ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2023; 19(11):3412-3427. doi:10.7150/ijbs.80351 This issue Cite
Research Paper
DBF4 Dependent Kinase Inhibition Suppresses Hepatocellular Carcinoma Progression and Potentiates Anti-Programmed Cell Death-1 Therapy
1. Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China.
2. Key Laboratory of Organ Transplantation, NHC Key Laboratory of Combined Multi-organ Transplantation, Hangzhou, China.
3. Department of Hepatobiliary and Pancreatic Surgery, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Research Center of Diagnosis and Treatment Technology for Hepatocellular Carcinoma of Zhejiang Province, Hangzhou, China.
4. Department of Medical Oncology, Sir Runrun Shaw Hospital, College of Medicine, Zhejiang University, Hangzhou, China.
Abstract
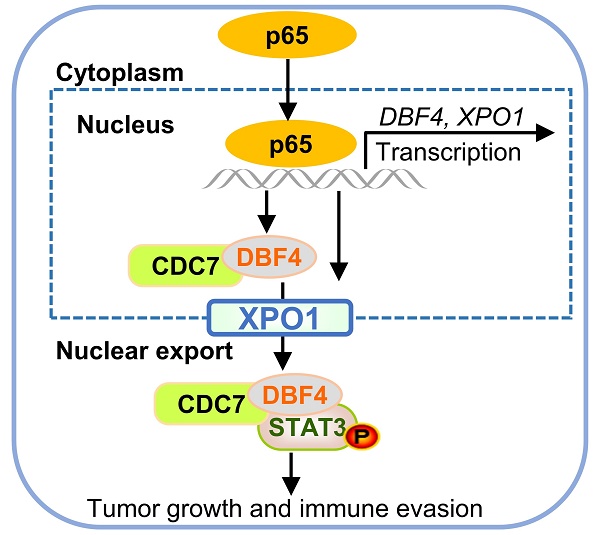
The progression of hepatocellular carcinoma (HCC) remains a huge clinical challenge, and elucidation of the underlying molecular mechanisms is critical to develop effective therapeutic strategy. Dumbbell former 4 (DBF4) complexes with cell division cycle 7 (CDC7) to form DBF4-dependent kinase (DDK), playing instrumental roles in tumor cell survival, whereas its roles in HCC remain elusive. This study revealed that DBF4 expression was upregulated in HCC and constituted an independent prognostic factor of patient survival. We identified p65 as an upstream inducer which increased DBF4 expression by directly binding to its promoter. DBF4 accelerated HCC cell proliferation and tumorigenesis in vitro and in vivo. Mechanistically, DBF4 complexed with CDC7 to bind to the coiled coil domain of STAT3 and activate STAT3 signaling through XPO1-mediated nuclear exportation. Notably, p65 enhanced the nuclear transport of DDK and DDK-STAT3 interaction by transcriptionally upregulating XPO1. DBF4 expression positively correlated with activated STAT3 and XPO1 in HCC tissues. Furthermore, combining DDK inhibitor XL413 with anti-PD-1 immunotherapy dramatically suppressed HCC growth and prolonged the survival of HCC-bearing mouse. Our findings reveal that DDK activates STAT3 pathway and facilitates HCC progression, and demonstrate the proof of the concept of targeting DDK to improve the efficacy of HCC immunotherapy.
Keywords: DDK, STAT3, XPO1, liver cancer, combined therapy
