ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2023; 19(13):4166-4180. doi:10.7150/ijbs.86855 This issue Cite
Review
Molecular mechanisms of pyroptosis and its role in anti-tumor immunity
1. The First Clinical College, Guangdong Medical University, Zhanjiang, 524023, Guangdong, China.
2. Department of Rheumatism and Immunology, Peking University Shenzhen Hospital, Shenzhen, Guangdong, 518036, China; Shenzhen Key Laboratory of Inflammatory and Immunology Diseases, Shenzhen, Guangdong, 518036, China.
3. The Marine Biomedical Research Institute, Guangdong Medical University, Zhanjiang, 524023, Guangdong, China.
4. The Marine Biomedical Research Institute of Guangdong Zhanjiang, Zhanjiang, 524023, Guangdong, China.
# Equal contribution.
Abstract
Pyroptosis is a form of cell death that is characterized by the destruction of the cell, and it has implications in both the immune system and cancer immunotherapy. The gasdermin family is responsible for the activation of pyroptosis, which involves the formation of pores in the cellular membrane that permit the discharge of inflammatory factors. The inflammasome response is a powerful mechanism that helps to eliminate bacteria and cancer cells when cellular damage occurs. As tumor cells become more resilient to apoptosis, other treatments for cancer are becoming more popular. It is essential to gain a thorough understanding of pyroptosis in order to use it in cancer treatment, considering the intricate association between pyroptosis and the immune system's defensive reaction against tumors. This review offers an overview of the mechanisms of pyroptosis, the relationship between the gasdermin family and pyroptosis, and the interplay between pyroptosis and anti-tumor immunity. In addition, the potential implications of pyroptosis in cancer immunotherapy are discussed. Additionally, we explore future research possibilities and introduce a novel approach to tumor treatment.
Keywords: pyroptosis, tumor microenvironment, Gasdermin, inflammasome, antitumor immunity
Introduction
Pyroptosis, also known as a type of programmed cell death (PCD), is vital to immune responses in the human immune system. As part of innate immunity, PCD not only contributes to guarding against pathogens, but also to eliminating tumor cells, which has attracted the attention of a growing number of scientists. Based on changes in cell morphology, PCD is usually divided into lytic and nonlytic cell death[1]. Apoptosis is a relatively "quiet" immune clearance called non-lytic PCD, while pyroptosis is a relatively "robust" clearance called lytic PCD. Inflammasomes activation and the release of various pro-inflammatory factors are two extremely crucial processes in pyroptosis to guard against infections. Generally, gasdermins (GSDMs), the executor of pyroptosis, have been believed to exist as a novel therapeutic target for the development of cancer medications[2].
Pyroptosis was first used in 2001 to describe a unique way of PCD[3]. Pyroptosis is characterized by cell swelling and volume enlargement. When swelling reaches a certain level, the cells rupture and break down[4]. Researchers have demonstrated that there are a large number of bubble-like protrusions related to GSDMD on the cell membrane[4]. Gasdermine D (GSDMD) is cleaved into a C-terminal domain (GSDMD-C) and an N-terminal domain (GSDMD-N) translocated to the cell membrane to form oligomers, also known as "pyroptotic bodies". The cell membranes form non-selective ion-permeable pores through "pyroptotic bodies", allowing small molecules to pass, resulting in an unbalanced osmotic pressure inside and outside the cell, giving rise to water inflow, usually accompanied by ions outflow[5-7].
Morphologically, pyroptosis shares feature with apoptosis and necrosis. Similar to apoptosis, pyroptosis is characterized by nuclear shrinkage resulting from DNA damage[8, 9]. However, distinct from apoptotic cells, pyroptotic cells maintain mitochondrial integrity and cytochrome c is not released[5, 6]. Similar to necrosis, cell membrane integrity is disrupted. Cytoplasmic inflammatory contents containing mature interleukin-1β (IL-1β) and interleukin-18 (IL-18) are rapidly released and induce an inflammatory response[8]. Distinct from necrosis, pyroptosis is usually mediated by caspase-1 associated with the release of IL-1β and IL-18[5]. Further research related to pyroptosis has shown that other caspases also mediate pyroptosis, and pyroptosis is usually dependent of caspases[10].
Recent investigations have demonstrated that pyroptosis is strongly linked to anti-tumor immunity and inflammatory reactions. Pyroptosis has the capacity to impede cancer growth, invasion, and metastasis, yet it may also have a tumor-promoting effect in a number of cancers. Consequently, pyroptosis may be employed in novel approaches for cancer therapy. Pyroptosis is a molecular mechanism that is involved in anti-tumor immunity. This process involves the activation of caspases, resulting in the release of pro-inflammatory cytokines and other molecules. We likewise examine the use of pyroptosis in cancer therapy, as well as its potential future directions.
The molecular mechanisms of Pyroptosis
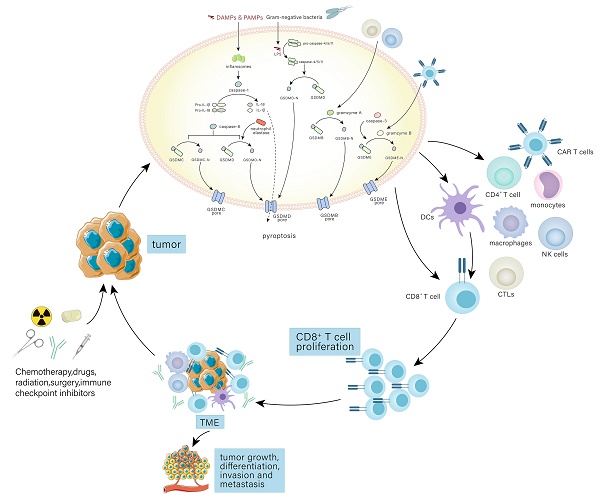
Pyroptosis has multiple pathways through which it can be initiated, such as the standard inflammasome pathway, the non-canonical inflammasome pathway, and the alternative signaling pathways (Fig. 1).
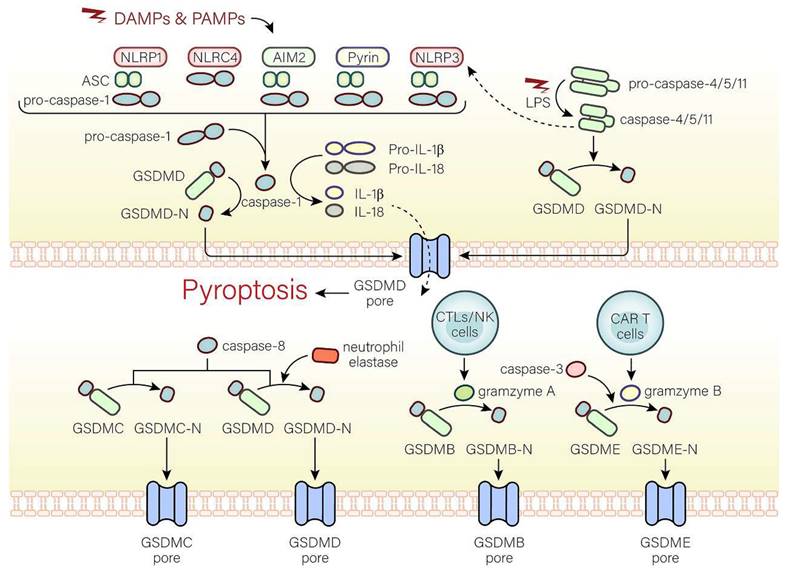
The molecular mechanisms of pyroptosis. In the canonical pathway, the canonical inflammasome pathway (NLRP1, NLRP3, NLRC4, AIM2, Pyrin) is activated and assembled first. Subsequently, the classical inflammasome triggers GSDMD-dependent pyroptosis through caspase-1. In the non-canonical pathway, the non-canonical inflammasome activated by LPS begins to assemble and also trigger GSDMD-dependent pyroptosis. Among alternative signaling pathways, CTLs, NK cells, and CAR T cells can deliver granzymes to target cells through the pores formed by perforin on target cells, thereby cleaving GSDMs, and triggering pyroptosis. Caspase-3 and caspase-8 triggers pyroptosis through pores on the cell membrane which are caused by GSDME-N and GSDMC-N respectively. Neutrophil elastase can mediate GSDMD-dependent pyroptosis.
The canonical inflammasome pathway
The canonical inflammasome pathway is mediated by pattern-recognition receptors (PRRs), including NOD-like receptor (NLR) family pyrin domain-containing (NLRP1), NLR protein 3 (NLRP3), NLR protein C4 (NLRC4), absent in melanoma 2 (AMI2), and pyrin, which can identify pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs). PRRs are stimulated and activated, recruiting caspase-1 and combining with caspase-1 and an adaptor protein (ASC), both of which are assembled into inflammasomes[11, 12]. Caspase-1, the key to pyroptosis in the canonical inflammasome pathway, is activated by inflammasomes and cleaves not only pro-IL-18 and pro-IL-1β to form active IL-18 and IL-1β, but also GSDMD into GSDMD-C and GSDMD-N[11]. GSDMD-N binds to phospholipid proteins on cell membranes, forming pores and triggering pyroptosis and inflammatory responses[4, 11]. However, there are huge differences in pyroptosis induced by different inflammasomes. In the following, we will discuss the activation of inflammasomes.
NLRP1 can form inflammasomes that trigger pyroptosis and inflammatory reactions[13]. The human genomes only contain NLRP1 gene, while three NLRP1 orthologs (NLRP1a, NLRP1b, NLRP1c) are present in mice[14]. After pathogen recognition, NLRP1, NLRP1a, or NLRP1b subsequently assembles with ASC, and pro-caspase-1 via caspase recruitment domain (CARD) to form the NLRP1 inflammasome, whereas ASC isn't essential to the NLRP1 inflammasome in mice[15-17]. In addition, the inhibition of the dipeptidyl peptidases 8 and 9 (DPP8 and DPP9), cytosolic serine dipeptidyl peptidases, serves to activate and assemble the NLRP1b inflammasome, thus triggers caspase-1-dependent pyroptosis[17].
