10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2018; 14(14):1993-2002. doi:10.7150/ijbs.27459 This issue Cite
Research Paper
SAK-HV Promotes RAW264.7 cells Migration Mediated by MCP-1 via JNK and NF-κB Pathways
1. Institute of Military Cognitive and Brain Sciences, Beijing, 100850, China.
2. Division of Oncology Research, Mayo Clinic, Rochester, MN 55905, USA.
Received 2018-5-24; Accepted 2018-9-25; Published 2018-11-2
Abstract
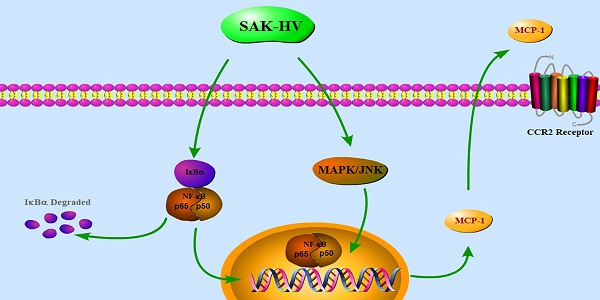
Macrophage migration plays an essential role in immune system and is also involved in many pathological situations. However, the regulatory mechanism of macrophage migration remains to be elucidated due to its diverse responses to various stimuli. SAK-HV, a multifunctional protein possessing thrombolytic and lipid-lowering activity, can selectively induce the macrophage proliferation. Here, we reported SAK-HV significantly triggered RAW264.7 cells migration through its functional domain of SAK-mutant by activating both c-jun N-terminal kinases (JNK) and nuclear factor-κB (NF-κB) pathways. Meanwhile, SAK-HV upregulated the expression of some effector proteins, among which only the expression of Monocyte chemoattractant protein-1 (MCP-1) was inhibited by the blockade of JNK and NF-κB pathways. Further research showed that MCP-1 promoted migration ultimately by interacting with Chemokine (C-C motif) Receptor 2 (CCR2) in an autocrine manner. In summary, SAK-HV induced RAW264.7 cells migration through its SAK-mutant domain, during which MCP-1 chemokine mediated by JNK and NF-κB pathways played a key role. These results revealed a novel effect of SAK-HV on modulating macrophage migration and also deepened the understanding of its pharmacodynamics.
Keywords: SAK-HV, macrophage migration, MCP-1, JNK, NF-κB
Introduction
Macrophage is an important component of the innate immune system, and its migration ability is a prerequisite to participate in immune and inflammatory responses. Macrophages migrate into tissues as part of the constitutive or steady-state efflux from blood, and are also recruited into foci of active inflammation after tissue injury or during infection, which is a hallmark of the innate immune responses [1]. However, deregulated migration of macrophages has been implicated in a variety of human disorders, such as chronic inflammation, neurodegenerative diseases and tumor metastasis. Therefore, the challenge of regulating macrophage migration needs to be overcome by modern medical treatments [2, 3].
Cellular migration is a complex process regulated by multiple signaling pathways[4]. Mitogen-activated protein (MAP) kinase pathway plays a pivotal role in cellular migration caused by stimuli of pathogens and organisms [5, 6]. Involved in several phosphorylation steps of the MAPK pathway, nuclear factor-κB (NF-κB) can elicit the transcription of macrophage-related cytokines and regulate cellular migration [7, 8]. Moreover, phosphatidylinositol 3-kinase (PI3K)/AKT is another pathway closely related to migration [9].
It is known to all, macrophages exposed to external stimuli secret a variety of cytokines, chemokine and growth factors that strengthen their migration ability and promote innate and adaptive immunity. Among them, MCP-1, also known as chemokine (C-C motif) ligand 2 (CCL2), is one of the primary chemokines for the recruitment of monocytes/macrophages while prompting the expression of various inflammatory cytokines [10]. Macrophage inflammatory protein-1α (MIP-1α), also known as CCL3, is another critical chemokine for macrophages recruitment [11]. In addition to chemokines, growth factors, such as Transforming growth factor-beta (TGF-β) and Macrophage Colony-Stimulating Factor (M-CSF), also play important role when macrophages migrate to the inflammatory sites and tumor tissues [12-14]. Besides, matrix metalloproteinases (MMPs) are essential for the degradation and remodeling of extracellular matrix (ECM) barriers in order to create space allowing cells to move [15-17].
A recombinant fusion protein SAK-HV possessing thrombolytic and lipid-lowering activity was constructed and purified by our lab. It has been shown that macrophage is the target cell line of SAK-HV and its proliferation can be selectively triggered by SAK-HV in our previous study [18-20]. To explore its impact on macrophages thoroughly, we further observed cellular migration stimulated by SAK-HV in both RAW264.7 cells and primary peritoneal macrophages, and found that the migration was promoted obviously by SAK-HV through its SAK-mutant functional domain. Additionally, the upregulation of MCP-1 mediated by JNK and NF-κB signaling pathways played a major role in SAK-HV-induced migration of RAW264.7 cells, suggesting that SAK-HV maybe the potential immune stimulator participating in modulation of immune response.
