ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2024; 20(2):621-642. doi:10.7150/ijbs.89376 This issue Cite
Review
Uncovering the flip side of immune checkpoint inhibitors: a comprehensive review of immune-related adverse events and predictive biomarkers
1. Cancer Center, Yantai Affiliated Hospital of Binzhou Medical University, The 2 nd Medical College of Binzhou Medical University, Yantai 264100, China.
2. Yantai Affiliated Hospital of Binzhou Medical University, The 2 nd Medical College of Binzhou Medical University, Yantai 264100, China.
3. The First Clinical Medical College, Lanzhou University, Lanzhou 730000, China.
4. Department of Thyroid and Breast Surgery, Yantai Affiliated Hospital of Binzhou Medical University, The 2 nd Medical College of Binzhou Medical University, Yantai 264100, China.
5. Department of Gastroenterology, Yantai Affiliated Hospital of Binzhou Medical University, The 2 nd Medical College of Binzhou Medical University, Yantai 264100, China.
Abstract
Immune checkpoint inhibitors (ICIs) have generated considerable excitement as a novel class of immunotherapeutic agents due to their remarkable efficacy in treating various types of cancer. However, the widespread use of ICIs has brought about a number of safety concerns, especially the development of immune-related adverse events (irAEs). These serious complications could result in treatment discontinuation and even life-threatening consequences, making it critical to identify high-risk groups and predictive markers of irAEs before initiating therapy. To this end, the current article examines several potential predictive markers of irAEs in important organs affected by ICIs. While retrospective studies have yielded some promising results, limitations such as small sample sizes, variable patient populations, and specific cancer types and ICIs studied make it difficult to generalize the findings. Therefore, prospective cohort studies and real-world investigations are needed to validate the potential of different biomarkers in predicting irAEs risk. Overall, identifying predictive markers of irAEs is a crucial step towards improving patient safety and enhancing the management of irAEs. With ongoing research efforts, it is hoped that more accurate and reliable biomarkers will be identified and incorporated into clinical practice to guide treatment decisions and prevent the development of irAEs in susceptible patients.
Keywords: Cancer, Immunotherapy, Immune Checkpoint Inhibitors, Immune-Related Adverse Effects, Markers
Introduction
Normally, the body's immune system is a complex and powerful defense mechanism that can usually identify and eradicate tumor cells within the tumor microenvironment. However, the survival and growth of tumor cells are facilitated via their adoption of various strategies that allow them to evade the immune system's detection and attack. As a result, the immune system becomes suppressed and incapable of effectively eliminating the tumor cells, leading to their survival throughout the different stages of the anti-tumor immune response [1]. The above trait of tumor cells is known as immune escape. In order to gain a deeper understanding of the complexity of multi-link and multi-step tumor immunity, the concept of the tumor-immune cycle was introduced by Chen, et al [2]. Various types of tumors can interfere with the immune system's capability to effectively recognize and eliminate cancer cells by disrupting different stages of the cycle, thereby promoting immune tolerance and contributing to the development of tumors.
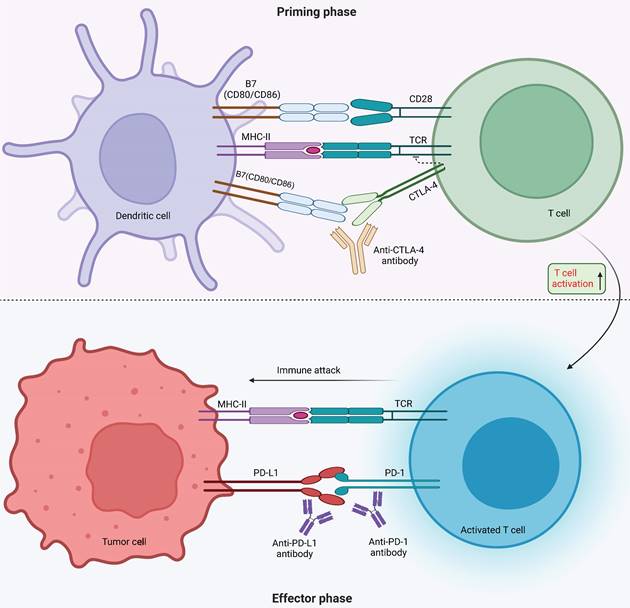
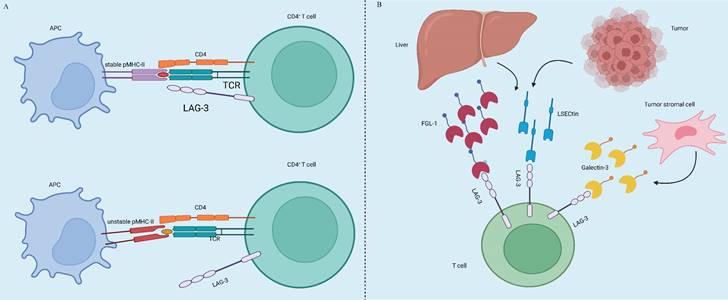
Tumor immunotherapy aims to manage and eliminate tumors by reinitiating and sustaining the tumor-immune cycle, thereby reinstating the body's typical anti-tumor immune response [3]. It includes immune checkpoint inhibitors (ICIs), cell therapy, small molecule inhibitors and cancer vaccines. Among the many immunotherapeutic strategies, ICIs are widely used and have shown remarkable efficacy in managing various types of solid tumors, including but not limited to non-small cell lung cancer (NSCLC), melanoma, bladder cancer, kidney cancer, and prostate cancer, among others. By using antibodies against cytotoxic T lymphocyte-associated protein 4 (CTLA-4), and inhibiting programmed cell death 1 (PD-1) or its ligand 1 (PD-L1), ICIs improve the immune response against tumors [4]. CTLA4, the initial immune-checkpoint receptor to undergo clinical targeting, is exclusively expressed on T cells, where its primary role is to modulate the magnitude of early T cell activation [5]. CTLA-4 molecules exhibit a stronger binding affinity to CD80 and CD86 compared to CD28, and they function as competitive inhibitors of CD28 in antigen-presenting cells (APCs) [6]. Cancer cells frequently co-opt the PD-1 signaling pathway as a means to evade immune surveillance. Moreover, apart from inhibiting specific early activation pathways of T cells, PD-1 directly impairs antigen recognition by disrupting the trimolecular interaction involving TCR-pMHC-CD8 (Figure 1). In addition to these immune checkpoints, there are many immune checkpoints that exhibit immunotherapeutic properties, including V structural domain Ig suppressor of T cell activation (VISTA, also known as VSIR), TIM-3 (also known as HAVCR2), lymphocyte-activated gene-3 (LAG-3, also known as CD223), T cell Ig and ITIM domain (TIGIT, WUCAM, Vstm3, VSIG9), Signal regulatory proteins (SIRP), BTLA (CD272), Siglec-7, LILRB, et al [7, 8]. LAG-3 is currently considered a prime target after PD-1/L1 (Figure 2). Several clinical trials are validating the effectiveness of LAG-3-targeted therapy [9], and Opdualag, the initial fixed-dose combination of Relatlimab, a LAG-3 inhibitor, and nivolumab, a PD-1 inhibitor, has been authorized by the Food and Drug Administration (FDA) on March 19, 2022, for treating unresectable or metastatic melanoma in patients aged 12 or above. This is the first combination regimen of a LAG-3 inhibitor and a PD-1 inhibitor to receive formal marketing approval, based on the results of RELATIVITY-047 phase II/III trial [10]. Multiple ICIs can significantly improve overall survival in patients with various cancers and have been approved for clinical use by the FDA and National Medical Products Administration (NMPA) (Table 1).