The NLRP3 inflammasome is characterized by NLRP3, ASC, and pro-caspase-1 and activated by two pathways[18]. In the typical activation of the NLRP3 inflammasome, two key signals, namely, the initiation signal (signal 1) and the activation signal (signal 2), are necessary. The function of signal 1 is to increase the NLRP3 and pro-IL-1β expression level, whereas the function of the activation signal is to promote the formation of the NLRP3 inflammasome. With permission from signal 1, signal 2 can work. In signal 1, after Toll-like receptor 4 (TLR4) receptors, the myeloid differentiation primary response 88 (MyD88) receptors, or time-restricted feeding (TRF) receptors accept the stimulation of microorganisms or the effect of pro-inflammatory factors, NLRP3 and pro-IL-1β transcription will increase through the IL-1 receptor-associated kinase (IRAK) or nuclear factor-kappa B (NF-κB) pathway[19, 20]. Different NLRP3 activators (such as mitochondrial reactive oxygen species (ROS) production, cholesterol crystals, calcium mobilization, etc.) can trigger signal 2[19-22]. In the pathway of the non-canonical NLRP3 inflammasome activation, caspase-4/5 in humans and caspase-11 in mice mediate the activation of NLRP3 inflammasome[23-28].
The NLRC4 inflammasome can be activated by Salmonella, the flagellin of the pathogen Legionella pneumophila, and the rod-shaped portion of the type III secretion system (TTSS). Notably, flagellin and TTSS rod-shaped proteins are not directly recognized by NLRC4 but by NAIP and apoptosis inhibitory proteins in the NLR family[29-31].
AIM2 is believed to exist as a DNA sensor that recognizes cytoplasmic DNA, especially double-stranded DNA[32, 33]. Notably, only dsDNA of at least 80 bp in length can connect to the HIN-200 domain[34, 35]. Negatively-charged bacterial dsDNA can replace the PYD domain of AIM2 and bind to the positively-charged HIN-200 domain through electrostatic effects, activating the PYD domain. AIM2 is subsequently assembled with ASC and caspase-1 to form the AIM2 inflammasome[33, 34, 36, 37].
Pyrin can indirectly detect inactive proteins under the action of many bacteria[38, 39]. In humans, Protein Kinase N1 (PKN1) and Protein Kinase N2 (PKN2), members of the protein kinase C (PKC) superfamily of kinases, are activated by Ras homolog gene family member A (RhoA), connect to human pyrin and phosphorylate S208 and S242, which are subsequently assembled with 14-3-3ε or 14-3-3τ to inhibit pyrin activation[39]. In mice, the combination between phosphorylated Ser-205 and Ser241 and the 14-3-3 protein inhibits pyrin activation[40]. However, with the inactivation of the Rho-modified protein, the interaction between the 14-3-3 protein and pyrin is reduced, and the inhibitory effect is reduced, promoting the activation and assembly of the pyrin inflammasome that is characterized by pyrin, ASC, and caspase-1[41, 42].
The non-canonical inflammasome pathway
The caspase-4/5/11-LPS complex is believed to exist as the non-canonical inflammasome that mediates the non-canonical pathway. In the cytoplasm, pathogenicity-associated lipopolysaccharides (LPS) of some gram-negative bacteria can bind to caspase-4/5/11 without inflammasomes by CARD fragments[28]. In addition, CARD fragments of caspase-4/5 are in humans and CARD fragments of caspase-11 are in mice[28]. Moreover, caspase-4 activation is dependent on guanylate-binding proteins that recruit and activate caspase-4 at the bacterial membranes, while caspase-11 activation is mediated by high mobility group box 1 (HMGB1) [43, 44]. The activated caspase-4/5/11 cleave GSDMD at ASP276 and subsequently release the GSDMD-C fragment and GSDMD-N pore-forming fragment that disrupts the membrane integrity and triggers pyroptosis. In addition, the non-canonical inflammasome can also activate the NLRP3 inflammasome, suggesting that the non-canonical pathway is associated with the canonical pathway[23, 25, 28]. However, the relationship between the non-canonical pathway and the canonical pathway is still unknown and worth investigating.
Alternative signaling pathways
Pyroptosis can also be activated by other pathways. Caspase-3 induces pyroptosis through the pores on the cell membrane caused by gasdermine E (GSDME)[45]. During Yersinia infection, the receptor interacting protein 1 (RIPK1)-caspase-8 complex cleaves GSDMD and induces pyroptosis dependent on Folliculin-Folliculin-interacting protein2-Rag-Ragulator complex[46]. Moreover, active caspase-8 can cleave oxidized death receptor DR6-dependent gasdermine C (GSDMC) to form GSDMC-N segments and cause pyroptosis[47]. Besides caspases, pyroptosis can be mediated by other substances. GSDMD cleavage in neutrophils is dependent on neutrophil elastase and involved in pyroptosis[48]. In cells undergoing apoptosis, cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells can produce granzyme A (GZMA) that transports into cells. If gasdermine B (GSDMB) is present in the cytoplasm, GSDMB will be cleaved by GZMA into GSDMB-N-terminal to trigger pyroptosis[49]. In addition, granzyme B (GZMB) released from chimeric antigen receptor (CAR T) cells mediates the cleavage of GSDME to cause pyroptosis[50].
Gasdermin family and pyroptosis
GSDMs, a family of intracellular proteins, can mediate pyroptosis. Six GSDMs proteins (GSDMA, GSDMB, GSDMC, GSDMD, GSDME, DFNB59) are present in the human system, while ten GSDMs proteins (GSDMA1, GSDMA2, GSDMA3, GSDMC1, GSDMC2, GSDMC3, GSDMC4, GSDMD, GSDME, DFNB59) are edited in mice[51, 52]. The Gasdermin family (except DFNB59) consists of the C-terminal inhibitory domain associated with the intermediate transition region and the N-terminal domain that binds to lipids in the cell membrane to generate pores, interrupt the integrity of cell membrane integrity and liberate cellular contents, resulting in the extracellular release and pyroptosis[52, 53]. Of note, GSDMD is regarded as the strongest substrate of inflammatory caspases and the only caspase-1 substrate for inducing pyroptosis[54-57]. In mouse D276 and human D275, the linker between GSDMD-C and GSDMD-N has a caspase-1-targeted cleavage site that is activated by the downstream inflammasome complexes. After cleavage, this weakens the inhibitory of GSDMD-C on GSDMD-N that freely oligomerizes at the cell membrane, forming pyroptosis-inducing pores and triggering pyroptosis[58].
Immune cells and pyroptosis
The immune system is characterized by innate and adaptive immunity and believed to exist as a defense against pathogens and a useful tool to eliminate cancer cells. The immune system is also composed of immune organs, immune cells and immune molecules substances and of importance to triggering an antimicrobial response to maintain a relatively stable internal environment. Immune cells are composed of macrophage, dendritic cells (DCs), NK cells, T cells, B cells, CTLs, Myeloid-derived suppressor cells (MDSCs), CAR T cells etc. The innate immune system can identify and fight foreign pathogens. For example, DCs, macrophages, and mast cells perform immune surveillance and release immune mediators when the host is attacked. However, the adaptive immune system requires DCs and NK cells to ingest foreign antigens and to remember previous attacks[59]. Upon identification of PAMPs and DAMPs by PRRS, inflammasomes are activated, which facilitate inflammatory factors maturation, including IL-1β and IL-18, and N-terminal fragment formation through proteolysis of GSDMs. It lyses cells and causes pyroptosis, and immune cells (such as NK cells, CD8+ T cells, B cells, CTLs, CAR T cells, Macrophage, etc.) are subsequently recruited to protect the host, accompanied by increased immune activity[60]. Nevertheless, pyroptosis can serve to suppress immunoreaction and the mechanisms of pyroptosis in immune regulation are still not clear[60].
The obscure tie between immune cells and pyroptosis seem to be related to inflammasomes, GSDMs and inflammatory factors, such as HMGB1, IL-1β and IL-18. For example, following the NLRC4 inflammasome activation in DCs through recognition of flagellin, noncognate memory CD8+ T cells are activated to promote interferon-γ (IFN-γ) secretion, accompanied by release of IL-18[61]. Interestingly, recent research has shown that during Salmonella infection, the NLRP3 inflammasome is inhibited, which reduces CD4+ T cells activity, suggesting that inflammasomes may be a potential target for immunotherapy[62]. Recently, researchers have found that, in breast cancer, the GSDME expression level is increased and caspase-8 can cleave GSDME, triggering pyroptosis and increasing DCs, CD4+ and CD8+ T cells[63]. Moreover, researchers have revealed that the expression of GSDMB have a relationship with CD4+ T cells and neutrophils in clear cell renal cell carcinoma, suggesting that GSDMB shows potential in controlling immune infiltrates[64]. It has been reported that IL-18 released by pyroptotic cells recruits or differentiates some immune cells, including NK cells, T cells, and monocytes, to regulate the immune system[65]. Following the cleavage of pyroptotic cells, however, HMGB1 is released and inhibits immune activity by facilitating MDSCs accumulation that are implicated in the inhibition of CD4+ and CD8+ T cells activation[66]. In addition, IL-1β contributes to recruiting monocytes and producing macrophages, resulting in the decline of DCs and inhibiting the immune activity[67]. All the evidence embodies that pyroptosis has a dual role in immune cells and exerts effects on quantity and quality of immune cells. Taken together, pyroptosis can regulate immune cells through inflammasomes, GSDMs and inflammatory factors and these may have the potential to enhance immune response. Of note, some immune cells, including CTLs, NK cells, and CAR T cells, can promote or regulate pyroptosis though special proteins, such as neutrophil elastase and granzymes[48-50].
Pyroptosis affects anti-tumor immunity through the tumor microenvironment
When exerting anti-tumor immunity, pyroptosis is inextricably linked with the tumor microenvironment (TME). The cellular parts of the tumor immune microenvironment (TIME) mainly contain tumor cells, endothelial cells and immune cells, among which the most relevant to anti-tumor activity is immune cells[68]. CTLs and NK cells can inhibit the development of tumor cells, while infiltrating immune cells such as macrophages and neutrophils can support tumor growth and escape. Regulatory T cells and MDSCs can inhibit immune response on the surface of tumor cells[69].