Results
SAK-HV triggered macrophages migration
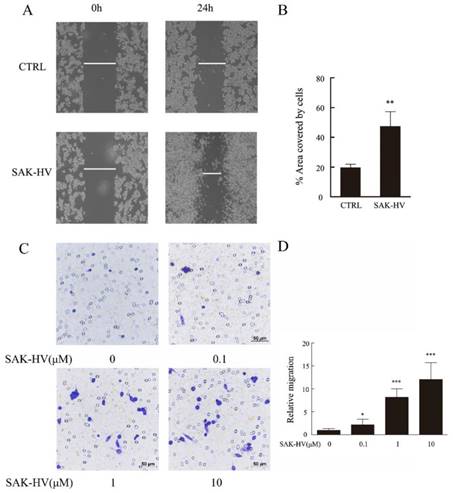
We conducted scratch healing and transwell assays to investigate the effects of SAK-HV on the migration of RAW264.7 cells and primary peritoneal macrophage cells. The results showed that scratch healing after incubation with 1μM SAK-HV for 24 h was much better than that in the control group in RAW264.7 cells. The area covered by cells in SAK-HV treatment group (47.37±9.79)% was significantly enlarged compared with control group (19.60±2.28)% (P<0.01) (Figure 1A, B). Similarly, the results of transwell assays showed that relative migration rate was 2.18±1.21 (P<0.05) when treated with 0.1μM SAK-HV and it increased to 8.18±1.87 and 12.09±3.67 (P<0.001) when treated with 1μM and 10μM SAK-HV separately (Figure 1C, D). These results indicated that SAK-HV remarkably promoted RAW264.7 cells migration in a concentration-dependent manner.
SAK-HV significantly induced migration in RAW264.7 cells. (A) The migration of RAW264.7 cells was analysed using the cell scratch assay, and cells were photographed after 1μM SAK-HV treatment for 24 h (n=3). SAK-HV treatment increased the area covered by cells compared to control group. (C) The migration of RAW264.7 cells was analysed using transwell assay, and cells were photographed after increasing concentrations of SAK-HV (0, 0.1, 1, and 10 µM) treatment for 12 h , respectively (n=3). Compared with control group, SAK-HV treatment increased the number of migrated cells. (B, D) The quantification for cell scratch assay and transwell assay above. * P<0.05 versus control group, ** P<0.01 versus control group. Abbreviation: CTRL, control.
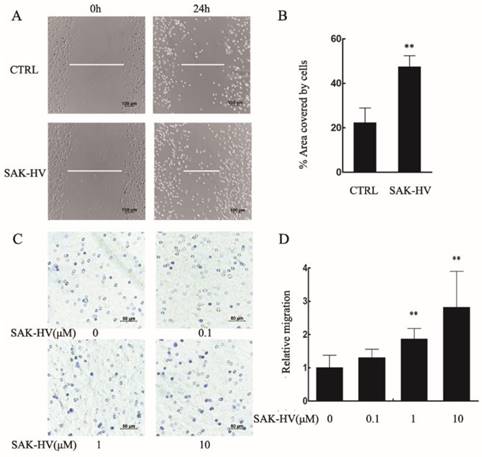
Further, we examined the effect of SAK-HV on the migration of primary peritoneal macrophages. The area covered by cells in SAK-HV treatment group (47.38±4.99)% was enlarged compared with control group (22.31±6.62)% (Figure 2A, B). As is shown in Figure 2C and 2D, although the relative migration rate was not affected by 0.1μM of SAK-HV, it was increased significantly by 1μM and 10μM SAK-HV treatment, indicating that SAK-HV had migration-promoting effect on both RAW264.7cells and primary peritoneal macrophages.
SAK-HV significantly induced migration in primary peritoneal macrophages. (A) The migration was analysed using the cell scratch assay, and cells were photographed after 1μM SAK-HV treatment for 24 h (n=3). SAK-HV treatment increased the area covered by cells compared to control group. (C) The migration of primary peritoneal macrophages was analysed using transwell assay, and cells were photographed after increasing concentrations of SAK-HV(0, 0.1, 1, and 10 µM) treatment for 12 h , respectively (n=3). Compared with control group, SAK-HV treatment increased the number of migrated cells. (B, D) The quantification for cell scratch assay and transwell assay above. * P<0.05 versus control group, ** P<0.01 versus control group. Abbreviation: CTRL, control.
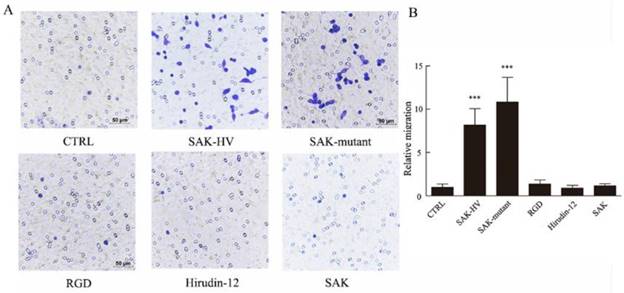
SAK-HV promoted cellular migration through its SAK-mutant domain
SAK-KV recombinant protein consists of three functional domains: an SAK-mutated domain, an Arg-Gly-Asp (RGD) sequence, and a 12-amino acid peptide from the C-terminus of hirudin (hirudin-12). Among them, SAK-mutated domain is originated from the deletion mutation of the wild-type SAK protein to lower its immunogenicity [18]. To validate the specific functional domain of SAK-HV to trigger cellular migration, the three individual functional domains and the wild type SAK at the same concentration (1μM) were used to stimulate cells separately. Strikingly, no significant differences in the relative migration rate were observed in groups treated with RGD domain, hirudin-12 and wild-type SAK (1.36±0.45, 0.91±0.32, 1.18±0.25) compared with control group (1±0.38) (Figure 3), but the SAK-mutant domain of SAK-HV (10.82±2.85) distinctly induced migration and the effect was comparable to SAK-HV treatment (8.12±1.87). These results suggested that SAK-HV promoted cellular migration through its SAK-mutant functional domain.