Immune checkpoint inhibitors approved by the Food and Drug Administration and the China National Drug Administration
| Drug | Target | Indications | ||
|---|---|---|---|---|
| FDA | NMPA | |||
| Pembrolizumab | PD-1 | Melanoma | Melanoma | |
| Advanced non-small-cell lung cancer | Non-small-cell lung cancer | |||
| Squamous-cell carcinoma of the head and neck | Squamous-cell carcinoma of the head and neck | |||
| Non-muscle invasive bladder cancer | Esophageal squamous cell carcinoma | |||
| Advanced renal-cell carcinoma | Hepatocellular carcinoma | |||
| Advanced colorectal cancer with MSI-H or dMMR | Colorectal cancer with MSI-H or dMMR | |||
| Solid tumors with MSI-H or dMMR | ||||
| High-risk early-stage or advanced triple-negative breast cancer | ||||
| Classic Hodgkin's lymphoma | ||||
| Advanced gastric cancer | ||||
| Advanced endometrial cancer | ||||
| Primary mediastinal B-cell lymphoma | ||||
| Advanced hepatocellular carcinoma | ||||
| Advanced esophageal carcinoma | ||||
| Uroepithelial carcinoma | ||||
| Advanced Merkel cell carcinoma | ||||
| Squamous-cell carcinoma of skin | ||||
| Nivolumab | PD-1 | Advanced non-small-cell lung cancer | Non-small-cell lung cancer | |
| Advanced Melanoma | Squamous-cell carcinoma of the head and neck | |||
| Squamous-cell carcinoma of the head and neck | Gastric cancer/adenocarcinoma of esophagogastric junction | |||
| Advanced renal-cell carcinoma | Advanced gastric cancer | |||
| Advanced hepatocellular carcinoma | Malignant pleural mesothelioma | |||
| Advanced bladder cancer | Esophageal squamous cell carcinoma | |||
| Classic Hodgkin's lymphoma | ||||
| Colorectal cancer with MSI-H or dMMR | ||||
| Malignant pleural mesothelioma | ||||
| Gastric or gastroesophageal junction cancer or esophageal adenocarcinoma | ||||
| Cemiplimab | PD-1 | Cutaneous Squamous Cell Carcinoma, | ||
| Non-small cell lung cancer | ||||
| Toripalimab | PD-1 | Melanoma, | ||
| Nasopharyngeal carcinoma | ||||
| Esophageal cancer | ||||
| Uroepithelial carcinoma | ||||
| Sintilimab | PD-1 | Hodgkin's lymphoma,lung cancer | ||
| Esophageal Cancer | ||||
| Hepatocellular carcinoma | ||||
| Gastric cancer/adenocarcinoma of esophagogastric junction | ||||
| Hepatocellular carcinoma | ||||
| Camrelizumab | PD-1 | Classic Hodgkin's lymphoma | ||
| Esophageal squamous carcinoma | ||||
| non-small-cell lung cancer | ||||
| Hepatocellular carcinoma | ||||
| Nasopharyngeal carcinoma | ||||
| Tislelizumab | PD-1 | Hodgkin's lymphoma | ||
| Lung cancer | ||||
| Hepatocellular carcinoma | ||||
| Uroepithelial carcinoma | ||||
| Solid tumors with MSI-H or dMMR | ||||
| Penpulimab | PD-1 | Hodgkin's lymphoma | ||
| Serplulimab | PD-1 | Solid tumors with MSI-H or dMMR | ||
| Pucotenlimab | PD-1 | Solid tumors with MSI-H or dMMR | ||
| Retifanlimab | PD-1 | Metastatic or recurrent locally advanced Merkel cell carcinoma | ||
| Atezolizumab | PD-L1 | Extensive stage small cell lung cancer | ||
| Metastatic non-small-cell lung cancer | ||||
| Advanced triple-negative breast cancer | ||||
| Locally advanced or metastatic uroepithelial carcinoma | ||||
| Advanced Melanoma | ||||
| Durvalumab | PD-L1 | Non-small-cell lung cancer | ||
| Small cell lung cancer | ||||
| Uroepithelial carcinoma. | ||||
| Cholangiocarcinoma | ||||
| Envafolimab | PD-L1 | Solid tumors with MSI-H or dMMR | ||
| Zimberelimab | PD-L1 | Hodgkin's lymphoma | ||
| Sugemalimab | PD-L1 | Hepatocellular carcinoma Non-Small-Cell Lung Cancer | ||
| Solid tumors with MSI-H or dMMR | ||||
| Avelumab | PD-L1 | Uroepithelial carcinoma | ||
| Advanced renal-cell carcinoma | ||||
| Merkel cell carcinoma | ||||
| Ipilimumab | CTLA-4 | Melanoma | ||
| Tremelimumab | CTLA-4 | Hepatocellular carcinoma | ||
| Non-small-cell lung cancer | ||||
| Cadonilimab | PD-1/CTLA4 | Cervical Cancer | ||
| Relatlimab | LAG-3 | Melanoma | ||
Note: FDA, Food and Drug Administration; NMPA, National Medical Products Administration; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; CTLA-4, cytotoxic T-lymphocyte antigen 4; LAG-3, lymphocyte activation gene-3. Data as of: March 2023.