Currently, it is revealed that pyroptosis can neutralize tumor immunity though TME (Table 1). On the one hand, pyroptosis affects anti-tumor immunity by releasing various cytokines. When tumor cells undergo pyroptosis, it is often attended by the release of the inflammatory cytokines IL-1β and IL-18, which possess pro-tumor and tumor-suppressive effects[70]. IL-18 takes a significant role in tumor growth, angiogenesis, invasion, and metastasis. It has been reported that IL-18 induces the differentiation of T-helper 1 (Th1) and T-helper 17 (Th17) cells, resulting in anti-tumor effects[71]. IL-18 modulates both innate and adaptable immune responses by recruiting or differentiating NK cells, T cells, monocytes, and other immune cells, hampering the growth and metastasis of various types of tumors. IL-18 also increases interferon (IFN) release and the killing roles of CTLs, neutrophils, and NK cells[65]. On the other hand, granzymes can activate certain GSDMs family proteins to affect anti-tumor immunity. GZMA can activate GSDMB, while GZMB and caspase-3 can activate GSDME[49, 72]. CTLs can induce pyroptosis in human GSDME or GSDMB-sensitized tumor cells. Cytolysis causes target cell death, and pyroptosis may enhance the killing effect of cytotoxic T lymphocytes in cancer cells. In particular, pyroptosis induction overcomes immunosuppression and activates systemic anti-tumor immunity. Tumor cells will produce a great number of neoantigens that stimulate the responses of systemic immune and conspicuously inhibit the tumor progression, which together with tumor cell eradication, is an important pathway to achieve long-term tumor control[73].
Tumor cells often express immune checkpoint molecules that suppress T cells' function in tumors. For example, the interaction of programmed death ligand 1 (PD-L1) and programmed death-1 (PD-1) on T cells inhibit target recognition. Despite the fact that neither blocking the PD-1-PD-L1 pathway nor the temporary pyroptosis induction could inhibit the growth of 4T-1 tumor alone, combination therapy exerts a great inhibitory effect on tumor growth. Furthermore, human GSDMB expresses PD-L1 in mouse colon cancer and melanoma cells without affecting tumor growth in immunocompetent mice. Nevertheless, it enhanced tumor growth inhibition by blocking immune checkpoints with anti-PD-1 antibodies[49, 74]. The effectiveness of current immunotherapies depends on the existence of anti-tumor immunity, and the suppressive TIME inhibits the ability of T cells to fight tumor cells. Abnormalities in tumor vasculature can create physical barriers to T cell trafficking, which is detrimental to tumor immunity. Therefore, the precondition of initiating anti-tumor immunity is the normalization of microenvironment[75, 76].
The role of pyroptosis in cancer immunotherapy
| Cancer type | Pyroptosis signaling | Immune factors | Increased Immune cells | References |
|---|---|---|---|---|
| Breast cancer | GSDMA3; NLRP3; GSDMD-mediated | Increase IL‐1β; IL‐18; HMGB1; IFNg; Gzm‐A, Gzm‐B; Gzm‐K; Fasl; IFN-γ. Decrease TAN, MDSC, and TAM populations. | Macrophage; CD4+T cells; CD8+ T cells; NK cells; CTLs; Long‐term memory T cell; leukocyte; DCs | [63, 74, 77] |
| Non‐small cell lung cancer cells | Caspase-1; caspase-3-mediated | Increase IL‐1β | RAW264.7 cells; peritoneal macrophages; THP‐1 cells; Tfh cells | [78-80] |
| Cholangiocarcinoma | Caspase-3-mediated | Increase IL‐1β | Macrophages | [81] |
| Melanoma | Caspase-1-mediated | Increase HMGB1 | CD4+ T cells; CD8+T cells | [82] |
| Colon adenocarcino | Caspase-3-mediated | Increase IL‐1β and IL‐18 | CD3+T cells; CD4+ T cells; CD8+T cells; CTLs; DCs | [83] |
| Cervical cancer | GSDMA3-mediated | Increase IL‐1β; IL‐18; HMGB1; | CD4+ T cells; CD8+T cells; NK cells; | [74] |
| Acute leukemia | Caspase-3; caspase-8; GSDME-mediated | Increase IL‐1β and IL‐18 | THP-1 cells | [84, 85] |
| Gastric cancer | Caspase-3; GSDME-mediated | Increase TNF and IL‐17 | CD4+T cells; CD8+ T cells; NK cells; TH1 cells | [86, 87] |
| Kidney Renal Clear Cell Carcinoma | Caspase-3; caspase-1; GSDME-mediated | Increase IL‐1β and IL‐18 | CD4+T cells; CD8+ T cells; Tfh cells;Treg cells | [88] |
Inflammasomes in anti-tumor immunity
Inflammasomes have become the focus of many researchers due to their significant effect on anti-tumor immunity. Just like inflammation, inflammasomes have a two-sided impact on anti-tumor immunity. Inflammasomes can both inhibit and facilitate tumor growth, differentiation, invasion, and metastasis, depending on the factors that are yet to be explored. The relatively mature role of inflammasomes in tumors and anti-tumor immunity are listed in Table 2.
Inflammasomes are of importance to the regulation of various tumors and there is a close association between the expression of inflammasomes and the prognosis of patients[89, 90]. The regulation of NLRP3 may be a potential tool to control tumor development due to the inhibitory effect of NLRP3 on the progression and development of cancer cells in liver cancer and gastric cancer. NLRP3 expression is down-regulated in liver cancer and closely related to prognosis, as evidenced by work, suggesting that NLRP3 exert effects on liver cancer[91]. Recent evidence has shown that pyroptosis triggered by the NLRP3 inflammasome inhibits gastric cancer progression, which may provide a new therapeutic approach for gastric cancer patients in the future[92]. Similar to the NLRP3 inflammasome in liver cancer, the AIM2 inflammasome also possesses similar anti-tumor effects. In liver cancer, the expression level of AIM2 is often decreased, and exogenous overexpression of AIM2 can suppress cancer cells progression and development by inducing pyroptosis and inhibiting the mTOR-S6K1 pathway[93]. Moreover, AIM2 expression level is also significantly decreased or severely absent in colorectal cancer, and expression of the AIM2 inflammasome is negatively correlated with colorectal cancer-specific death and disease recurrence in patients, suggesting that there is a negative relationship between the AIM2 inflammasome and colorectal cancer[94]. It has been shown that in breast cancer, the NLRC4 inflammation expression is upregulated, triggering inflammation and subsequently inducing tumor invasion[95]. Interestingly, Zhai et al. [96] has found that along with increased levels of IL‐1β, NLRP1 serves to facilitate tumor development by inhibiting caspase-2/9-mediated apoptosis reliant on caspase-3/7 in metastatic melanoma. Overall, these studies indicate that roles of inflammasomes in tumors are complex and related to the type of inflammasomes and tumor, and TME.
Inflammasomes can regulate immune cells and inflammatory factors, such as IL-1β and IL-18, to inhibit tumor growth, differentiation, and metastasis. Recent research has demonstrated the way inflammasomes protect against tumors by activating anti-tumor immunity through IL-1β and IL-18. Evidence embodies that the release of IL-1β by activated NLRP3 inflammasome can promote the expansion of CD8+ T cells and improve the activity of immune checkpoint blockers to regulate immune functions[97]. In agreement, Dania et al. [98] have found that IL-1β released by activated inflammasomes in DCs is beneficial to the hyperactivation of DCs and stimulated powerful CTLs responses to strengthen anti-tumor immunity, thereby eradicating tumors in whole organisms. Li et al.[99] have shown that the NLRP3 inflammasome exerts effects on the anti-tumor process of the CD39 antibody. The release of IL-18 mediated by the NLRP3 inflammasome increases the expression level of effector T cells, enhancing the effect of anti-tumor immunity. Moreover, the NLRP3 inflammasome blockade suppresses head and neck squamous cell carcinomas (HNSCC) growth by downregulating IL-1βexpression and reducing MDSCs, PD-1 and tumor-associated Macrophages (TAMs) in head and neck squamous cell carcinoma[100]. Interestingly, the NLRP3 inflammasome expression is negatively correlated to the number of effective CD4+ and CD8+ T cells that exert effects on anti-tumor immunity. In metastatic breast cancer, the NLRP3 inflammasome inhibition delays tumor growth by reducing IL-1β in metastatic breast cancer[101]. Of note, the NLRP3 inflammasome inhibition during cancer treatment plays a synergistic in anti-PD-1 treatment. Apart from the NLRP3 inflammasome, other inflammasomes are of importance to suppressing tumor progression. Lin et al. [102] have found that NLRC4/neuronal apoptosis inhibitor protein 5 (NAIP5) activated by flagellin is thought to be involved in anti-tumor induced by CD8+ T cells. Chai et al. [103] have demonstrated that the combination of a DNA vaccine and AIM2 in H1 Nanoparticles is important for the expansion of CD8+ T cells to suppress tumor growth in renal carcinoma. In summary, inflammasomes inhibit tumor development by the regulation of immune cells or inflammatory factors and may be a target for therapy against tumors.
However, cancer cells can also evade anti-tumor immunity through immunosuppression induced by inflammasomes. A deeper understanding of immunosuppression induced by inflammasomes has important implications for new ideas in cancer therapy. In melanoma cell line B16-F10, the NLRP3 inflammasome promotes the MDSCs accumulation to counteract anti-tumor immunity[104]. In pancreatic ductal adenocarcinoma (PDA), the NLRP3 inflammasome is beneficial to the expansion of immune-suppressive macrophages that promote PDA growth[105]. Lu et al. [106] have found that the NLRP3 inflammasome exerts effects on the up-regulation of PD-L1 to facilitate lymphoma growth in diffuse large B cell lymphoma. What's more, the NLRP3 inflammasome blockade during cancer treatment plays an antagonistic role in anti-PD-L1 therapy due to an imbalance in T cell proportion[106]. The NLRP3 inflammasome appears to have different immunosuppressive effects depending on the cancer type, suggesting a connection with cancer type specificity. This suggests that targeting NLRP3 may be a potential way of augmenting anti-tumor immunity. The inflammasomes of pyroptosis have a dual effect on anti-tumor immunity, potentially offering a new outlook on tumor immunotherapy.