Effects of SAK-HV treatment on migration-relative pathways
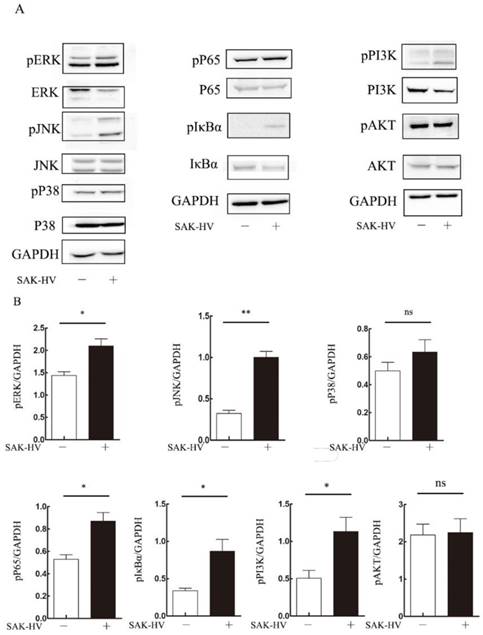
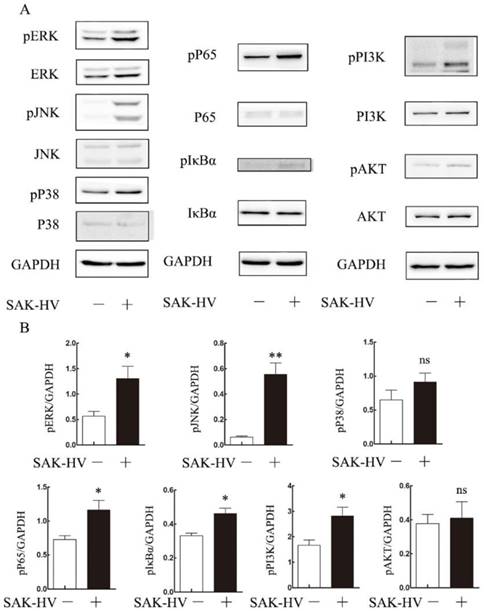
To explore the molecular mechanism of SAK-HV-augmented macrophage migration, we screened the pathways closely related to cellular migration including NF-κB, PI3K/AKT pathways and three major members of MAPK family: extracellular signal regulated kinases (ERKs), JNKs and p38 MAPKs both in RAW264.7 cells and primary peritoneal macrophages[21]. The time point at 12h was in line with that of migration detection in transwell assay as detailed previously. As shown in Figure 4, the phosphorylation levels of JNK and ERK were strengthened at 12h in RAW264.7 cells except for that of P38. We also observed evoked phosphorylation levels of p65, IκBα, and also the degradation of IκBα at 12h, suggesting the release of NF-κB from the cytoplasmic NF-κB/IκBα complex and its subsequent translocation to the nucleus. The phosphorylation of PI3K was increased while its downstream AKT pathway was not affected. Besides, ERK, JNK and NF-κB pathways were activated in primary peritoneal macrophages from C57BL/6J mice after SAK-HV treatment, while P38 and AKT pathways were not affected, which was in accordance with RAW264.7 cells (Figure 5). Since PI3K activation was shown to be transient in our previous study and its downstream AKT pathway was not affected [20], we did not make further research on this signaling. In all, these results showed that the activation of JNK, ERK and NF-κB pathways closely correlated with SAK-HV-induced macrophage migration. This also suggested the great consistency between RAW264.7 cells and primary peritoneal macrophages in migration mechanism, thus RAW264.7 cells were used as model in further studies.
Effects of four domains of SAK-HV on migration of macrophages. (A) The migration of RAW264.7 cells was analyzed using the transwell assay, and cells were photographed after SAK-HV, the three individual functional domains of SAK-HV or the wild-type SAK at 1 µM for 12 h (n=3). Only the SAK-mutant domain could induce RAW264.7 cell migration but not the other domains or wild-type SAK. (B) The quantification for transwell assay above. *** P<0.001 versus control group. Abbreviation: hirudin-12, a 12-amino acid peptide from the C-terminus of hirudin.
The effects of SAK-HV treatment on migration-relative pathways (p38 MAPK, ERK, JNK, NF-κB and PI3K/AKT) in RAW264.7 cells at 12h time points. (A) Western blot analysis for the migration-related pathways (n=3). The phosphorylation levels of JNK, ERK and NF-κB pathways but not P38 or PI3K/AKT were upregulated with SAK-HV (1µM) treatment for 12h. (B) The bands quantification for Western blot. * P<0.05 versus control group, ** P<0.01 versus control group. ns, no significance.
The effects of SAK-HV treatment on migration-relative pathways (p38 MAPK, ERK, JNK, NF-κB and PI3K/AKT) in primary peritoneal macrophages at 12h time points. (A) Western blot analysis for the migration-related pathways (n=3). The phosphorylation levels of JNK, ERK and NF-κB pathways but not P38 or PI3K/AKT were upregulated with SAK-HV (1µM) treatment for 12h. (B) The bands quantification for Western blot. * P<0.05 versus control group, ** P<0.01 versus control group. ns, no significance.
