10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2019; 15(4):738-748. doi:10.7150/ijbs.30106 This issue Cite
Research Paper
USP17 Suppresses Tumorigenesis and Tumor Growth through Deubiquitinating AEP
1. Department of Neurosurgery, Renji Hospital, School of Medicine, Shanghai Jiao-tong University, Shanghai, 200127, China.
2. CAS key laboratory of Tissue Microenvironment and Tumor, Shanghai Institute of Nutrition and Health, Shanghai Institutes for Biological Sciences, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200025, China.
3. Shanghai Institute of Advanced Immunochemical Studies, ShanghaiTech University, Shanghai 201210, China.
4. Department of Radiation Oncology, Ruijin Hospital, School of Medicine, Shanghai Jiao-tong University, Shanghai 200025, China.
*These authors contributed equally to this work.
Abstract
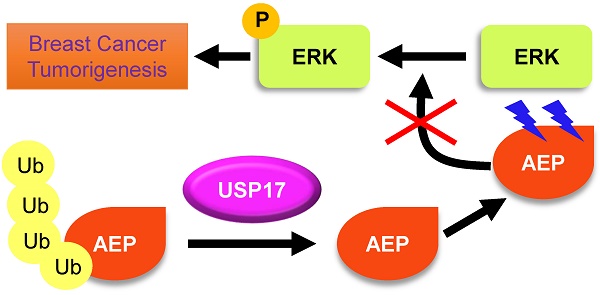
Ubiquitin-specific protease 17 (USP17), a novel member of deubiquitinase, is reported to play essential roles in several solid tumors. However, the expression and function of USP17 in breast cancer tumorigenesis remains ambiguity. Here we found that the mRNA level of USP17 was lower in breast cancer tissues than normal tissues. Meanwhile, higher USP17 level was detected in normal epithelial cell MCF-10A and a less-malignant cell MCF-7 than malignant cell line MDA-MB-231. Inhibition of USP17 in MCF7 cells enhanced tumorigenesis and tumor growth while overexpression of USP17 in malignant MDA-MB-231 cells reduced its tumorigenesis and growth ability in vitro and in vivo. Further study revealed that USP17 interacted with and deubiquitinated Asparaginyl endopeptidase (AEP), resulting in decreased protein levels of AEP. Moreover, knockdown of AEP inhibited breast cancer tumorigenesis and growth in vitro and in vivo through the inactivation of ERK signaling. Taken together, our works indicate that USP17 deubiquitinates AEP, down-regulates its protein level, and inhibits breast cancer tumorigenesis through disturbing ERK signaling. Thus, our data suggests that USP17 is a potential tumor suppressor in breast cancer and AEP is a promising target in breast cancer therapy.
Keywords: Breast cancer, AEP, USP17, Deubiquitination, ERK

 Global reach, higher impact
Global reach, higher impact