Gasdermin family in anti-tumor immunity
Increasing evidence proves that the GSDM family exert effects on anti-tumor immunity. Here, we will focus on the latest studies based on the GSDM proteins involved and the pyroptosis in anti-tumor immunity.
GSDME in anti-tumor immunity
Research on both in vitro and in vivo models has shown GSDME to have a tumor-repressive role. When GSDME was overexpressed, it resulted in a decrease in cancer cell proliferation, migration, colony formation and invasion, while its decrease had the opposite effect. In the latter, tumors in GSDME knockout mice were found to be growing faster than those found in their non-knockout counterparts[107]. This is enough to prove that GSDME has a tumor-suppressive effect.
The role of inflammasomes in tumors and anti-tumor immunity.
| Cancer types | The function of inflammasomes | References |
|---|---|---|
| Liver cancer | The NLRP3 inflammasome resists liver cancer development. The AIM2 inflammasome suppress the proliferation and metastasis of cancer cells by inducing pyroptosis and inhibiting the mTOR-S6K1 pathway. | [91] [93] |
| Gastric cancer | The NLRP3 inflammasome resists the development of cancer cells. | [92] |
| Colorectal cancer | The expression of AIM2 inflammasome is negatively correlated with colorectal cancer-specific death and disease recurrence in patients. | [94] |
| Breast cancer | The NLRC4 inflammation triggers inflammation and subsequently induces tumor invasion. The NLRP3 inflammasome inhibition delayed tumor growth through the reduction in IL-1β. | [95] [101] |
| Melanoma | NLRP1 serves to facilitate tumor development by inhibiting caspase-2/9-mediated apoptosis reliant on caspase-3/7 in metastatic melanoma. The release of IL-1β by activated NLRP3 inflammasome can promote anti-tumor immunity. The NLRP3 inflammasome promotes the accumulation of MDSCs to resist anti-tumor immunity. | [96] [97, 98], [104] |
| B cell lymphoma | The release of IL-18 reliant on the NLRP3 inflammasome serve to stimulate anti-tumor immunity. The NLRP3 inflammasome is critical for the up-regulation of PD-L1 to promote lymphoma growth. | [99] [106] |
| Renal carcinoma | DNA vaccine combined with AIM2 in H1 Nanoparticles is important to the expansion of CD8+ T cells to suppress tumor growth. | [103] |
| Pancreatic ductal adenocarcinoma | The NLRP3 inflammasome plays a vital role in the expansion of immune-suppressive macrophages to promote PDA growth. | [105] |
| Head and neck squamous cell carcinoma | The NLRP3 inflammasome blockade suppresses HNSCC growth by downregulating the expression of IL-1β and reducing MDSCs, PD-1 and TAMs. | [100] |
The tumor-suppressive function of GSDME can be reflected in three aspects (Fig. 2). Caspase-3 can be induced by intrinsic or external factors, like chemotherapy and drugs, resulting in GSDME-dependent pyroptosis. Anti-cancer drugs, such as pacisplatin, Lobaplatin, and triptolide, induce pyroptosis through the GSDME/caspase-3 pathway[108-111]. In addition, GSDME expression is associated with the proliferation and function of tumor-associated CD4+ T cells, CD8+ T cells, TAMs, NK cells, etc[50]. It has been demonstrated that GSDME expression is positively related to tumor-infiltrating CD8+ T cells, GZMB, and macrophages with an M1 phenotype[112]. The killing of target cells by CTLs is primarily triggered by the discharge of cytotoxic granules containing granzymes and perforin. NK cells release GZMB and activate GSDME by cleavage at D270. The expression of GSDME increases the phagocytosis of TAMs, enhances the function of tumor-infiltrating natural-killer, and then inhibits tumor growth[50, 72]. Furthermore, cleavage of GSDME and release of HMGB1 activate DCs, eventually leading to T cell proliferation, thereby exerting anti-tumor effects. Combined treatment with the v-raf murine sarcoma viral oncogene homolog B1 inhibitor, and MEK inhibitor may achieve the anti-tumor goal by promoting HMGB1 release and GSDME cleavage in DCs[113].
The other members of gasdermin family in anti-tumor immunity
The GSDME is not alone in its efforts against anti-tumor immunity; other members of the GSDM family are just as important. If trifluorophenylalanine (PHF-BF3) is present, GSDMA3 induces tumor cell death and elicits potent antitumor immunity, mainly regulated by CD8+ T cells, thereby enhancing tumor clearance[74, 114](Fig. 2). Wang et al.[74] established a biorthogonal chemical system, a system based on demethylation of Phe-BF3, which was used to selectively control the release of GSDMA3 from nanoparticle conjugates in mouse 4T1 breast tumors. This model demonstrated that a mere 15% of the tumor cells undergoing pyroptosis was sufficient to eradicate the 4T1 breast tumor graft. Furthermore, the tumors remained in both immunodeficient and T cell-depleted mice, indicating that GSDMA3 triggers powerful anti-tumor immunity and possesses potent tumor suppressor properties.
Mechanisms of the gasdermin family involved in anti-tumor immunity. In the GSDME pathway, GZMB directly cleaves GSDME to GSDME-N and GSDME-C at site D270 or activates caspase-3 to cleave GSDME into GSDME-N and GSDME-C. In the GSDMB pathway, GSDMB can be cleaved into GSDMB-N and GSDMB-C by GZMA at site Ly244. In the GSDMA3 pathway, extracellular Phe-BF3 enters the cell and cleaves GSDMA3 to GSDMA3-N and GSDMA3-C. GSDM-N produced by three pathways induces pyroptosis in tumor cells. The release of HGMB1 and GSDM-N produced in pyroptosis prompts the release of IL-1β, IFN-γ, and GZMB from DCs, CD8+ T cells, and NK cells to enhance the anti-tumor immune activity, and ultimately acts on tumor cells.
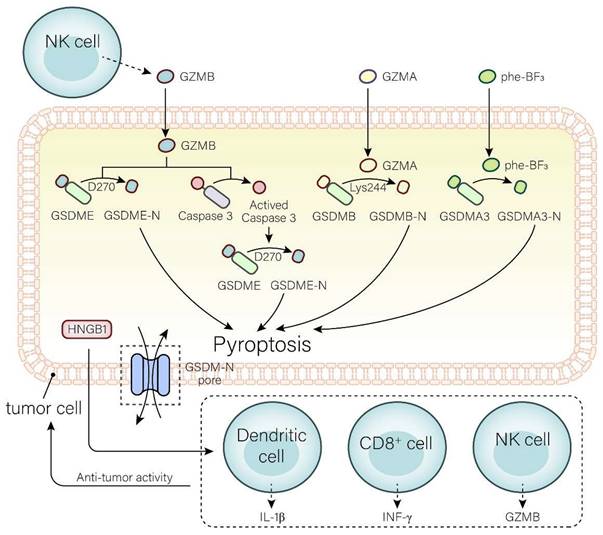
GSDMB is highly expressed in tumor cells and is able to destroy them by triggering pyroptosis, lysing cells with GZMA, and releasing active pore-forming fragments. It is well known that cytotoxic lymphocyte-mediated immunity depends on granzymes. GZMA released by NK cells and CTLs can cause pyroptosis in GSDMB-positive cells. In addition, IFN-γ upregulates GSDMB expression and promotes pyroptosis[115-119].
GSDMD is a major component in regulating pyroptosis. K3ZrF7:Yb/Er upconversion nanoparticles (ZrNPs), a pyroptosis inducer, induces tumor cell pyroptosis through the GSDMD/IL-1β pathway, leading to cell lysis. Pyroptosis induced by this pathway enhances the maturation of DCs, the frequency of effector memory T cells, and the inhibition of tumor growth, demonstrating superior antitumor immunity[120]. Su et al.[121] constructed a carbonic anhydrase IX (CAIX)-anchored rhenium(I) photosensitizer (CA-Re) that activated GSDMD, triggering pyroptosis and an anti-tumor response. Simultaneously, it promoted DCs maturation and antigen presentation capacity, and completely activated T cell-dependent adaptive immune responses in vivo. Eventually, remote tumors were eliminated while destroying primary tumors.
Pyroptosis in the clinical anti-tumor strategy
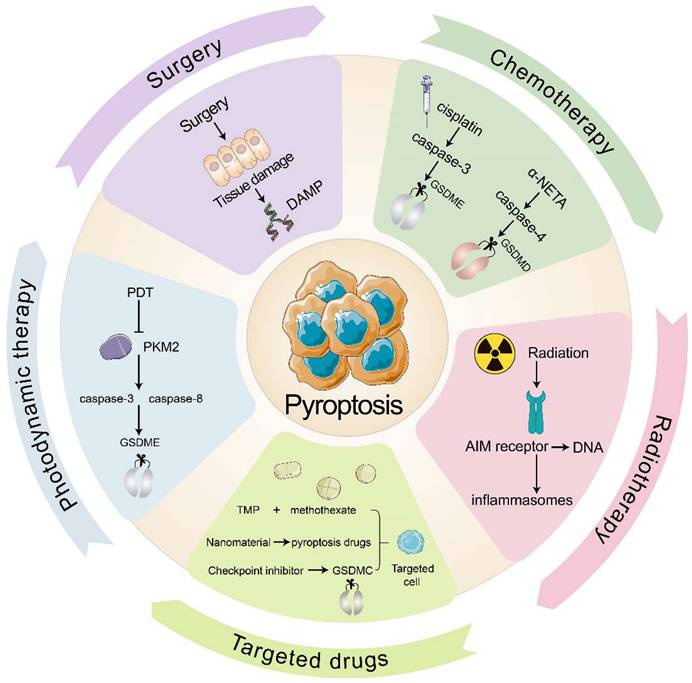
In clinical practice, the primary anti-tumor therapies are surgery, chemotherapy, radiotherapy, immune checkpoint inhibitors, and targeted therapy. Several clinical cancer therapies also start from pyroptosis and TIME to promote tumor immunity (Fig. 3). Many chemotherapeutic drugs, including dihydroartemisinin (DHA), doxorubicin, and cisplatin, have been shown to induce pyroptosis and kill tumor cells (Table 3). In esophageal squamous cell carcinoma (ESCC), the pyruvate kinase M2 (PKM2)-caspase 8/3-GSDME pathway induces pyroptosis in ESCC cells[122]. Pyroptosis that doxorubicin induces in melanoma cells goes through the eucharyotic elongation factor 2 kinase (eEF-2K) /GSDME pathway. Inhibition of eEF-2K inhibits autophagy and enhances pyroptosis, thereby increasing the sensitivity of melanoma to doxorubicin[123]. Although most people believe that cisplatin kills tumors by apoptosis, recent evidence showed that cisplatin kills A549 cells by inducing pyroptosis through the caspase-3 / GSDME pathway, and that knockdown of GSDME reduced the sensitivity of A549 to cisplatin[108]. Taken together, these consequences reveal that pyroptosis may provide new insights into anti-tumor therapy.