SAK-HV promoted macrophage migration via JNK and NF-κB pathways not via ERK pathways. (A-C) The effects of inhibitor U0126(1μM), SP600125(1μM), and BAY11-7082(0.5μM) on the SAK-HV-triggered (0.1μM) migration (n = 3) respectively. The migration was analyzed using the transwell assay, and cells were photographed after SAK-HV and inhibitors treatment. Migration induced by SAK-HV could be inhibited by U0126 and SP600125, not by U0126. (D-F) The quantification for transwell assay. Abbreviation: SP, SP600125. BAY, BAY11-7082. ** P<0.01 versus SAK-HV group, *** P<0.001 versus SAK-HV group. ns, no significance.
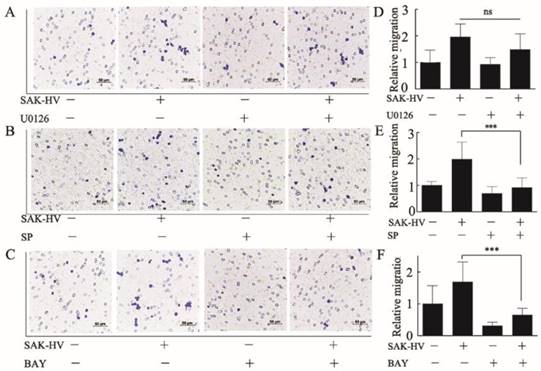
SAK-HV promoted migration via JNK and NF-κB pathways
Furthermore, we used specific inhibitors of the activated pathways mentioned above to determine their critical roles in triggering migration. U0126 (1μM), SP600125 (1μM), BAY11-7082 (0.5μM) were used to inhibit ERK, JNK, and NF-κB respectively. Results showed that SP600125 and BAY11-7082 had obvious inhibitory effects on the migration induced by SAK-HV, while U0126 could not inhibit migration (Figure 6). These results indicated that activation of JNK and NF-κB were critical in SAK-HV-induced macrophages migration.
Effects of SAK-HV treatment on migration-relative effector proteins
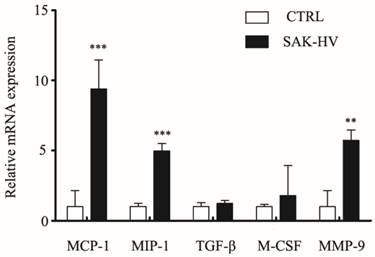
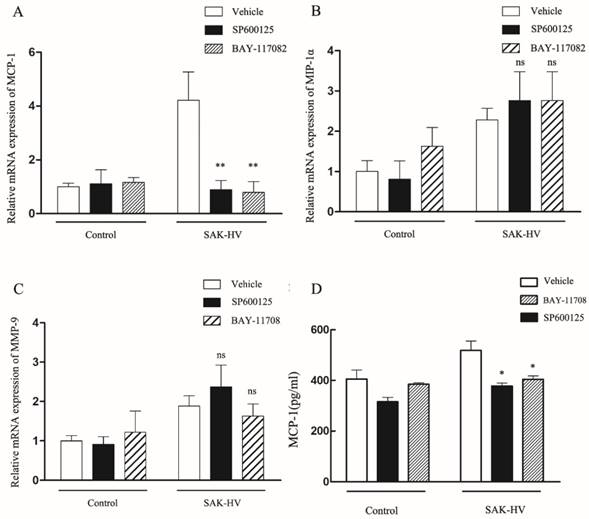
Cytokines like MCP-1, MIP-1α, TGF-β, and M-CSF are reported to regulate macrophage migration. MMPs, especially Matrix Metallopeptidase 9 (MMP-9), are reported to be closely associated with cell migration [22, 23]. In order to determine if migration may be correlated with expression of these effector protein, levels of above five genes were measured using RT-qPCR. As shown in Figure 7, the treatment for 12 h with SAK-HV (1 μM) significantly (P<0.05) increased the transcriptional levels of MCP-1, MIP-1α and MMP-9 without any remarkable effects on levels of TGF-β and M-CSF, suggesting the upregulation of MCP-1, MIP-1α and MMP-9 may play active role in SAK-HV stimulated migration.
Effects of SAK-HV on mRNA expression levels of MCP-1, MIP-1α, TGF-β, M-SCF, MMP-9 in RAW264.7 cells. The relative mRNA expression of candidate genes (as indicated) was assessed by RT-qPCR after SAK-HV (1μM) treatment for 12h (n = 3, three independent experiments). At 12h, the transcriptional level of MCP-1, MIP-1α, MMP-9 were increased, whereas expressions of TGF-β and M-SCF were not altered. ** P<0.01 versus control group, *** P<0.001 versus control group.
SAK-HV promoted MCP-1 expression through JNK/NF-κB pathways
Since it has been shown that migration was mediated by JNK/NF-κB pathways, we further screened the potential effector proteins of them from the transcriptionally upregulated MCP-1, MIP-1α and MMP-9 via the combinationary adoption of the inhibitors of JNK/NF-κB. As shown in Figure 8A-C, only mRNA level of MCP-1 decreased after combined treatment compared with SAK-HV treatment alone, while the levels of MIP-1α and MMP-9 were not reduced. We further detected the protein concentration of MCP-1 in supernatants of SAK-HV-stimulated cells and observed a higher MCP-1 level compared with that of the control group. Similarly, SP600125 and BAY11-7082 inhibited the upregulation of MCP-1 induced by SAK-HV in protein level (P<0.05, Figure 8D). From these experiments, we may conclude that JNK/NF-κB mediated MCP-1 expression in SAK-HV-treated RAW264.7 cells.