Immunotherapy kills tumors by improving the body's own immune function, but an over-activated immune system can also damage normal cells due to complex mechanisms, resulting in uncomfortable symptoms in the relevant organs, called immunotherapy-related adverse events (irAEs). The use of ICIs for cancer treatment has resulted in a sharp rise in the cumulative annual incidence of irAEs, with almost 13,000 cases reported in 2018 [14]. ICIs exhibit a distinct spectrum of adverse effects compared to conventional chemotherapy and other biological treatments. The majority of these adverse events stem from an overactive immune response targeting healthy tissues, affecting nearly every organ system [4, 15]. Notably, more than two-thirds of documented cases are irAEs, with three specific drugs (ipilimumab, nivolumab, and pembrolizumab) accounting for nearly 60% of these incidents [14]. The severity of these toxicities varies from mild to life-threatening, creating a clinical dilemma regarding whether to discontinue treatment or manage the condition through frequent hospitalization and immunosuppressive therapy [16-18]. In this review, we mainly discuss the irAEs and predictive biomarkers.
Mechanism of immune checkpoint inhibitors. Monoclonal antibodies targeting CTLA-4 and PD-1 receptors, as well as PD-L1, are ICIs that regulate T cell activation. During the priming phase, antigen presentation by MHC class II molecules on antigen-presenting cells triggers T cell activation by the T-cell receptor (TCR) recognizing antigen, followed by CD28 receptor binding with B7 (CD80 or CD86). The surface receptor of CTLA-4 on T cells inhibits T cell activation through competing with CD28 for CD80 or CD86 binding. The use of CTLA-4 inhibitor antibodies blocks the CTLA-4-CD80 or CTLA-4-CD86 binding, thus promoting T cell activation (indicated by a dashed line). During the effector phase, PD-1 expressed by T cells interacts with PD-L1 expressed by tumor and myeloid cells, promoting apoptosis of antigen-specific T cells while reducing regulatory T cell apoptosis. Under normal circumstances, this mechanism serves to protect against autoimmune disorders. However, cancer cells take advantage of it by increasing the expression of PD-L1, which helps them evade the immune system. To counteract this, inhibitors of PD-1 and PD-L1 can be used to block their interaction and promote activation of T-cells.
Immunotherapy-related adverse events
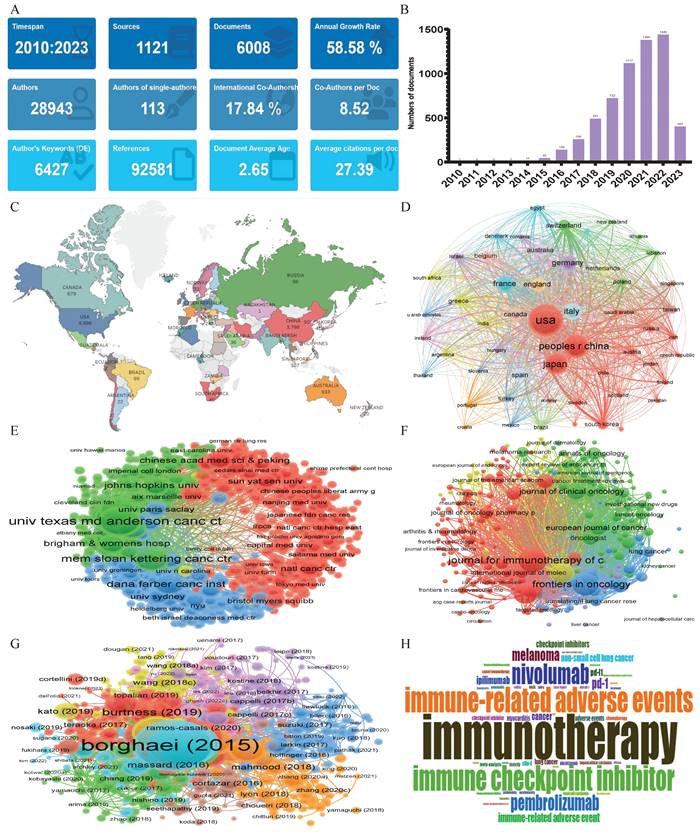
As shown in Figure 3, the bibliometric method was used to search the Web of Science core collection (WOSCC) literature database- Science Citation Index-Expanded (SCI-Expanded), based on the subject terms of “adverse event*” and “immune checkpoint inhibitor*”. 6008 English articles and reviews were published from January 2010 to April 2023 with an annual growth rate of 58.58%, particularly after 2019, signaling a rapid escalation in this field of research (Figure 3A, B).The most frequent countries' Scientific Production were the United States of America (8896), followed by China (3798) and Japan (3194), Moreover, the nations with the highest volume of publications in this field were the United States of America (1,886 articles, accounting for 31.39% of the total), China (1,115 articles, representing 18.56%), and Japan (780 articles, 12.98%), securing the top three positions respectively (Figure 3C, D). Between January 2010 and April 2023, the leading institutions in terms of the volume of global publications in the field of “adverse events related to immune checkpoint inhibitors” are illustrated in Figure 3E. “The University of Texas MD Anderson Cancer Center” and “Harvard Medical School” held the first two places, with a total of 613 and 303 publications, respectively. “The Memorial Sloan Kettering Cancer Center”, having published 287 articles, occupied the third position. The “Journal for Immunotherapy of Cancer”, with 259 publications, was the periodical that carried the most literature on the subject. “Frontiers in Oncology”, publishing 226 articles, and “Cancers”, with 175 publications, took the second and third positions respectively (Figure 3F). Figure 3G provides a schematic representation of the literature's co-citation network. The most frequently cited work was Borghaei H, 2015[19], with 6,497 citations, followed by Brahmer J, 2015[20] (4,676 citations), and Robert C, 2015 [21] (3,896 citations) ranking second and third, respectively. An analysis based on keywords can reveal trends in theme evolution and research hotspots within a particular field over a given period. As shown in Figure 3H, the five most frequently occurring keywords were: “immunotherapy, immune checkpoint inhibitors, immune-related adverse events, immune checkpoint inhibitor, and nivolumab”.