Radiation therapy is an effective way to eliminate tumor cells with the help of high-energy radiation. Additionally, ionizing radiation can induce tumor immunity through pyroptosis, which is mediated by GSDME. Di et al.[124]has found that radiation causes pyroptosis that GSDME induces in nasopharyngeal carcinoma (NPC) cells, and the deubiquitination of GSDME enhanced the radiosensitivity of NPC. After pyroptosis occurs, cytokines begin to be released and CTLs appear. While radiation will kill immunogenic cells and promote anti-tumor immunity. Furthermore, ionizing radiation kill cells directly by destroying DNA. After AIM2 receptor recognizes DNA fragment, inflammasomes will be triggered, resulting in pyroptosis. Then inflammatory mediators liberating aggravate immune cells infiltrating in tumor and enhance anti-tumor immunity[125].
In recent times, multiple studies have indicated that pyroptosis can be utilized in anti-tumor immunity, with the help of various drug targeting and delivery techniques. Nanomaterials have great benefits by diminishing their collection in healthy cells and adverse effects of targeted drugs. For example, the combination of cisplatin-containing tumor-targeting nanoliposomes and DNA methyltransferase inhibitors (DACs) inhibits DFNA5 gene methylation while activating caspase-3-mediated coagulation, thereby reducing tumor growth and transfer[83, 126]. In addition, tumor cell-derived microparticles (TMPs) also have a good targeting effect. TMPs inject methotrexate into cholangiocarcinoma cells (CCA), induce GSDME-mediated pyroptosis, activate patient-derived macrophages to produce pro-inflammatory cytokines, and recruit neutrophils to tumor sites for performing drug-directed tumor destruction[81]. Furthermore, the combination of various traditional and anti-inflammatory therapies may also provide new medical breakthroughs. For instance, photodynamic therapy (PDT) induces GSDME-mediated pyroptosis in ESCC. Mechanistically, PDT inhibits PKM2, which activates caspase-8 and caspase-3, eventually releasing GSDME-N and triggering pyroptosis in ESCC[127].
To increase targeted immune responses to tumor antigens, immune checkpoint inhibitors are employed, with pyroptosis playing a major role. PD-1 and PD-L1 are the main targets of these inhibitors[128]. Clinical testing has demonstrated that a PD-L1 inhibitor coupled with chemotherapy or radiotherapy can bring about pyroptosis and put tumor cells to death. In comparison with PD-L1 inhibitors group, the survival of the combination therapy group has improved. This may be related to the effect of nuclear PD-L1 in promoting the transition from apoptosis to pyroptosis after GSDMC expression at the transcriptional level[129, 130].
Lu et al.[131] devised Natural Killer-92 (NK92) cells including a novel chimeric co-stimulatory transforming receptor (CCCR). This receptor could change PD-1/PD-L1 signal from negative to activated, enhancing the inhibiting immunity efficiency of PD-1. CCCR-NK92 cells were targeted to eliminate tumor cells through triggering pyroptosis in H1299 cells which is PD-L1-positive. If PD-1 and PD-L1 inhibitors play an initial role, the subsequent lesion induction will further improve the efficacy. At the same time, the inflammatory response changes the tumor microenvironment, thereby affecting the treatment of immune checkpoint inhibitors and making tumor immunity more sensitive. It can provide PD-1 and PD-L1 inhibitors along with other anti-tumor treatments. In conclusion, the prospect of pyroptosis in anti-tumor therapy is considerable. The continuous in-depth research and clinical application of pyroptosis will bring good news to the medical industry and patients.
The role of chemotherapeutics in anti-tunor treatment.
| Chemotherapeutics | Cancer types | Pyroptosis signaling | References |
|---|---|---|---|
| Paclitaxel | Lung cancer | Caspase-3/GSDME | [108] |
| α-NETA | Ovarian cancer | Caspase-4/GSDMD | [132] |
| Doxorubicin | Melanoma | eEF-2K/GSDME | [123] |
| Lobaplatin | cervical cancer | Caspase-3/GSDME | [109] |
| 5-FU | Gastric cancer | Caspase-3/GSDME | [86] |
| Cisplatin | ESCC | Caspase-3/GSDME | [133] |
| Carboplatin | Retinoblastoma | Caspase-3/GSDME | [134] |
| DHA | ESCC | PKM2-caspase-8/3-GSDME | [122] |
Pyroptosis in the clinical anti-tumor strategy. Surgery damages the tissue to release DAMP and then induces pyroptosis. Chemotherapeutic agents cisplatin and α-NETA induce pyroptosis through caspase-3/GSDME and caspase-4/GSDMD respectively. Radiotherapy allows the AIM receptor to recognize DNA by radiation, activate the inflammasome and induce pyroptosis. Targeted drugs induce pyroptosis in targeted cells. PDT inhibits PKM2 and induces pyroptosis through caspase-8, caspase-3/GSDME pathway.
Conclusions and future perspectives
Pyroptosis is distinct from other cell death as it is associated with a powerful inflammatory response and cell rupture. We provide an in-depth explanation of the molecular process of pyroptosis, the function of the inflammasome and GSDMs, the relationship between pyroptosis and anti-tumor immunity, and the clinical applications of pyroptosis.
Pyroptosis is a major factor in the stimulation of anti-tumor immunity and the inhibition of tumors. However, the effects of pyroptosis on tumor cells are intricate. Pyroptosis activation in tumor and immune cells may promote the liberation of inflammatory chemicals, thus amplifying the efficacy of immunotherapy. At the same time, the activity of the inflammasomes and cytokines created by pyroptosis can result in chronic inflammation, which assists tumor cells in evading the immune system and advancing tumor progression. Current evidence indicates that pyroptosis is more likely to cause tumor promotion than inhibition, though different pathways can result in different cancers. Remarkably, reducing pyroptosis can actually promote tumor growth. It was discovered that a significant positive correlation exists between the downregulation of GSDMD and cancer cell proliferation. It is essential to delve deeper into the role of pyroptosis on the immune system, examining the association between other potential mechanisms and tumors to uncover more cancer therapies.
Recent evidence has revealed that particular virus-induced pyroptosis can lead to strong immune responses, for instance, NLRP3 was activated by SARS-CoV-2, prompting caspase-1 to induce pyroptosis in human monocytes. Moreover, encephalomyocarditis virus, an RNA virus that causes potassium outflow, activates the NLRP3 inflammasome in other cells to cause pyroptosis. Appropriate regulation of viral infection may be advantageous for tumor immunotherapy.
If pyroptosis induction is not managed correctly, it can harm the healthy tissue near the tumor. Nanomaterials may be a potential answer. By combining pyroptosis inducers and certain antibodies on the surface of nanoparticles, the delivery of nanoparticles in the TME can be improved, targeting cancer cells more accurately. This lowers the side effects of pyroptosis activation and allows for a more efficient distribution of inducers in the TME. The utilization of pyroptosis in a new manner is predicted to dramatically improve the effect of the treatment.
The mechanisms of action of GSDM family in pyroptosis have been gradually uncovered, yet the exact mechanisms of other GSDM families apart from GSDMD remain obscure. Notably, GSDMC has not been identified as a GSDMC-associated disease as its expression is limited. It has been reported that under hypoxic conditions, nuclear PD-L1 increases GSDMC expression, which is linked to poorer outcomes. This suggests that pyroptosis of chronic tumor cells in the central hypoxic zone, induced by GSDMC, may contribute to tumor progression. Despite this, the exact process remains unknown and requires further investigation.
Exploring the use of nanomaterials to induce pyroptosis is an area of development in the realm of anti-tumor immunity. Current strategies to induce pyroptosis come with a number of disadvantages, including severe systemic adverse effects, low bioavailability of drugs, limitation of immunosuppressive TME, and ineffective induction of immunogenic cell death. Fortunately, nanomaterials have been developed to address these issues, providing benefits like prolonged blood circulation, controlled drug release, and low toxic side effects. Additionally, a nanotherapeutic system that combines internal and external synergistic stimulation can induce pyroptosis in tumor cells, thereby activating a powerful systemic anti-tumor immune response that eliminates primary tumors and produces a memory effect to prevent metastasis of distant tumors[135]. Additionally, pyroptosis has pro-cancer and anti-cancer effects, but in the stage of cancer development, its anticancer effect is stronger than that of primary cancer[136]. It is crucial to grasp the balance between different tumor types and stages to achieve the best results in anti-tumor immunotherapy.