MCP-1 was essential for SAK-HV-stimulated macrophage migration
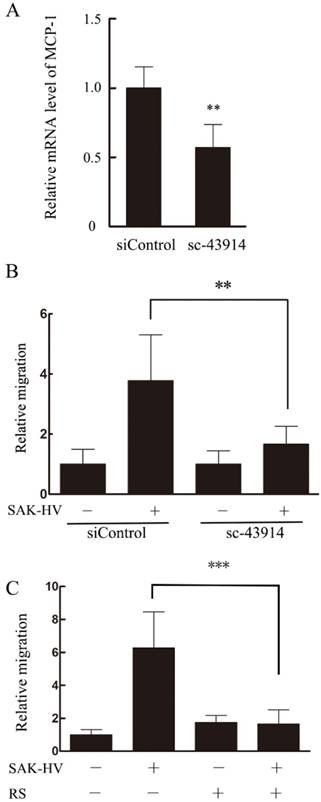
Based on the results above, we hypothesized that MCP-1 might be the key mediator in SAK-HV-stimulated migration. Therefore, specific MCP-1 siRNA (sc-43914) was used prior to treatment with SAK-HV. Since MCP-1 acts mainly through the G-protein-coupled receptor CCR2 to exert its biological effects [24], a selective inhibitor of CCR2b RS102895 was used to detect the CCR2 function as further confirmation of the MCP-1 knocking down. As shown in Figure 9B and C, both of these treatments partly blocked SAK-HV-triggered macrophage migration, thereby indicating that MCP-1 was a critical mediator in migration, and SAK-HV increased RAW264.7 cells migration at least partially via MCP-1 secretion induced by JNK and NF-κB pathways.
Discussion
Macrophages migration to the inflamed tissues is a hallmark of inflammation, which is a prerequisite for rapid phagocytosis of pathogens and apoptotic materials in protective immunity [25]. However, in several pathological conditions the movement of macrophages can also be detrimental, such as aggravating tumor growth and damaging the tissues in chronic inflammation. Although cellular migration is the subject of intense investigation, the mechanisms involved in its regulation are not completely understood [26]. We previously showed that SAK-HV promoted proliferation of macrophages, and obtained similar results in RAW264.7 and primary peritoneal macrophage cells [20]. In this work, we further investigated the effect of SAK-HV on macrophage migration and addressed mechanism by which SAK-HV induced migration basing on the cell model of RAW264.7.
Effects of SAK-HV on mRNA or protein expression levels of MCP-1, MIP-1α, MMP-9 in macrophages with or without SP600125 and BAY11-7082. (A-C) The relative mRNA expression of candidate genes (as indicated) were assessed by RT-qPCR after SAK-HV (0.1μM) treatment for 12h with or without SP600125 (1μM) or BAY11-7082 (0.5μM). Only the mRNA expression level of MCP-1 was decreased by SP and BAY treatment. (D) The protein content of MCP-1 in culture supernatant of cells treated by SAK-HV (0.1μM) with or without SP600125 or BAY11-7082 was analyzed by ELISA. The protein levels of MCP-1 decreased after inhibitors treatment. Abbreviation: SP, SP600125. BAY, BAY11-7082. * P<0.05 versus SAK-HV group, ** P<0.01 versus SAK-HV group. ns, no significance.
Transwell assay showed that SAK-HV induced migration in a concentration-dependent manner in RAW264.7 cells and primary peritoneal macrophage cells. Interestingly, this function was gained from SAK-mutant domain not the other functional domains of SAK-HV or wild-type SAK. Next, we asked how SAK-HV could trigger cellular migration. After analyzing multiple migration-related pathways, we found among the activated pathways, only JNK and NF-κB pathways served central roles in SAK-HV-induced migration in RAW264.7 cells. JNK activation has been shown to be critical for the modulation of cellular migration [27-30], while NF-κB is involved in enhanced expression of several macrophage migration-related genes [31-34], all of which support our findings. Although some studies suggested that ERK, P38 and PI3K/AKT had some role in this process [10, 35-37], they were not involved in our model.
Migratory process is closely regulated by the cytokines production. So, we next asked whether SAK-HV facilitated migration by the production of key cytokines. Given the important function of MMPs family in ECM remodeling, we also focused on MMP-9, which have been regarded as a hallmark of mesenchymal migration mode that macrophages adopt[38]. In SAK-HV-treated cells, MCP-1, MIP-1α as well as MMP-9 were significantly elevated in mRNA levels. Intriguingly, among the upregulated effectors, only MCP-1, but not MIP-1α and MMP-9, could be specifically reduced by chemical inhibition of JNK and NF-κB pathways, implying MCP-1 was the downstream target of JNK and NF-κB activation. Similarly, other studies have confirmed the pivotal roles of JNK and NF-κB in transcription and secretion of MCP-1 in response to IFN-γ and hydrogen peroxide respectively [39, 40].
Based on the results, we hypothesized that MCP-1 could be the determinant mediator and it activated CCR2 thus accelerated migration in an autocrine way. The results that SAK-HV-induced migration could be partly abolished by either interfering MCP-1 expression or inhibiting CCR2 were basically in agreement with our hypothesis. As one of the major chemokines, MCP-1 is known to direct macrophages migration to infection site and activate their function [24, 41]. MCP-1 was shown to elicit PI3K-Rac-lamellipodium protrusion cascade and subsequent chemotaxis in human myelomonocytic leukemia THP-1 cell [42]. In endometrial stromal cells, MCP-1 regulated migration through AKT and Erk1/2 [43], while in mouse bone marrow-derived stromal cells MCP-1-initiated migration was mediated by PI3K/Akt, PAK, and ERK signaling [44]. These reports proved the important role of MCP-1 in migration, which may function as initiative factor as well as effector protein.