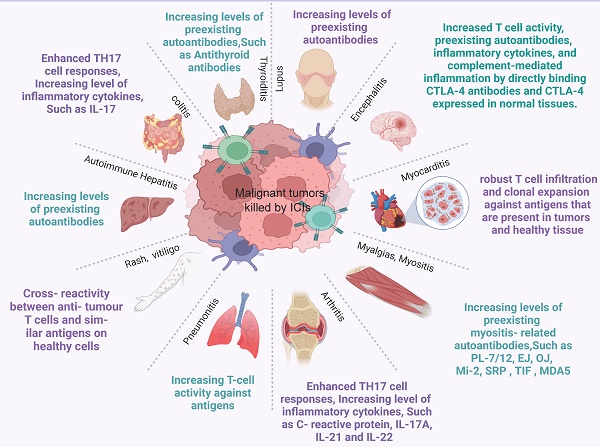
irAEs can manifest in various organs, with the skin, gastrointestinal tract, kidney, endocrine gland, liver, and other organs being the most commonly affected (Figure 4, Figure S1). Also, potential irAEs involve a wide range of organs and require multidisciplinary and collaborative management by physicians from various disciplines in the clinical setting. When ICIs are used in tumor treatment, predicting the risk of irAEs before drug administration can effectively help identify high-risk groups, enabling enhanced surveillance, early intervention, and, in some cases, irAE prevention. It is particularly important to discover predictive markers for irAEs. For these reasons, this article also reviews the predictive markers of irAEs in several important organs caused by ICIs. Common irAEs and potential biomarkers for various irAEs are summarized in Table 2.
Dermatologic-related Toxicity
Among the adverse events related to ICIs, dermatological toxicity is frequently observed, particularly in patients receiving CTLA-4 or PD-1/PD-L1 blockade [23]. Incidences of dermatological toxicity (of all grades) have been reported in around 30-40% of patients taking PD-1/PD-L1 inhibitors and in about 50% ipilimumab treated patients [24]. Dermatologic-related toxicity appears earlier compared to other irAEs, with cases being observed around one month after the commencement of anti-CTLA-4 treatment and approximately two months after starting PD-1/PD-L1 inhibitors [25, 26]. The main clinical manifestations of dermatologic -related toxicity are macular papules, pruritus and vitiligo [4, 14, 27, 28]. Other types include lichenoid dermatitis, psoriasis, autoimmune skin diseases (e.g., herpetiform aspergillosis, dermatomyositis, pemphigus), acne-like rash, and vascular disease-like changes [4, 14, 27].
LAG-3 expression and ligands. (A) LAG-3 is not initially present on primary T cells but can be induced upon antigen stimulation on both CD4+ and CD8+ T cells. It is also expressed in a particular subset of CD4+ T cells with suppressive capabilities. LAG-3 can selectively bind to stable pMHCII7, distinguishing the conformation of pMHCII[11]. (B) Additionally, FGL1 protein is a significant functional ligand for LAG-3. Upregulation of FGL1 expression by tumor cells can regulate the inhibitory function of LAG-3, thereby affecting T cell immune activity [12]. Furthermore, Galectin-3 and LSECtin can interact with the glycans on LAG-3 [9, 13].
A bibliometric analysis of adverse events related to immune checkpoint inhibitors. (A) The overview of adverse events related to immune checkpoint inhibitors in WOSCC by 'Bibiometrix' package. (B) Analysis of annual publication trends in the field of adverse events related to immune checkpoint inhibitors. (C) World map of Conutries's scientific production. (D) A geographical distribution of scholarly papers originating from various nations, elucidated through bibliographic coupling analysis facilitated by VOSviewer (version 1.6.18). Every circle symbolizes a nation, with the circle's magnitude corresponding to the volume of scholarly production from that nation. The connecting lines imply inter-country collaborations; the thicker the line, the more intimate the cooperation. Various hues signify distinct clusters. € An elucidation of the distribution of scholarly output emanating from different institutions, facilitated via bibliographic coupling analysis using VOSviewer (version 1.6.18). Every circle represents an institution, and the circle's magnitude embodies the volume of scholarly output from that institution. Interconnecting lines suggest institutional collaborations; the broader the line, the more intimate the association. Diverse shades indicate distinct clusters. (F) A VOSviewer visualization chart of bibliographic coupling sources. Each circle embodies a journal, with the circle's size reflecting the volume of publications in that particular journal as per the bibliographic coupling analysis. The greater the circle, the more voluminous the publications. Lines connecting the circles signify inter-journal relationships, and differently hued connection networks suggest cooperative clusters between distinct journals. Diverse shades represent different clusters [22]. (G) A VOSviewer visualization diagram of cited manuscripts. Each circle represents a manuscript, with the circle's size mirroring the citation count in the citation analysis. The greater the circle, the more citations. Interconnecting lines illustrate relationships between manuscripts, and distinctively hued connection networks indicate collaborative clusters between different documents. Various colors symbolize different clusters. (H) A word cloud representing the frequency of authors' keywords within the realm of unfavorable outcomes linked to immune checkpoint inhibitors. The more prominent the font, the greater its prevalence.