Several issues remain unresolved. Initially, the design of new nanomaterials is necessary, and further research is needed to comprehend the molecular mechanism of nanomaterials-induced pyroptosis and its potential consequences. Moreover, the changes in cellular functional changes caused by nanomaterials-mediated pyroptosis and signaling pathways have not been evaluated[137]. Additionally, the irregular expression and functioning of pyroptosis-related components is a major issue that needs to be addressed in different cancers or within cancers[138]. We anticipate that these issues will be resolved in the future as technology advances and molecular, genetic and epigenetic targeting/delivery systems become more precise and personalized.
Abbreviations
AIM2: absent in melanoma 2; ASC: apoptosis-associated speck-like protein containing a caspase recruit domain; CARD: caspase recruitment domain; CAR T cells: chimeric antigen receptor T cells; CA-Re: a carbonic anhydrase IX (CAIX)-anchored rhenium(I) photosensitizer; CCA: cholangiocarcinoma cells; CCCR: chimeric co-stimulatory transforming receptor; CTLs: cytotoxic T lymphocytes; DACs: DNA methyltransferase inhibitors; DAMPs: damage-associated molecular patterns; DCs: dendritic cells; DHA: dihydroartemisinic; DPP8: dipeptidyl peptidases 8; DPP9: dipeptidyl peptidases 9; eEF2K: eucharyotic elongation factor 2 kinase; ESCC: esophageal squamous cell carcinoma; GSDMB: Gasdermin B; GSDMC: Gasdermin C; GSDMD: Gasdermin D; GSDMD-C: a C-terminal domain of GSDMD; GSDMD-N: an N-terminal domain of GSDMD; GSDME: Gasdermin E; GSDMs: Gasdermins; GZMA: granzyme A; GZMB: granzyme B; HMGB1: high mobility group box 1; HNSCC: head and neck squamous cell carcinomas; IFN: interferon; IFN-γ: interferon-γ; IL-18: interleukin-18; IL-1β: interleukin-1β; IRAK: IL-1 receptor-associated kinase; LPS: lipopolysaccharides; MDSCs: Myeloid-derived suppressor cells; MyD88: myeloid differentiation primary response 88; NAIP5: neuronal apoptosis inhibitor protein 5; NF-κB: nuclear factor-kappa B; NK cells: natural killer cells; NK92: Natural Killer-92; NLR: NOD-like receptor; NLRC4: NOD-like receptor protein C4; NLRP1: NOD-like receptor family pyrin domain-containing 1; NLRP3: NOD-like receptor protein 3; NPC: nasopharyngeal carcinoma; PAMPs: pathogen-associated molecular patterns; PCD: programmed cell death; PD-1: programmed death-1; PDA: pancreatic ductal adenocarcinoma; PD-L1: programmed death ligand 1; PDT: photodynamic therapy; PHF-BF3: trifluorophenylalanine; PKC: protein kinase C; PKM2: pyruvate kinase M2; PKN1: Protein Kinase N1; PKN2: Protein Kinase N2; PRRs: pattern-recognition receptors; RhoA: Ras homolog gene family member A; RIPK1: receptor interacting protein 1; ROS: reactive oxygen species; TAMs: tumor-associated Macrophages; Th1: T-helper 1; Th17: T-helper 17; TIME: tumor immune microenvironment; TLR4: Toll-like receptor 4; TME: tumor microenvironment; TMPs: tumor cell-derived microparticles; TRF: time-restricted feeding; TTSS: type III secretion system; ZrNPs: K3ZrF7:Yb/Er upconversion nanoparticles.
Acknowledgements
We thank the Public Service Platform of South China Sea for R&D Marine Biomedicine Resources for support.
Funding
This research was funded by the Science and Technology Special Project of Zhanjiang (2022A01034); The Guangdong Provincial Department of Education Research Project (2022KTSCX); The Science and technology program of Guangdong Province (2023A1515010850); Key Discipline Construction Project of Guangdong Medical University (4SG22004G); Shenzhen Science and Technology Program (No. JCYJ20210324110209026).
Author contributions
LX Luo conceived and designed the review. HY Huang and YM Weng wrote the manuscript. W Tian drawn pictures; LX Luo, X Lin and J Chen reviewed the paper and provided comments. All authors contributed to this manuscript and approved the submitted version.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bedoui S, Herold M J, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol. 2020;21:678-695
2. Green D R. The Coming Decade of Cell Death Research: Five Riddles. Cell. 2019;177:1094-1107
3. Cookson B T, Brennan M A. Pro-inflammatory programmed cell death. Trends in microbiology. 2001;9:113-114
4. Chen X, He W T, Hu L, Li J, Fang Y, Wang X. et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26:1007-1020
5. Bergsbaken T, Fink S L, Cookson B T. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99-109
6. Jesenberger V, Procyk K J, Yuan J, Reipert S, Baccarini M. Salmonella-induced caspase-2 activation in macrophages: a novel mechanism in pathogen-mediated apoptosis. J Exp Med. 2000;192:1035-1046
7. Cervantes J, Nagata T, Uchijima M, Shibata K, Koide Y. Intracytosolic Listeria monocytogenes induces cell death through caspase-1 activation in murine macrophages. Cell Microbiol. 2008;10:41-52
8. Fink S L, Cookson B T. Pyroptosis and host cell death responses during Salmonella infection. Cell Microbiol. 2007;9:2562-2570
9. Fink S, Bergsbaken T, Cookson B J P o t N A o S o t U S o A. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. 2008; 105: 4312-4317.
10. Galluzzi L, Vitale I, Aaronson S A, Abrams J M, Adam D, Agostinis P. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486-541
11. Man S M, Kanneganti T D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol. 2016;16:7-21
12. Takeuchi O, Akira S J C. Pattern recognition receptors and inflammation. 2010; 140: 805-820.
13. Tan M S, Tan L, Jiang T, Zhu X C, Wang H F, Jia C D. et al. Amyloid-beta induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer's disease. Cell Death Dis. 2014;5:e1382
14. Boyden E D, Dietrich W F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240-244
15. Masters Seth L, Gerlic M, Metcalf D, Preston S, Pellegrini M, O'Donnell Joanne A. et al. NLRP1 Inflammasome Activation Induces Pyroptosis of Hematopoietic Progenitor Cells. Immunity. 2012;37:1009-1023
16. Broz P, von Moltke J, Jones J W, Vance R E, Monack D M. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010;8:471-483
17. Zhong F L, Mamai O, Sborgi L, Boussofara L, Hopkins R, Robinson K. et al. Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation. Cell. 2016;167:187-202 e117
18. Duncan J A, Bergstralh D T, Wang Y, Willingham S B, Ye Z, Zimmermann A G. et al. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Natl Acad Sci U S A. 2007;104:8041-8046
19. Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cellular & molecular immunology. 2021;18:2114-2127
20. Wang L, Hauenstein A V. The NLRP3 inflammasome: Mechanism of action, role in disease and therapies. Mol Aspects Med. 2020;76:100889
21. Sharma B R, Kanneganti T D. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22:550-559
22. Paik S, Kim J K, Silwal P, Sasakawa C, Jo E K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cellular & molecular immunology. 2021;18:1141-1160
23. Schmid-Burgk J L, Gaidt M M, Schmidt T, Ebert T S, Bartok E, Hornung V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur J Immunol. 2015;45:2911-2917
24. Ruhl S, Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol. 2015;45:2927-2936
25. Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J. et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117-121
26. Matikainen S, Nyman T A, Cypryk W. Function and Regulation of Noncanonical Caspase-4/5/11 Inflammasome. J Immunol. 2020;204:3063-3069
27. Baker P J, Boucher D, Bierschenk D, Tebartz C, Whitney P G, D'Silva D B. et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur J Immunol. 2015;45:2918-2926
28. Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187-192
29. Franchi L, Amer A, Body-Malapel M, Kanneganti T D, Ozoren N, Jagirdar R. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576-582
30. Ren T, Zamboni D S, Roy C R, Dietrich W F, Vance R E. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18
31. Zhao Y, Yang J, Shi J, Gong Y N, Lu Q, Xu H. et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596-600
32. Brunette R L, Young J M, Whitley D G, Brodsky I E, Malik H S, Stetson D B. Extensive evolutionary and functional diversity among mammalian AIM2-like receptors. J Exp Med. 2012;209:1969-1983
33. Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266-272
34. Jin T, Perry A, Jiang J, Smith P, Curry J A, Unterholzner L. et al. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity. 2012;36:561-571
35. Caneparo V, Landolfo S, Gariglio M, De Andrea M. The Absent in Melanoma 2-Like Receptor IFN-Inducible Protein 16 as an Inflammasome Regulator in Systemic Lupus Erythematosus: The Dark Side of Sensing Microbes. Front Immunol. 2018;9:1180
36. Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey D R. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514-518
37. Fernandes-Alnemri T, Yu J W, Datta P, Wu J, Alnemri E S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509-513
38. Zhao Y, Shao F. Diverse mechanisms for inflammasome sensing of cytosolic bacteria and bacterial virulence. Curr Opin Microbiol. 2016;29:37-42
39. Park Y H, Wood G, Kastner D L, Chae J J. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol. 2016;17:914-921
40. Gao W, Yang J, Liu W, Wang Y, Shao F. Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proc Natl Acad Sci U S A. 2016;113:E4857-4866
41. Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660-665
42. Yu J W, Wu J, Zhang Z, Datta P, Ibrahimi I, Taniguchi S. et al. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ. 2006;13:236-249
43. Wandel M P, Kim B H, Park E S, Boyle K B, Nayak K, Lagrange B. et al. Guanylate-binding proteins convert cytosolic bacteria into caspase-4 signaling platforms. Nat Immunol. 2020;21:880-891
44. Deng M, Tang Y, Li W, Wang X, Zhang R, Zhang X. et al. The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. 2018; 49: 740-753.e747.