MCP-1 was important for SAK-HV induced macrophage migration. (A)The transcriptional level of MCP-1 was reduced significantly in sc-43914 siRNA transfected cells. RAW264.7 cells were transfected with control or sc-43914 48h prior to detection, and siRNA inhibitory efficiency was confirmed by RT-qPCR. **P<0.01 vs. control siRNA-treated cells. (B) The SAK-HV-induced migration could be blocked partly by sc-43914 siRNA. Cells were treated with and without SAK-HV (0.5 μM) for 12 h and cell migration were measured by transwell assay. The relative migration rate decreased after sc-43914 transfection. *P<0.05 vs. control siRNA-treated cells. (C) The migration induced by SAK-HV could be inhibited by CCR2 inhibitor RS. RAW264.7 cells were treated with RS 1h prior to treatment with and without SAK-HV (1 μM) for 12 h and cell migration were measured by transwell assay. *** P<0.001 versus SAK-HV group. Abbreviation: RS, RS102895.
In all, our findings suggest that SAK-HV is a potent stimulator for macrophage recruitment to inflammatory sites. SAK-mutant domain as the functional domain is determinant for migration. Further investigation of mechanism proved that SAK-HV may enhance MCP-1 expression through activating JNK/NF-κB pathways, and thus initiate RAW264.7 cells migration in an autocrine manner, which might be applied to primary macrophages. Given the major role of macrophages in immune system and their correlation with thrombolytic and lipid-lowering effects, it is valuable to clarify the contribution and underlying molecular mechanisms of SAK-HV-mediated regulation of macrophage migration.
Supplementary Material
Supplementary figures.
Methods
Preparation of SAK-HV fusion protein
The preparation process was as indicated in [18]. Briefly, E.coli BL21(DE3) harboring the recombinant plasmids were disrupted by sonication to obtain crude extract, and the clear supernatant of crude extract was loaded onto a Q-Sepharose Fast flow column equilibrated with phosphate buffer and eluted with a linear gradient of NaCl. Fractions containing SAK-HV activity, determined by fibrinolytic activity assay, were collected and then loaded onto a Sephacryl S-200 column equilibrated with 50 mM Phosphate buffer (pH7.4). The column was eluted with Phosphate buffer and fractions containing SAK-HV activity were collected for further analysis.
Cell culture and reagents
RAW264.7 cells were purchased from The National Experimental Cell Resource Sharing Platform, and were cultured in DMEM (Gibco, USA) supplemented with 10 % fetal bovine serum (FBS), in a humidified atmosphere with 5 % CO2.
The Extraction of Primary Peritoneal Macrophage Cells from C57BL/6J Mice
The procedure was as indicated in [20]. Male C57BL/6J mice aged 6-8 weeks were executed followed by soaking in alcohol for 5 min. Then, about 7 mL saline was injected into the abdominal cavity through syringes. After having washed for 3 times, the saline was collected, and the cells were separated by a centrifuge (1200 r/min) for 5 min. Then, cells were transferred into the cell culture dishes with RPMI supplemented with 10% FBS for 4h of incubation. Following this, cells were collected by scraper for further experiments.
Wound Healing
The experiments were performed as described in [45]. Briefly, RAW264.7 cells (3×105 cells / well) were seeded in a 6-well culture plate (Corning-Costar, USA) for 24 h to reach the confluences of 80% before serum starvation for 12 h. Scratched wound lines were created using a 200 μL micropipette tip. Then, cells treated with or without SAK-HV were cultured for 24 h. The wounded area was visualized using a Nikon Eclipse TS2-LS microscope equipped with NIS-Elements 3.0 software (Tokyo, Japan) and was calculated by ImageJ software. Cell motility was estimated by the quantification of the % of recovery using the equation: R (%) = [1 - (wound area at Tt/wound area at T0)] × 100, where T0 is the wounded area at 0 h and Tt is the wounded area after 24 h.
Transwell migration assay
RAW264.7 cell suspensions of 8×104 cells in 100 μL DMEM were added to the upper wells of chemotaxis chambers (Corning-Costar, USA) while 600 μL of medium containing 0.5% FBS were added to the lower wells. Indicated concentration of inhibitors were added to both the upper and lower wells and preincubated for 1 h prior to SAK-HV addition. After 12h, cells in upper wells were removed with a cotton swab, the filters were fixed with Paraformaldehyde (4% in PBS) and stained with crystal violet. Cell migration was assessed by counting the number of migrated cells in five randomly selected microscopy fields per well at 400 magnification.
Cell Transfection
25 pmol of small interfering RNAs (siRNAs) sc-43914 (Santa Cruz Biotechnology, Germany) specific for MCP-1 and control siRNA were added to 2 × 105 cells cultured per well of a 6-well plate. After siRNA transfection for 48 h, cells were seeded in chambers or collected for RNA extraction. Transfections were performed using Lipofectamine RNAiMAX Reagent (Life Technologies, USA) according to manufacturer instructions.
Western Blot
The procedures were described as previous[20]. Briefly, equal amounts of cell protein per sample were electrophoresed through SDS-PAGE (12% polyacrylamide), and then were transferred onto a PVDF membrane (Roche, USA). Blots were incubated overnight with primary antibodies listed below. The primary antibodies included ERK1/2, p-ERK1/2, JNK, p-JNK, PI3K, p-PI3K (Tyr458), AKT, p-AKT, P38, p-P38, P65, p-P65, IκBα, p-IκBα (Cell Signaling Technology, USA), and GAPDH (CWBIO, China). Then, after incubation with secondary HRP-anti-rabbit IgG or HRP-anti-mouse IgG (CWBIO), the PVDF membrane were visualized with the ECL luminescence reagent (Thermo Fisher Scientific, USA). The immunoreactive bands were quantified using ImageJ.