The various irAEs in patients with cancer treated with ICIs and potential biomarkers for various irAEs.
| irAEs | Type | Potential predictive biomarkers |
|---|---|---|
| Dermatologic-related Toxicity | Macular Papules, Pruritus, Vitiligo, Lichenoid Dermatitis, Psoriasis, Herpetiform Aspergillosis, Dermatomyositis, Pemphigus, Acne-like Rash, Vascular Disease-like Changes, | sCD163, CXCL5, BP180 IgG autoantibody, IL17A |
| Gastrointestinal adverse events | Colitis | Combine of IL-17, neutrophils, eosinophils, and leukocytes, GCSF, CD177, CEACAM1, Intestinal microbiota (Fusarium, Bacteroides fragilis, Phascolarctobacterium genus, Enterobacteriaceae family, Akkermansia muciniphila, Firmicutes phylum, Veillonela, S. fragilis, Bifidobacterium, Lactobacillus. |
| Hepatic Toxicity | ICI-associated hepatitis | ANA, AMA |
| Endocrine System Diseases | Hypophysitis, Hyperthyroidism Hypothyroidism, Thyroiditis Primary adrenal insufficiency, Insulin-dependent diabetes mellitus | anti-GNAL, anti-ITM2B, APA, AHA, HLA-I, HLA-DQ7, HLA-DR4, TgAb, ALB, FCGR2B, CD44, LCN2, CD74 |
| Respiratory Toxicity | ICI-associated Pneumonitis | NLR, HLA-I, IL-10 |
| Cardiovascular Toxicity | Myocarditis | CK, CK-MB, NT-proBNP, IL-6, CXCL9, CXCL10, CXCL13, CD31, CCL5, CCL4, CCL4L2 |
| Neurological irAEs | Neuromuscular disease, Neuropathy, Noninfectious meningitis, Encephalitis, Vasculitis, Myelitis, Cranial neuropathy, Peripheral neuropathy, Myasthenia gravis, Guillain-Barre syndrome | IL17A |
| CK | ||
| ADA | ||
| Rheumatic irAEs | Arthritis, Sicca and Sjogren's, Systemic lupus erythematosus, Myositis, Vasculitis, Polymyalgia rheumatic | IL17, TNF-α, |
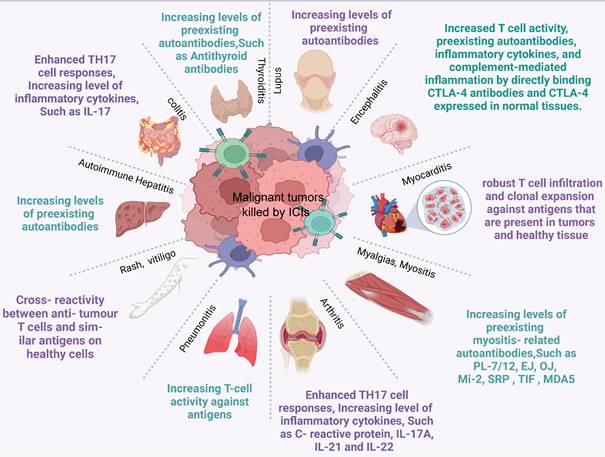
Common immune-related adverse events and possible mechanisms. ICI therapy may result in adverse events in any organ. This diagram shows the most common irAEs encountered by clinicians in patients treated with ICIs and their possible mechanisms.
A network meta-analysis of ICIs for 14 randomized clinical trials (RCTs) including 9572 advanced NSCLC patients demonstrated that combination of two ICIs or combination with chemotherapy drug has a higher incidence of severe dermatologic toxicity, such as nivolumab + ipilimumab + platinum (79.1%), nivolumab + ipilimumab (72.9%), camrelizumab + platinum (64.9%), atezolizumab + platinum (47.4%). Pembrolizumab single drug induced dermatologic toxicity incidence (75.2%), followed by nivolumab (44.2%), durvalumab (40.5%), et al [29]. Similarly, another network meta-analysis, include 12 RCTs including 8,453 NSCLC patients found that the incidences of severe dermatologic irAEs was ranked nivolumab + ipilimumab (97.4%), pembrolizumab (80.1%), nivolumab (67.1%), pembrolizumab + platinum (43.3%) [30]. Another meta-analysis of irAEs with pembrolizumab and nivolumab for the treatment of multiple solid tumors reported an incidence of 16.7% and 14.3% for all grades of rash, respectively [27]. A case report by Patil and colleagues described a patient with metastatic urothelial carcinoma who experienced a maculopapular rash during treatment with a PD-L1 inhibitor [31]. Interestingly, a meta-analysis that included 137 randomized controlled studies identified the development of vitiligo to be related to improvements in progression-free survival and overall survival time [32]. According to a retrospective study, the presence of spongiotic dermatitis and lichenoid eruptions in patients receiving PD-1/PD-L1 inhibitors may indicate a strong immune response and improved oncological outcomes [33]. Although most of these dermatologic-related irAEs are self-limiting, early identification and management are essential to prevent progression, improve the quality of patient survival, and prevent interruption of treatment. A number of indicators are available for earlier identification of such dermatologic-related irAEs. The principal constituent of tumor-infiltrating lymphocytes is CD163+ macrophages, which have the capacity to generate diverse chemokines by activating cancer-specific stromal factors, including IL-4, POSTN, and RANKL [34]. The dermis of various inflammatory cutaneous disorders, including autoimmune conditions like pemphigus vulgaris, scleroderma, and herpetiform aspergillosis, contains POSTN, which is an extracellular matrix protein [35]. Increased tumor mesenchymal POSTN expression in melanoma, and POSTN stimulates CXCL5 release from tumor-associated macrophages [36]. In a study conducted by Taku and colleagues, 46 patients with advanced melanoma who were treated with nivolumab were examined for their serum sCD163 and CXCL5 levels. The researchers observed that the patients who developed irAEs had significantly elevated absolute serum sCD163 values [37]. Despite the lack of significant changes in serum CXCL5 levels, the absolute value of CXCL5 can be utilized as a supplementary marker for the absolute value of serum sCD163. Therefore, both serum sCD163 and CXCL5 levels can be valuable predictors of