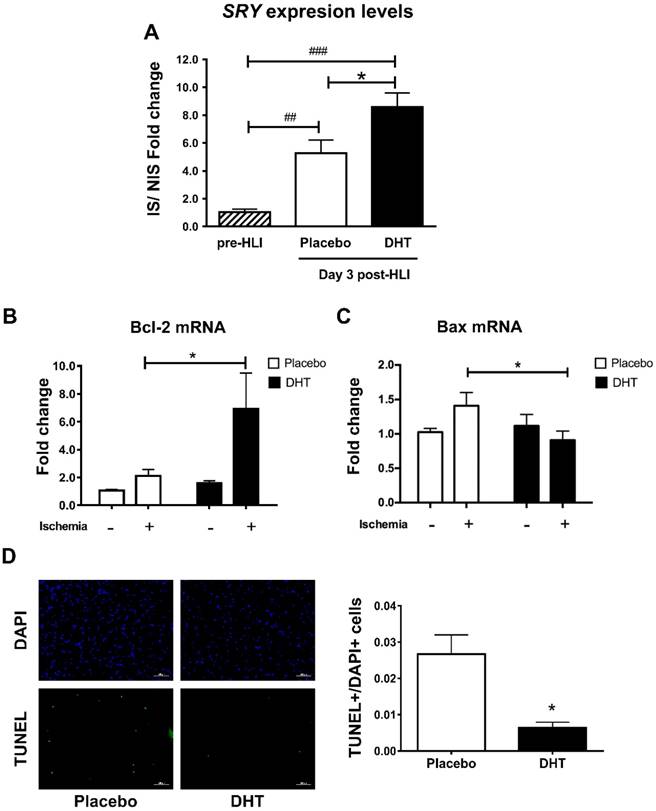
Having demonstrated that androgens augment progenitor cell production and mobilization, we next determined whether androgens promote cell homing to ischemic sites by examining male-specific SRY expression levels in female ischemic muscle tissues. Hindlimb ischemia induced BMC homing in placebo-treated female recipients with a significant 5.26-fold increase in the SRY levels detected at day 3 post-HLI (Day 3: 5.26 ± 0.952 vs. pre-HLI: 1.03 ± 0.226, P=0.007). Remarkably, DHT further augmented BMC homing with an 8.59-fold increase in the SRY levels, which was significantly higher than that in placebo-treated recipients (DHT 8.59 ± 0.997 vs. placebo: 5.26 ± 0.952, P=0.023) (Figure 3A). Cell homing has previously been associated with reduced apoptosis and improved tissue salvage [23]. Anti-apoptotic Bcl-2 mRNA expression was significantly higher, whereas apoptotic Bax levels were significantly lower, in ischemic tissue of DHT-treated compared with placebo-treated recipients (Bcl-2, DHT: 5.886 ± 2.185 vs. placebo: 2.109 ± 0.447, P=0.047; Bax, DHT: 0.910 ± 0.131 vs. placebo: 1.409 ± 0.193, P=0.036) (Figure 3B and 3C). Consistently, TUNEL staining revealed that the number of apoptotic cells in ischemic tissues of DHT-treated recipients was significantly lower compared to placebo-treated mice at day 14 post-HLI (Figure 3D). These results indicate that androgens promote cell homing in response to ischemia and is associated with improved tissue salvage due to increased cell survival.
Stromal cell-derived factor-1 (SDF-1) mRNA expression levels in muscle tissues of female recipients transplanted with male bone marrow. Total RNA was extracted from non-ischemic and ischemic tissues of female recipients at day 3 post-HLI. SDF-1 expression was normalized to internal control 36B4. Data are presented as mean ± SEM. n=6
In conclusion, this study definitively shows that androgen enhancement of vasculogenesis makes a direct contribution to neovascularization following ischemia. Androgens augment bone marrow-derived progenitor cell production and mobilization upon ischemic induction. Importantly, androgens also promote cell homing to the ischemic sites and improve ischemic tissue integrity. However, whether androgen-mediated homing of male progenitor cells incorporate directly into ischemic vasculature or stimulate angiogenesis of resident cells through paracrine release of proangiogenic factors is unclear and requires further investigation. Evidence from animal and preliminary human stem cell therapy studies indicates that very low numbers of progenitor cells are incorporated into ischemic vasculature, suggesting that the levels of progenitor cell incorporation do not account for the vascular improvement following ischemia [27]. Alternatively, it has been proposed that proangiogenic factors, such as vascular endothelial growth factor and basic fibroblast growth factor, are released by progenitor cells to stimulate angiogenesis of resident cells after homing to the ischemic sites [28]. Using a gender mismatch transplantation, we also showed that, for the first time, androgens modulate ischemia-induced vasculogenic response in recipients of male bone marrow without altering host tissue response. These findings highlight the role of androgens in contributing to ischemia-induced neovascularization and further support its potential in therapeutic implications.
Quantification of male SRY gene expression in ischemic tissues of female recipients transplanted with male bone marrow. DNA extracted from the gastrocnemius muscles of DHT- and placebo-treated female recipients with male bone marrow, pre-HLI and at day 3 post-HLI. (A) The levels of male-specific SRY gene were normalized to autosomal NME1 gene. Data are presented as mean ± SEM. n=6. ## p<0.01, ### p<0.001 vs. pre-HLI. * p<0.05 vs. placebo-treated recipient mice. One-way ANOVA with Bonferroni adjustment post-test. Expression levels of (B) Bcl-2 and (C) Bax in the ischemic and non-ischemic tissues of female recipients. * P<0.05 vs. ischemic tissues of placebo controls. Two-way ANOVA with Bonferroni adjustment post-test. (D) Representative images and percentage of TUNEL+/DAPI+ cells in muscle tissues. * P<0.05. Unpaired Student's t-test.