RT-qPCR
Total RNA was extracted from cultured cells after treatment with or without SAK-HV and inhibitors using RNAprep pure Kit (Tiangen, China). The cDNA was synthesized from 5μg of total RNA with 0.5 ng oligo-dT primers using Reverse Transcriptase (Thermo Fisher Scientific). Quantitative real-time polymerase chain reaction was performed using a 7500 Real Time PCR System (Applied Biosystems, USA) and SYBR™ Select Master Mix (Thermo Fisher Scientific). The levels of mRNA were normalized to GAPDH mRNA. Primers for GAPDH were obtained from BBI Life Sciences (China). Primers for MCP-1 were 5'-CCTGCTGCTACTCATTCACCA-3' and 5'-ATTCCTTCTTGGGGTCAGCA-3'. Primers for MIP-1α were 5'-ACCATGACACTCTGCAACCA-3' and 5'-GATGAATTGGCGTGGAATCT-3'. Primers for TGF-β were 5'- CTGCTGACCCCCACTGATAC-3' and 5'-AGCCCTGTATTCCGTCTCCT-3'. Primers for M-CSF were 5'- TTGCCAAGGAGGTGTCAGAAC-3' and 5'- AAAGGCAATCTGGCATGAAGTC-3'. Primers for MMP-9 were 5'- GCCGACTTTTGTGGTCTTCC-3' and 5'- GGTACAAGTATGCCTCTGCCA-3'.
ELISA
The amount of MCP-1 in the culture supernatants was determined using a Mouse CCL2 (MCP-1) ELISA Ready-SET-Go! kit (Neobioscience, China) according to the manufacturer's instructions.
Statistics
All data are presented as the means ± Standard Error of Mean (SEM). Statistical analysis between 2 samples was performed using the Student's t-test. Statistical significance of more than 2 groups was checked with one-way analysis of variance (ANOVA) and Tukey's multiple comparison post hoc tests. In all cases, P<0.05 was considered significant.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (No.81770857) and National Science and Technology Major Project (No.2016YFC1202904).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Osma-Garcia IC, Punzon C, Fresno M. et al. Dose-dependent effects of prostaglandin E2 in macrophage adhesion and migration. European journal of immunology. 2016;46:677-88
2. Wiesner C, Le-Cabec V, El Azzouzi K. et al. Podosomes in space: macrophage migration and matrix degradation in 2D and 3D settings. Cell adhesion & migration. 2014;8:179-91
3. Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263-6
4. Jones GE. Cellular signaling in macrophage migration and chemotaxis. Journal of leukocyte biology. 2000;68:593-602
5. Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. Journal of cell science. 2004;117:4619-28
6. Tarcic G, Yarden Y. MAP Kinase activation by receptor tyrosine kinases: in control of cell migration. Methods in molecular biology. 2010;661:125-35
7. Hirasawa M, Takubo K, Osada H. et al. Angiopoietin-like Protein 2 Is a Multistep Regulator of Inflammatory Neovascularization in a Murine Model of Age-related Macular Degeneration. The Journal of biological chemistry. 2016;291:7373-85
8. Cheng CI, Chen PH, Lin YC. et al. High glucose activates Raw264.7 macrophages through RhoA kinase-mediated signaling pathway. Cellular signalling. 2015;27:283-92
9. Ouro A, Arana L, Rivera IG. et al. Phosphatidic acid inhibits ceramide 1-phosphate-stimulated macrophage migration. Biochemical pharmacology. 2014;92:642-50
10. Arana L, Ordonez M, Ouro A. et al. Ceramide 1-phosphate induces macrophage chemoattractant protein-1 release: involvement in ceramide 1-phosphate-stimulated cell migration. American journal of physiology Endocrinology and metabolism. 2013;304:E1213-26
11. Schall TJ, Bacon K, Camp RD. et al. Goeddel DV. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. The Journal of experimental medicine. 1993;177:1821-6
12. Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends in cell biology. 2004;14:628-38
13. Kim JS, Kim JG, Moon MY. et al. Transforming growth factor-beta1 regulates macrophage migration via RhoA. Blood. 2006;108:1821-9
14. Pixley FJ. Macrophage Migration and Its Regulation by CSF-1. International journal of cell biology. 2012;2012:501962
15. Gong Y, Hart E, Shchurin A. et al. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. The Journal of clinical investigation. 2008;118:3012-24
16. Wolf K, Mazo I, Leung H. et al. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. The Journal of cell biology. 2003;160:267-77
17. Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nature reviews Immunology. 2004;4:617-29
18. Wang M, Wang Y, Wang J. et al. Construction and characterization of a novel staphylokinase variant with thrombin-inhibitory activity. Biotechnology letters. 2009;31:1923-7
19. Zhang C, Huang Z, Jing H. et al. SAK-HV Triggered a Short-period Lipid-lowering Biotherapy Based on the Energy Model of Liver Proliferation via a Novel Pathway. Theranostics. 2017;7:1749-69
20. Zhang C, Chen Y, Gan X. et al. SAK-HV Decreases the Self-Ubiquitination of MEKK1 to Promote Macrophage Proliferation via MAPK/ERK and JNK Pathways. International journal of molecular sciences. 2017;18:1-19
21. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911-2
