10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2018; 14(14):2023-2036. doi:10.7150/ijbs.28302 This issue Cite
Research Paper
The intracellular NADH level regulates atrophic nonunion pathogenesis through the CtBP2-p300-Runx2 transcriptional complex
1. Department of Orthopedics, the Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710005, Shaanxi, China;
2. Department of Orthopaedics, Hong-Hui Hospital, Xi'an Jiaotong University, Xi'an 710054, Shaanxi, China;
3. The second department of surgery room, Shaanxi Provincial Tumor Hospital, Xi'an 710061, Shaanxi, China.
* These authors contribute equally to this work.
Abstract
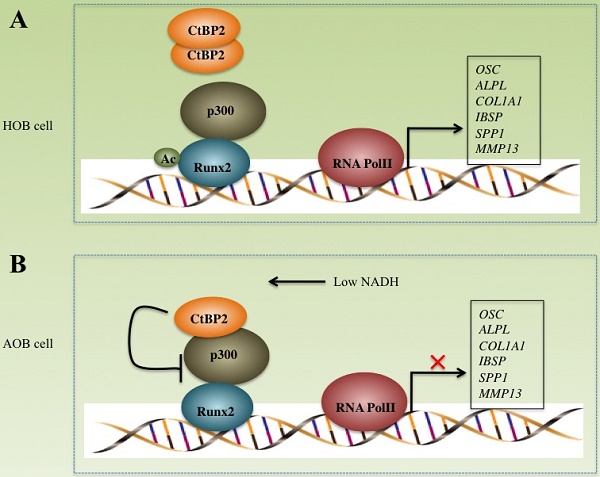
Atrophic nonunion, a complicated failure of fracture healing, is still obscure regarding its molecular pathological mechanisms. Carboxyl-terminal binding proteins (CtBPs), an NADH-sensitive transcriptional corepressor family, are involved in many diseases, such as cancer and inflammation. Here, we found that CtBP2, but not CtBP1, was significantly overexpressed in atrophic nonunion tissues compared to healthy controls. Using a mass spectrometry assay, we found that CtBP2 can form a complex with histone acetyltransferase p300 and transcription factor Runx2. The lower NADH level in atrophic nonunion tissues disrupted CtBP2 dimerization and enhanced the blockage of the accessibility of the p300-Runx2 complex to the promoters of a series of bone-related target genes, such as OSC, ALPL, COL1A1, IBSP, SPP1 and MMP13. The expression of these genes can be reversed by a forced increase in NADH with CoCl2 treatment. In conclusion, our study revealed that NADH levels determine the expression of bone formation and development of related genes through affecting the dissociation or binding of CtBP2 to the p300-Runx2 complex. These results represent a conserved mechanism, by which CtBP2 serves as a NADH-dependent repressor of the p300-Runx2 transcriptional complex and thus affects bone formation.
Keywords: atrophic nonunion, CtBP2, p300, Runx2, NADH/NAD ratio

 Global reach, higher impact
Global reach, higher impact