Acknowledgements
We thank Ms Jenny Spaliviero and Mr Mark Jimenez (ANZAC Research Institute, Sydney, Australia) for their assistance with implant and animal preparation, handling and transportation. We also thank Dr Jeff Crosbie (Centenary Institute, Sydney, Australia) for his assistance with the Gammacell 40 Exactor for the bone marrow transplant study. This study was supported by National Health and Medical Research Council (NHMRC) Project Grant No. 1011111 awarded to Martin K. C. Ng.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Corrigan FE 3rd, Al Mheid I, Eapen DJ. et al. Low testosterone in men predicts impaired arterial elasticity and microvascular function. Int J Cardiol. 2015;194:94-9
2. Canpolat U, Tokgozoglu L, Aydin K. et al. Impaired aortic elastic properties in patients with adult-onset hypogonadism. Blood Press. 2013;22(2):114-9
3. Khaw KT, Dowsett M, Folkerd E. et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116(23):2694-701
4. Martin-Merino E, Johansson S, Morris T, Garcia Rodriguez LA. Androgen deprivation therapy and the risk of coronary heart disease and heart failure in patients with prostate cancer: a nested case-control study in UK primary care. Drug Saf. 2011;34(11):1061-77
5. Muller M, van den Beld AW, Bots ML. et al. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation. 2004;109(17):2074-9
6. Yeap BB, Hyde Z, Almeida OP. et al. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J Clin Endocrinol Metab. 2009;94(7):2353-9
7. Yeap BB, Alfonso H, Paul Chubb SA. et al. In older men, higher plasma testosterone or dihydrotestosterone are independent predictors for reduced incidence of stroke but not myocardial infarction. J Clin Endocrinol Metab. 2014;99(12):4565-73
8. Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93(1):68-75
9. Hsu B, Cumming RG, Naganathan V. et al. Temporal Changes in Androgens and Estrogens are associated with All-Cause and Cause-Specific Mortality in Older Men. J Clin Endocrinol Metab. 2016;101(5):2201-2210 jc20161025
10. Sieveking DP, Lim P, Chow RW. et al. A sex-specific role for androgens in angiogenesis. J Exp Med. 2010;207(2):345-52
11. Chen Y, Fu L, Han Y. et al. Testosterone replacement therapy promotes angiogenesis after acute myocardial infarction by enhancing expression of cytokines HIF-1a, SDF-1a and VEGF. Eur J Pharmacol. 2012;684(1-3):116-24
12. Rehman J, Li J, Orschell CM, March KL. Peripheral blood "endothelial progenitor cells" are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107(8):1164-9
13. Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51(6):660-8
14. Vasa M, Fichtlscherer S, Aicher A. et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89(1):E1-7
15. Hill JM, Zalos G, Halcox JP. et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593-600
16. Fadini GP, de Kreutzenberg SV, Coracina A. et al. Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. Eur Heart J. 2006;27(18):2247-55
17. Foresta C, Caretta N, Lana A. et al. Reduced number of circulating endothelial progenitor cells in hypogonadal men. J Clin Endocrinol Metab. 2006;91(11):4599-602
18. Foresta C, Zuccarello D, De Toni L. et al. Androgens stimulate endothelial progenitor cells through an androgen receptor-mediated pathway. Clin Endocrinol (Oxf). 2008;68(2):284-9
19. Lam YT, Lecce L, Tan JT. et al. Androgen receptor mediated genomic androgen action augments ischemia-induced neovascularization. Endocrinology. 2016;157(12):4853-4864
20. Couffinhal T, Silver M, Zheng LP. et al. Mouse model of angiogenesis. Am J Pathol. 1998;152(6):1667-79
21. Singh J, O'Neill C, Handelsman DJ. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 1995;136(12):5311-21
22. Zhang X, Sarkar K, Rey S. et al. Aging impairs the mobilization and homing of bone marrow-derived angiogenic cells to burn wounds. J Mol Med (Berl). 2011;89(10):985-95
23. Rey S, Lee K, Wang CJ. et al. Synergistic effect of HIF-1alpha gene therapy and HIF-1-activated bone marrow-derived angiogenic cells in a mouse model of limb ischemia. Proc Natl Acad Sci U S A. 2009;106(48):20399-404
24. Ceradini DJ, Kulkarni AR, Callaghan MJ. et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10(8):858-64
25. Liu HB, Gong YF, Yu CJ. et al. Endothelial progenitor cells in cardiovascular diseases: from biomarker to therapeutic agent. Regen Med Res. 2013;1(1):9
26. Zhang X, Liu L, Wei X. et al. Impaired angiogenesis and mobilization of circulating angiogenic cells in HIF-1alpha heterozygous-null mice after burn wounding. Wound Repair Regen. 2010;18(2):193-201
27. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204-19
28. Kinnaird T, Stabile E, Burnett MS. et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543-9
Author contact
![]() Corresponding author: Martin K.C. Ng, Department of Cardiology, Royal Prince Alfred Hospital, Sydney 2050 Tel: +61 2 8208 8900 Fax: +61 2 9565 5584 mkcngusyd.edu.au
Corresponding author: Martin K.C. Ng, Department of Cardiology, Royal Prince Alfred Hospital, Sydney 2050 Tel: +61 2 8208 8900 Fax: +61 2 9565 5584 mkcngusyd.edu.au

 Global reach, higher impact
Global reach, higher impact