22. Verollet C, Charriere GM, Labrousse A. et al. Maridonneau-Parini I. Extracellular proteolysis in macrophage migration: losing grip for a breakthrough. European journal of immunology. 2011;41:2805-13
23. Kim YH, Kwon HJ, Kim DS. Matrix metalloproteinase 9 (MMP-9)-dependent processing of betaig-h3 protein regulates cell migration, invasion, and adhesion. The Journal of biological chemistry. 2012;287:38957-69
24. Deshmane SL, Kremlev S, Amini S. et al. Monocyte chemoattractant protein-1 (MCP-1): an overview. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2009;29:313-26
25. Ley K, Laudanna C, Cybulsky MI. et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature reviews Immunology. 2007;7:678-89
26. Ordonez M, Rivera IG, Presa N. et al. Implication of matrix metalloproteinases 2 and 9 in ceramide 1-phosphate-stimulated macrophage migration. Cellular signalling. 2016;28:1066-74
27. Huang Z, Yan DP, Ge BX. JNK regulates cell migration through promotion of tyrosine phosphorylation of paxillin. Cellular signalling. 2008;20:2002-12
28. Miyamoto Y, Torii T, Yamamori N. et al. Paxillin is the target of c-Jun N-terminal kinase in Schwann cells and regulates migration. Cellular signalling. 2012;24:2061-9
29. Jin J, Suzuki H, Hirai S. et al. JNK phosphorylates Ser332 of doublecortin and regulates its function in neurite extension and neuronal migration. Developmental neurobiology. 2010;70:929-42
30. Zhang Y, Bai XT, Zhu KY. et al. In vivo interstitial migration of primitive macrophages mediated by JNK-matrix metalloproteinase 13 signaling in response to acute injury. Journal of immunology. 2008;181:2155-64
31. Ma GF, Chen S, Yin L. et al. Exendin-4 ameliorates oxidized-LDL-induced inhibition of macrophage migration in vitro via the NF-kappaB pathway. Acta pharmacologica Sinica. 2014;35:195-202
32. Lee CY, Su MJ, Huang CY. et al. Macrophage migration inhibitory factor increases cell motility and up-regulates alphavbeta3 integrin in human chondrosarcoma cells. Journal of cellular biochemistry. 2012;113:1590-8
33. Lv W, Chen N, Lin Y. et al. Macrophage migration inhibitory factor promotes breast cancer metastasis via activation of HMGB1/TLR4/NF kappa B axis. Cancer letters. 2016;375:245-55
34. Wang H, Wang X, Li X. et al. CD68(+)HLA-DR(+) M1-like macrophages promote motility of HCC cells via NF-kappaB/FAK pathway. Cancer letters. 2014;345:91-9
35. Stephens L, Ellson C, Hawkins P. Roles of PI3Ks in leukocyte chemotaxis and phagocytosis. Current opinion in cell biology. 2002;14:203-13
36. Liu Z, Jiang Y, Li Y. et al. TLR4 Signaling augments monocyte chemotaxis by regulating G protein-coupled receptor kinase 2 translocation. Journal of immunology. 2013;191:857-64
37. Liu X, Ma B, Malik AB. et al. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nature immunology. 2012;13:457-64
38. Ferrer DG, Dato VA, Fincati JRJ. et al. Activated alpha2 -Macroglobulin Induces Mesenchymal Cellular Migration Of Raw264.7 Cells Through Low-Density Lipoprotein Receptor-Related Protein 1. Journal of cellular biochemistry. 2017;118:1810-8
39. Lee JH, Kim H, Woo JH. et al. 5, 8, 11, 14-eicosatetraynoic acid suppresses CCL2/MCP-1 expression in IFN-gamma-stimulated astrocytes by increasing MAPK phosphatase-1 mRNA stability. Journal of neuroinflammation. 2012;9:34
40. Jaramillo M, Olivier M. Hydrogen peroxide induces murine macrophage chemokine gene transcription via extracellular signal-regulated kinase- and cyclic adenosine 5'-monophosphate (cAMP)-dependent pathways: involvement of NF-kappa B, activator protein 1, and cAMP response element binding protein. Journal of immunology. 2002;169:7026-38
41. Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clinica chimica acta; international journal of clinical chemistry. 2010;411:1570-9
42. Terashima Y, Onai N, Murai M. et al. Pivotal function for cytoplasmic protein FROUNT in CCR2-mediated monocyte chemotaxis. Nature immunology. 2005;6:827-35
43. Li MQ, Li HP, Meng YH. et al. Chemokine CCL2 enhances survival and invasiveness of endometrial stromal cells in an autocrine manner by activating Akt and MAPK/Erk1/2 signal pathway. Fertility and sterility. 2012;97:919-29
44. Ryan CM, Brown JA, Bourke E. et al. ROCK activity and the Gbetagamma complex mediate chemotactic migration of mouse bone marrow-derived stromal cells. Stem cell research & therapy. 2015;6:136
45. Pendyala S, Moitra J, Kalari S. et al. Nrf2 regulates hyperoxia-induced Nox4 expression in human lung endothelium: identification of functional antioxidant response elements on the Nox4 promoter. Free radical biology & medicine. 2011;50:1749-59
Author contact
![]() Corresponding authors: Dong-Gang Xu, E-mail: xudgac.cn; Chao Zhang, E-mail: Zhang.Chaoedu
Corresponding authors: Dong-Gang Xu, E-mail: xudgac.cn; Chao Zhang, E-mail: Zhang.Chaoedu

 Global reach, higher impact
Global reach, higher impact