dermatologic-related irAEs in the context of nivolumab therapy. In addition, bullous pemphigoid (BP), another common type of dermatologic irAEs, appears to be the most commonly expressed antigen in NSCLC [38]. According to a report by Hasan and colleagues, NSCLC patients with higher levels of anti-BP180 IgG exhibited improved treatment response and overall survival, as well as an increased risk of cutaneous irAEs when undergoing anti-PD1/PD-L1 therapy [39]. Another study on pembrolizumab-induced BP showed that 20 of the 22 cases tested, the serum anti-BP180 autoantibodies were positive. However, in 10 cases (91.90%, 10/11) the circulating autoantibodies of anti-BP230 were negative [40]. IgE anti-BP180 were detected in 21 out of 36 BP serum samples, while IgE anti-BP230 were identified in 18 out of the 36 BP sera. Interestingly, IgG and IgE anti-BP180 antibodies were found to be associated with disease activity, whereas IgG and IgE anti-BP230 autoantibodies did not exhibit such a correlation [41]. Furthermore, the serum anti-BP180 IgG exhibited higher levels of sensitivity and specificity compared to anti-BP230 IgG [42]. It follows that anti-BP180 is more highly expressed in BP and correlates with disease activity. Therefore, the detection of BP180 IgG autoantibody levels is a guide to predict dermatologic irAEs. In addition, a case of psoriasiform dermatologic toxicity caused by pembrolizumab reported by Johnson et al. regressed after systemic interleukin IL17A blockade treatment, suggesting that IL17A has the potential to be one of the predictive markers of dermatologic -related irAEs [43].
Gastrointestinal adverse events
Immune checkpoint inhibitor-associated colitis has been the most extensively documented. ICIs combination with chemotherapy drug has a higher incidence of colitis, such as atezolizumab + platinum (77.1%), pembrolizumab + platinum (41.4%), however, ICIs alone, nivolumab resulted in the highest incidence of colitis (67.3%), followed by pembrolizumab (60.5%) and durvalumab (45.2%) [30]. Another meta-analysis yielded similar results, multi-drug combination: nivolumab + ipilimumab + platinum (72.4%), atezolizumab + platinum (56.9%), pembrolizumab + platinum (38.6%), ICIs alone: nivolumab (63.1%), durvalumab (56.6%), pembrolizumab (54.9%) [29]. The most prevalent clinical manifestation of ICI-associated colitis is diarrhea, accompanied by additional symptoms such as fever, abdominal pain, vomiting, blood in the stool, nausea, loss of appetite, and others [44]. A combined analysis of CheckMate 817, CheckMate 568, and CheckMate 227, evaluated the safety of first-line treatment with Nivolumab and Ipilimumab in metastatic NSCLC patients. The results indicated that in the patient population treated with the combination of nivolumab and ipilimumab, 78% experienced diarrhea as the most prevalent treatment-related adverse event (TRAE) of all grades (20%), followed by fatigue (18%) and pruritus (17%). TRAE, a treatment-associated adverse event, commonly known as an adverse event (AE) or adverse effect, is an undesired and detrimental consequence or secondary effect that arises due to a medical treatment, procedure, medication, or intervention. These occurrences can span the spectrum from mild and short-lived discomfort to severe and potentially life-threatening reactions. Adverse events have the potential to manifest in a variety of medical contexts, such as clinical trials, medical treatments, and the use of medications. The most frequent grade 3 or 4 TRAEs are increased lipase (6%), fatigue (2%), and diarrhea (2%) [45]. Typically, PD-1/PD-L1 inhibitors have a lower occurrence of severe gastrointestinal adverse effects than anti-CTLA-4 drugs [21, 46]. Based on a study involving 198 patients with renal cell carcinoma (RCC) or metastatic melanoma (MM) treated with CTLA4 inhibitors, small bowel colitis was identified as the primary major toxicity, observed in 21% of patients [47]. Watery diarrhea is commonly related to anti-CTLA-4 therapy (27-54%) [48]. A different study conducted in Japan involved 661 cancer patients, which revealed the occurrence of gastrointestinal irAEs (GI-irAEs). Among patients treated with anti-PD-1/PD-L1 antibodies, the incidence of GI-irAEs was 5.6%. In contrast, patients who received anti-CTLA-4 antibodies alone or in combination with anti-PD-1 and anti-CTLA-4 antibodies experienced a higher frequency of GI-irAEs, with a recorded incidence of 16.1%. Interestingly, MM patients with GI-irAEs had better OS with continued ICI treatment [49]. Severe immunodigestive adverse reactions are a common cause of ICI discontinuation. In addition, some rare but severe death-causing intestinal perforations have been associated with ICI-induced colitis or small bowel colitis [48]. Therefore, early diagnosis is essential to prevent the persistence or progression of colitis and to minimize the interruption of ICI treatment, and exploring predictive markers of ICI-associated GI-irAEs is a key.