45. Wang Y, Gao W, Shi X, Ding J, Liu W, He H. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. 2017; 547: 99-103.
46. Zheng Z, Deng W, Bai Y, Miao R, Mei S, Zhang Z. et al. The Lysosomal Rag-Ragulator Complex Licenses RIPK1 and Caspase-8-mediated Pyroptosis by Yersinia. Science. 2021 372
47. Zhang J Y, Zhou B, Sun R Y, Ai Y L, Cheng K, Li F N. et al. The metabolite α-KG induces GSDMC-dependent pyroptosis through death receptor 6-activated caspase-8. Cell Res. 2021;31:980-997
48. Kambara H, Liu F, Zhang X, Liu P, Bajrami B, Teng Y. et al. Gasdermin D Exerts Anti-inflammatory Effects by Promoting Neutrophil Death. Cell Reports. 2018;22:2924-2936
49. Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y. et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020 368
50. Liu Y, Fang Y, Chen X, Wang Z, Liang X, Zhang T. et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Science immunology. 2020 5
51. Tamura M, Tanaka S, Fujii T, Aoki A, Komiyama H, Ezawa K. et al. Members of a novel gene family, Gsdm, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics. 2007;89:618-629
52. Broz P, Pelegrín P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20:143-157
53. Ding J, Wang K, Liu W, She Y, Sun Q, Shi J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111-116
54. Liu X, Lieberman J. Knocking 'em Dead: Pore-Forming Proteins in Immune Defense. Annu Rev Immunol. 2020;38:455-485
55. Saeki N, Usui T, Aoyagi K, Kim D H, Sato M, Mabuchi T. et al. Distinctive expression and function of four GSDM family genes (GSDMA-D) in normal and malignant upper gastrointestinal epithelium. Genes, chromosomes & cancer. 2009;48:261-271
56. Katoh M, Katoh M. Identification and characterization of human DFNA5L, mouse Dfna5l, and rat Dfna5l genes in silico. International journal of oncology. 2004;25:765-770
57. Fujii T, Tamura M, Tanaka S, Kato Y, Yamamoto H, Mizushina Y. et al. Gasdermin D (Gsdmd) is dispensable for mouse intestinal epithelium development. Genesis (New York, N.Y.: 2000). 2008;46:418-423
58. Liu X, Zhang Z, Ruan J, Pan Y, Magupalli V G, Wu H. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153-158
59. Kennedy L B, Salama A K S. A review of cancer immunotherapy toxicity. CA: a cancer journal for clinicians. 2020;70:86-104
60. Du T, Gao J, Li P, Wang Y, Qi Q, Liu X. et al. Pyroptosis, metabolism, and tumor immune microenvironment. Clinical and translational medicine. 2021;11:e492
61. Kupz A, Guarda G, Gebhardt T, Sander L E, Short K R, Diavatopoulos D A. et al. NLRC4 inflammasomes in dendritic cells regulate noncognate effector function by memory CD8⁺ T cells. Nat Immunol. 2012;13:162-169
62. Tourlomousis P, Wright J A, Bittante A S, Hopkins L J, Webster S J, Bryant O J. et al. Modifying bacterial flagellin to evade Nod-like Receptor CARD 4 recognition enhances protective immunity against Salmonella. Nature microbiology. 2020;5:1588-1597
63. Zhao P, Wang M, Chen M, Chen Z, Peng X, Zhou F. et al. Programming cell pyroptosis with biomimetic nanoparticles for solid tumor immunotherapy. Biomaterials. 2020;254:120142
64. Cui Y, Zhou Z, Chai Y, Zhang Y. Upregulated GSDMB in Clear Cell Renal Cell Carcinoma Is Associated with Immune Infiltrates and Poor Prognosis. Journal of immunology research. 2021;2021:7753553
65. Kaplanski G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunological reviews. 2018;281:138-153
66. Parker K H, Sinha P, Horn L A, Clements V K, Yang H, Li J. et al. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer research. 2014;74:5723-5733
67. Kaplanov I, Carmi Y, Kornetsky R, Shemesh A, Shurin G V, Shurin M R. et al. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc Natl Acad Sci U S A. 2019;116:1361-1369
68. Kozlova N, Grossman J E, Iwanicki M P, Muranen T. The Interplay of the Extracellular Matrix and Stromal Cells as a Drug Target in Stroma-Rich Cancers. Trends in pharmacological sciences. 2020;41:183-198
69. Hanahan D, Coussens L M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309-322
70. Baker K J, Houston A, Brint E. IL-1 Family Members in Cancer; Two Sides to Every Story. Front Immunol. 2019;10:1197
71. Wawrocki S, Druszczynska M, Kowalewicz-Kulbat M, Rudnicka W. Interleukin 18 (IL-18) as a target for immune intervention. Acta biochimica Polonica. 2016;63:59-63
72. Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X. et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415-420
73. Tang R, Xu J, Zhang B, Liu J, Liang C, Hua J. et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. Journal of hematology & oncology. 2020;13:110
74. Wang Q, Wang Y, Ding J, Wang C, Zhou X, Gao W. et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature. 2020;579:421-426
75. Jenkins R W, Barbie D A, Flaherty K T. Mechanisms of resistance to immune checkpoint inhibitors. British journal of cancer. 2018;118:9-16
76. Mpekris F, Voutouri C, Baish J W, Duda D G, Munn L L, Stylianopoulos T. et al. Combining microenvironment normalization strategies to improve cancer immunotherapy. Proc Natl Acad Sci U S A. 2020;117:3728-3737
77. Ringel-Scaia V M, Beitel-White N, Lorenzo M F, Brock R M, Huie K E, Coutermarsh-Ott S. et al. High-frequency irreversible electroporation is an effective tumor ablation strategy that induces immunologic cell death and promotes systemic anti-tumor immunity. EBioMedicine. 2019;44:112-125
78. Nyiramana M M, Cho S B, Kim E J, Kim M J, Ryu J H, Nam H J. et al. Sea Hare Hydrolysate-Induced Reduction of Human Non-Small Cell Lung Cancer Cell Growth through Regulation of Macrophage Polarization and Non-Apoptotic Regulated Cell Death Pathways. Cancers. 2020 12
79. Peng Z, Wang P, Song W, Yao Q, Li Y, Liu L. et al. GSDME enhances Cisplatin sensitivity to regress non-small cell lung carcinoma by mediating pyroptosis to trigger antitumor immunocyte infiltration. Signal transduction and targeted therapy. 2020;5:159
80. Hou Y, Cao Y, Dong L, Huang Y, Zhang Z, Bi Y. et al. Regulation of follicular T helper cell differentiation in antitumor immunity. Int J Cancer. 2022 DOI: 10.1002/ijc.34368
81. Gao Y, Zhang H, Zhou N, Xu P, Wang J, Gao Y. et al. Methotrexate-loaded tumour-cell-derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nature biomedical engineering. 2020;4:743-753
82. Xiong H, Ma X, Wang X, Su W, Wu L, Zhang T. et al. Inspired Epigenetic Modulation Synergy with Adenosine Inhibition Elicits Pyroptosis and Potentiates Cancer Immunotherapy. Advanced Functional Materials. 2021 31
83. Fan J X, Deng R H, Wang H, Liu X H, Wang X N, Qin R. et al. Epigenetics-Based Tumor Cells Pyroptosis for Enhancing the Immunological Effect of Chemotherapeutic Nanocarriers. Nano letters. 2019;19:8049-8058
84. Yang W, Liu S, Li Y, Wang Y, Deng Y, Sun W. et al. Pyridoxine induces monocyte-macrophages death as specific treatment of acute myeloid leukemia. Cancer Lett. 2020;492:96-105
85. Jiang M, Qi L, Li L, Wu Y, Song D, Li Y. Caspase-8: A key protein of cross-talk signal way in "PANoptosis" in cancer. Int J Cancer. 2021;149:1408-1420
86. Wang Y, Yin B, Li D, Wang G, Han X, Sun X. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochemical and biophysical research communications. 2018;495:1418-1425
87. Yang W, Niu L, Zhao X, Duan L, Wang X, Li Y. et al. Pyroptosis impacts the prognosis and treatment response in gastric cancer via immune system modulation. American journal of cancer research. 2022;12:1511-1534
88. Sun Z, Jing C, Guo X, Zhang M, Kong F, Wang Z. et al. Comprehensive Analysis of the Immune Infiltrates of Pyroptosis in Kidney Renal Clear Cell Carcinoma. Frontiers in oncology. 2021;11:716854
89. Lee C, Do H T T, Her J, Kim Y, Seo D, Rhee I. Inflammasome as a promising therapeutic target for cancer. Life Sci. 2019;231:116593
90. Karki R, Kanneganti T D. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat Rev Cancer. 2019;19:197-214
91. Wei Q, Mu K, Li T, Zhang Y, Yang Z, Jia X. et al. Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab Invest. 2014;94:52-62
92. Ren N, Jiang T, Wang C, Xie S, Xing Y, Piao D. et al. LncRNA ADAMTS9-AS2 inhibits gastric cancer (GC) development and sensitizes chemoresistant GC cells to cisplatin by regulating miR-223-3p/NLRP3 axis. Aging. 2020;12:11025-11041
93. Ma X, Guo P, Qiu Y, Mu K, Zhu L, Zhao W. et al. Loss of AIM2 expression promotes hepatocarcinoma progression through activation of mTOR-S6K1 pathway. Oncotarget. 2016;7:36185-36197
94. Dihlmann S, Tao S, Echterdiek F, Herpel E, Jansen L, Chang-Claude J. et al. Lack of Absent in Melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patients. Int J Cancer. 2014;135:2387-2396
95. Jin H, Kim H J. NLRC4, ASC and Caspase-1 Are Inflammasome Components that Are Mediated by P2Y2R Activation in Breast Cancer Cells. Int J Mol Sci. 2020 21
96. Zhai Z, Liu W, Kaur M, Luo Y, Domenico J, Samson J M. et al. NLRP1 promotes tumor growth by enhancing inflammasome activation and suppressing apoptosis in metastatic melanoma. Oncogene. 2017;36:3820-3830