The regulation of flora-gut barrier homeostasis by immunotherapy occurs through the apoptosis of intestinal epithelial cells (IECs) mediated by intraepithelial lymphocytes (IELs) [50]. Dysbiosis of the intestinal flora is the cause of GI-irAEs, in which the products of the microbiome trigger innate immune responses and activate auto-reactive immune cells [51]. Several intestinal bacteria have been shown to enhance the induction of Treg cells and thus maintain intestinal tolerance [52]. Increasing evidence suggests a potential correlation between gut microbiome traits and the incidence of ICI-associated colitis as well as tumor response to ICI treatment [44]. However, Chaput et al. performed a prospective study which demonstrated that ipilimumab has no impact on the microbiome's composition [53]. Fusarium, a specialized anaerobic bacterium, has a role in maintaining the integrity of the colonic mucosa, and its enrichment might be related to the development of colitis [53, 54]. Furthermore, it has the potential to elevate CTL concentrations within the tumor microenvironment, leading to prolonged overall survival and increased progression-free time [54]. The increase in Bacteroides fragilis, Phascolarctobacterium genus, Enterobacteriaceae family, and Akkermansia muciniphila are thought to be a protective factor for combined immunotherapy for cancer (CIC) and it exerts anti-inflammatory effects in the gastrointestinal tract [55-57]. On the contrary, Firmicutes phylum and Veillonela are a risk factor for ICIs-related colitis [53, 58, 59]. Bacteroides fragilis produces polysaccharide A to activate the CTLA-4 pathway, which plays a crucial role in immune regulation by upregulating IL-10 levels and ultimately reducing inflammation [60]. Furthermore, studies have indicated that blocking CTLA-4 can promote the proliferation of S. fragilis in the colon [61]. Additionally, an elevation in the population of Bacteroides fragilis may lead to a further decrease in tumor size among patients undergoing ipilimumab treatment [62]. Therapeutic effects of anti-CTLA-4 inhibitors can be restored via relay transfer of Bifidobacterium fragilis-specific T cells into germ-free mice [63]. Clinical trials using PD-L1 inhibitors have revealed that Bifidobacterium is capable of facilitating antitumor effectiveness, with oral administration of Bifidobacterium alone improving melanoma tumor control to a similar extent as PD-L1-specific antibody therapy, with combination therapy virtually eliminating tumor growth [64]. Interestingly, Bifidobacterium was able to attenuate Treg cell-mediated intestinal inflammation without impairing the antitumor response [65]. Wang et al. developed a model of colitis associated with ICIs and observed a reduction in the levels of Lactobacillus in the fecal samples of ICI-treated mice compared to the control group [66]. Similarly, a mouse model constructed using trinitrobenzenesulfonic acid showed that Lactobacillus royale strains suppressed intestinal inflammation [80]. In addition, Lactobacillus royale enhanced dendritic cell function and improved antitumor response after blockade of PD-L1[67]. Thus, the development of ICI-associated colitis may be influenced by the intestinal flora's type and relative abundance. Certain specific microorganisms may coordinate the initiation of inflammation, while other subgroups may perpetuate ICI-associated colitis. Specific bacteria may be biomarkers of ICI-associated colitis, and further exploration of the complex symbiotic relationship between ICI-induced colitis and bacteria is needed with the ultimate goal of manipulating the microbiota for tumor response and irAEs balance.
Furthermore, a combination of multiple molecular markers, including heightened levels of IL-17, neutrophils, eosinophils, and leukocytes, has been utilized to forecast the occurrence of ICI-induced colitis [68, 69]. Patients with ICI-associated colitis were observed to have lower baseline levels of granulocyte colony-stimulating factor (GCSF) [69, 70]. Shahabi et al [71] studied the whole blood gene expression profile of 162 patients treated with ipilimumab. Increased therapeutic expression of CD177 and CEACAM1, which are two indicators of neutrophil activation, was found to be strongly associated with GI-irAEs, suggesting that neutrophils may play a role in ipilimumab-associated GI-irAEs. Given the limited sensitivity of these biomarkers, they cannot be utilized in isolation for predicting the occurrence of GI-irAEs in patients. Therefore, it is essential to investigate these biomarkers further in a more extensive patient group.
ICI-associated hepatitis
The incidence of immunotherapy-induced hepatitis is relatively low, ranging from 5-10% in monotherapy ICI cases. Severe toxicity associated with this form of treatment is less than 2%. However, when combination therapy involving ipilimumab and nivolumab is used, the incidence of immunotherapy-related hepatitis increases substantially, affecting approximately 30% of patients [72, 73]. According to research data, the likelihood of experiencing hepatotoxicity is higher in patients treated with ipilimumab (ranging from 3% to 9%) compared to those treated with anti-PD-1/anti-PD-L1 antibodies (ranging from 1% to 2%) [74]. In a meta-analysis of 17 RCTs, it was found that CTLA-4 inhibitors (such as ipilimumab and tremelimumab) had a higher risk of total and advanced hepatotoxicity when compared to control regimens. On the other hand, PD-1 inhibitors (like nivolumab and pembrolizumab) were related to a lower risk of total and advanced hepatotoxicity compared to control regimens [75]. Besides, the incidence of immune hepatitis varies with use of different drugs and for different cancer types. Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST)are usually elevated in ICI-associated hepatitis [76]. The incidence of hepatotoxicity due to ICIs has been reported to be 1% to 17% in melanoma [77]. A comparative safety trial of nivolumab in combination with ipilimumab versus ipilimumab in untreated melanoma found that any-grade abnormal elevations in AST and ALT is 22% and 21%, respectively, in the combination nivolumab and ipilimumab group, with greater than grade 3-4 accounting for 11% and 7%. In contrast, the ipilimumab group had only 4% of any-grade AST and ALT elevations and no grade 3-4 toxicity [78]. In contrast, a recent study from Japan enrolled a total of 202 patients with multiple cancers who received monotherapy with different ICIs (nivolumab, pembrolizumab, ipilimumab, atezolizumab, and avelumab). The resultant incidence of immune hepatitis was 8.4% (17/202), of which the incidence of grade ≥3 immune hepatitis was 4% (8/202) [79]. Incorporating data from 11 clinical trials and 7,086 patients diagnosed with NSCLC, a meta-analysis revealed that the use of ICIs resulted in an overall incidence rate of 6.18% for ALT elevation, 4.99% for AST elevation, and 1.09% for hepatitis [80]. Several reports have shown that the median time to onset of immune hepatitis is approximately 6-14 weeks after use of ICIs and the median time of 10 weeks from the severe immune-related hepatitis [81]. However, the exact time is associated with the type of tumor and the type of drug used. For example, lung cancer patients treated with nivolumab developed hepatitis later than those treated with pembrolizumab, and also later than those treated with melanoma [82, 83]. Since the time of appearance of immune hepatitis is not quite the same as given in different reports, it is not reliable to identify whether it is immune hepatitis only by the time of appearance.