97. Segovia M, Russo S, Jeldres M, Mahmoud Y D, Perez V, Duhalde M. et al. Targeting TMEM176B Enhances Antitumor Immunity and Augments the Efficacy of Immune Checkpoint Blockers by Unleashing Inflammasome Activation. Cancer Cell. 2019;35:767-781 e766
98. Zhivaki D, Borriello F, Chow O A, Doran B, Fleming I, Theisen D J. et al. Inflammasomes within Hyperactive Murine Dendritic Cells Stimulate Long-Lived T Cell-Mediated Anti-tumor Immunity. Cell Rep. 2020;33:108381
99. Li X Y, Moesta A K, Xiao C, Nakamura K, Casey M, Zhang H. et al. Targeting CD39 in Cancer Reveals an Extracellular ATP- and Inflammasome-Driven Tumor Immunity. Cancer Discov. 2019;9:1754-1773
100. Chen L, Huang C F, Li Y C, Deng W W, Mao L, Wu L. et al. Blockage of the NLRP3 inflammasome by MCC950 improves anti-tumor immune responses in head and neck squamous cell carcinoma. Cell Mol Life Sci. 2018;75:2045-2058
101. Tengesdal I W, Li S, Powers N E, May M, Neff C P, Joosten L A B. et al. Activation of Host-NLRP3 Inflammasome in Myeloid Cells Dictates Response to Anti-PD-1 Therapy in Metastatic Breast Cancers. Pharmaceuticals (Basel). 2022 15
102. Lin K H, Chang L S, Tian C Y, Yeh Y C, Chen Y J, Chuang T H. et al. Carboxyl-terminal fusion of E7 into Flagellin shifts TLR5 activation to NLRC4/NAIP5 activation and induces TLR5-independent anti-tumor immunity. Sci Rep. 2016;6:24199
103. Chai D, Shan H, Wang G, Zhang Q, Li H, Fang L. et al. Combining DNA Vaccine and AIM2 in H1 Nanoparticles Exert Anti-Renal Carcinoma Effects via Enhancing Tumor-Specific Multi-functional CD8(+) T-cell Responses. Molecular cancer therapeutics. 2019;18:323-334
104. van Deventer H W, Burgents J E, Wu Q P, Woodford R M, Brickey W J, Allen I C. et al. The inflammasome component NLRP3 impairs antitumor vaccine by enhancing the accumulation of tumor-associated myeloid-derived suppressor cells. Cancer research. 2010;70:10161-10169
105. Daley D, Mani V R, Mohan N, Akkad N, Pandian G, Savadkar S. et al. NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma. J Exp Med. 2017;214:1711-1724
106. Lu F, Zhao Y, Pang Y, Ji M, Sun Y, Wang H. et al. NLRP3 inflammasome upregulates PD-L1 expression and contributes to immune suppression in lymphoma. Cancer Lett. 2021;497:178-189
107. Rogers C, Erkes D A, Nardone A, Aplin A E, Fernandes-Alnemri T, Alnemri E S. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nature communications. 2019;10:1689
108. Zhang C C, Li C G, Wang Y F, Xu L H, He X H, Zeng Q Z. et al. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis: an international journal on programmed cell death. 2019;24:312-325
109. Chen J, Ge L, Shi X, Liu J, Ruan H, Heng D. et al. Lobaplatin Induces Pyroptosis in Cervical Cancer Cells via the Caspase-3/GSDME Pathway. Anti-cancer agents in medicinal chemistry. 2022;22:2091-2097
110. Yang J, Guo W, Lu M. Confusion about the article: Natural product triptolide induces GSDME-mediated pyroptosis in head and neck cancer through suppressing mitochondrial hexokinase-ΙΙ. Cancer Lett. 2022;534:115568
111. Yu J, Li S, Qi J, Chen Z, Wu Y, Guo J. et al. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis. 2019;10:193
112. Wang S, Zhang M J, Wu Z Z, Zhu S W, Wan S C, Zhang B X. et al. GSDME Is Related to Prognosis and Response to Chemotherapy in Oral Cancer. Journal of dental research. 2022;101:848-858
113. Erkes D A, Cai W, Sanchez I M, Purwin T J, Rogers C, Field C O. et al. Mutant BRAF and MEK Inhibitors Regulate the Tumor Immune Microenvironment via Pyroptosis. Cancer Discov. 2020;10:254-269
114. Fu C. Gasdermin: a novel therapeutic target for tumour treatment by activating anti-tumour immunity. Signal transduction and targeted therapy. 2020;5:69
115. Robbins P F, Li Y F, El-Gamil M, Zhao Y, Wargo J A, Zheng Z. et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180:6116-6131
116. Wang C M, Wu Z Q, Wang Y, Guo Y L, Dai H R, Wang X H. et al. Autologous T Cells Expressing CD30 Chimeric Antigen Receptors for Relapsed or Refractory Hodgkin Lymphoma: An Open-Label Phase I Trial. Clinical cancer research: an official journal of the American Association for Cancer Research. 2017;23:1156-1166
117. Feng K C, Guo Y L, Liu Y, Dai H R, Wang Y, Lv H Y. et al. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. Journal of hematology & oncology. 2017;10:4
118. Wang Y, Zhang W Y, Han Q W, Liu Y, Dai H R, Guo Y L. et al. Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clinical immunology (Orlando, Fla.). 2014;155:160-175
119. Dotiwala F, Fellay I, Filgueira L, Martinvalet D, Lieberman J, Walch M. A High Yield and Cost-efficient Expression System of Human Granzymes in Mammalian Cells. Journal of visualized experiments: JoVE. 2015 DOI: 10.3791/52911: e52911
120. Ding B, Sheng J, Zheng P, Li C, Li D, Cheng Z. et al. Biodegradable Upconversion Nanoparticles Induce Pyroptosis for Cancer Immunotherapy. Nano letters. 2021;21:8281-8289
121. Su X, Wang W J, Cao Q, Zhang H, Liu B, Ling Y. et al. A Carbonic Anhydrase IX (CAIX)-Anchored Rhenium(I) Photosensitizer Evokes Pyroptosis for Enhanced Anti-Tumor Immunity. Angewandte Chemie (International ed. in English). 2022;61:e202115800
122. Jiang M, Wu Y, Qi L, Li L, Song D, Gan J. et al. Dihydroartemisinin mediating PKM2-caspase-8/3-GSDME axis for pyroptosis in esophageal squamous cell carcinoma. Chemico-biological interactions. 2021;350:109704
123. Yu P, Wang H Y, Tian M, Li A X, Chen X S, Wang X L. et al. Eukaryotic elongation factor-2 kinase regulates the cross-talk between autophagy and pyroptosis in doxorubicin-treated human melanoma cells in vitro. Acta pharmacologica Sinica. 2019;40:1237-1244
124. Di M, Miao J, Pan Q, Wu Z, Chen B, Wang M. et al. OTUD4-mediated GSDME deubiquitination enhances radiosensitivity in nasopharyngeal carcinoma by inducing pyroptosis. Journal of experimental & clinical cancer research: CR. 2022;41:328
125. McLaughlin M, Patin E C, Pedersen M, Wilkins A, Dillon M T, Melcher A A. et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. 2020;20:203-217
126. De Schutter E, Croes L, Ibrahim J, Pauwels P, Op de Beeck K, Vandenabeele P. et al. GSDME and its role in cancer: From behind the scenes to the front of the stage. Int J Cancer. 2021;148:2872-2883
127. Li L, Song D, Qi L, Jiang M, Wu Y, Gan J. et al. Photodynamic therapy induces human esophageal carcinoma cell pyroptosis by targeting the PKM2/caspase-8/caspase-3/GSDME axis. Cancer Lett. 2021;520:143-159
128. Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. American journal of cancer research. 2020;10:727-742
129. Reck M, Schenker M, Lee K H, Provencio M, Nishio M, Lesniewski-Kmak K. et al. Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non-small-cell lung cancer with high tumour mutational burden: patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. European journal of cancer (Oxford, England: 1990). 2019;116:137-147
130. Hou J, Zhao R, Xia W, Chang C W, You Y, Hsu J M. et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nature cell biology. 2020;22:1264-1275
131. Lu C, Guo C, Chen H, Zhang H, Zhi L, Lv T. et al. A novel chimeric PD1-NKG2D-41BB receptor enhances antitumor activity of NK92 cells against human lung cancer H1299 cells by triggering pyroptosis. Molecular immunology. 2020;122:200-206
132. Qiao L, Wu X, Zhang J, Liu L, Sui X, Zhang R. et al. α-NETA induces pyroptosis of epithelial ovarian cancer cells through the GSDMD/caspase-4 pathway. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2019;33:12760-12767
133. Wu M, Wang Y, Yang D, Gong Y, Rao F, Liu R. et al. A PLK1 kinase inhibitor enhances the chemosensitivity of cisplatin by inducing pyroptosis in oesophageal squamous cell carcinoma. EBioMedicine. 2019;41:244-255
134. Li F, Xia Q, Ren L, Nie Y, Ren H, Guo X. et al. GSDME Increases Chemotherapeutic Drug Sensitivity by Inducing Pyroptosis in Retinoblastoma Cells. Oxidative medicine and cellular longevity. 2022;2022:2371807
135. Sun Z-J, Wang W-D. Evoking pyroptosis with nanomaterials for cancer immunotherapy: Current boom and novel outlook. Nano TransMed. 2022 1
136. Li L, Jiang M, Qi L, Wu Y, Song D, Gan J. et al. Pyroptosis, a new bridge to tumor immunity. Cancer science. 2021;112:3979-3994
137. Lu L, Zhang Y, Tan X, Merkher Y, Leonov S, Zhu L. et al. Emerging mechanisms of pyroptosis and its therapeutic strategy in cancer. Cell death discovery. 2022;8:338
138. Loveless R, Bloomquist R, Teng Y. Pyroptosis at the forefront of anticancer immunity. Journal of experimental & clinical cancer research: CR. 2021;40:264
Author contact
![]() Corresponding authors: Email: luolianxiang321edu.cn (Lianxiang Luo); chenjiancom (Jian Chen); linxiangabrieledu.cn (Xian Lin).
Corresponding authors: Email: luolianxiang321edu.cn (Lianxiang Luo); chenjiancom (Jian Chen); linxiangabrieledu.cn (Xian Lin).
Received 2023-6-6
Accepted 2023-7-27
Published 2023-8-6