Although the precise mechanism behind ICI-induced hepatitis is not completely comprehended, it is believed to entail a multifaceted interaction between the immune system and liver cells. Here are some possible mechanisms: (1) activation of T cells: ICIs activate T cells, which are a type of immune cell that can attack cancer cells. However, in some cases, these activated T cells may also attack healthy liver cells, leading to hepatitis [84-86]. (2) Dysregulation of cytokines: ICIs can cause a dysregulation of cytokines, which are proteins that regulate the immune response. This dysregulation can lead to an overactive immune response against liver cells [87, 88]. (3) Direct toxicity: Some ICIs may have direct toxic effects on liver cells, leading to hepatitis [89-91]. (4) Pre-existing liver damage: Patients with pre-existing liver damage, such as hepatitis B or C, cirrhosis, or fatty liver disease, may be more susceptible to immunotherapy-related hepatitis [92, 93]. (5) Genetic factors: Certain genetic factors may increase the risk of developing immunotherapy-related hepatitis, although the exact genes involved are not yet known. For example, genetic variants such as HLA-DRB107, HLA-DQA102, HLA- DRB1*04:01 and HLA- DRB1*15:01-DQB1*06:02 have been associated with the development of immunotherapy-associated hepatitis [94-96]. It is essential to note that the exact mechanism of immunotherapy-related hepatitis may vary depending on the specific type of immune checkpoint inhibitor used, as well as individual patient factors.
There are fewer reports on predictors of immune hepatitis. Some studies have shown that female patients treated with ICIs have a higher probability of developing grade ≥3 immune hepatitis compared to men [79]. Certain underlying conditions might enhance the risk of immunotherapy-associated hepatitis. For example, patients with autoimmune diseases or infectious diseases may be more likely to develop immunotherapy-associated hepatitis [97]. ICI-associated hepatitis shares some similarities with classical autoimmune liver disease (AILD). In their study, Coukos et al. identified three distinct histological patterns of liver injury caused by ICIs treatment: hepatitic (52%), cholangitic (19%), and mixed (29%). Furthermore, when comparing the ICIs-associated group to the AILD group, the former showed a higher prevalence of centrilobular injury and granuloma formation. Besides, CD8+ T cells tended to be increased in ICIs-related hepatitis [98]. Patients with poor liver function are more likely to develop immunotherapy-associated hepatitis. In addition, diet and lifestyle may also influence the development of immunotherapy-associated hepatitis. For example, excessive alcohol consumption and obesity may increase a patient's risk of developing immunotherapy-associated hepatitis [99, 100]. In addition, induction of liver injury by ICIs may be related to certain antibodies. Taking NSCLC as an example, research has shown that antituberculous antibody (ANA) and antimitochondrial antibody (AMA) may help predict ICI-induced liver injury. The present study examined NSCLC patients who were administered either nivolumab (3 mg/kg every 2 weeks) (186 patients) or pembrolizumab (200 mg every 3 weeks) (66 patients) as monotherapy. The results indicated that for patients treated with nivolumab, positive ANA testing was identified as a risk factor for ICI-related hepatitis (risk ratio 2.133: 95% CI 1.085-4.194: p=0.0281). On the other hand, for patients receiving pembrolizumab, both positive ANA and age were found to be potential risk factors for ICIS-related hepatitis (risk ratios 7.834: 95% CI 1.743-35.21: p=0.0073 and 0.896: 95% CI 0.896: p=0.0050, respectively) [101]. Predicting immune-mediated hepatitis is relatively difficult, and primary liver diseases and other causes of liver function abnormalities should be excluded first. With the gradual application of ICIs in clinical practice, the incidence of immune-related hepatitis is increasing. Accurate prediction of immune-related adverse reactions is particularly important for the prognosis of patients treated with ICIs.
Endocrine system diseases
ICIs treatment can lead to the development of various endocrinopathies such as hypophysitis, hyperthyroidism or hypothyroidism, thyroiditis, primary adrenal insufficiency (PAI), and insulin-dependent diabetes mellitus (DM) [102]. Certain endocrinopathies have been observed to occur more frequently with specific ICIs. For instance, hypophysitis is more prevalent following treatment with anti-CTLA-4 drugs like ipilimumab, while thyroid dysfunction is more commonly associated with anti-PD-1 drugs such as nivolumab and pembrolizumab. Moreover, the co-administration of these drugs might increase the risk of developing ICI-associated endocrinopathies [102]. In a meta-analysis comprising 34 studies examining ICIs therapy, it was found that out of 6472 patients, 85 cases of hypophysitis were observed, with 34 cases classified as grade 3 or higher (constituting 0.5% of the total cases). During treatment, the ipilimumab-nivolumab combination therapy had the highest recorded incidence of pelvic inflammation (6.4%), whereas anti-CTLA-4 therapy had rates of 3.2%, anti-PD-1 therapy had rates of 0.4%, and anti-PD-L1 therapy had rates of less than 0.1% [102]. The frequency of hypophysitis caused by anti-CTLA-4 antibodies was found to be between 8.0% to 11.7% [103]. Individuals who underwent anti-PD-1 therapy had a significantly lower likelihood of developing hypophysitis in comparison to those who received ipilimumab treatment [104]. Thyroid dysfunction represents a commonly observed endocrine-related irAEs associated with the use of ICIs [105]. The ICIs-associated thyroid dysfunction is mostly characterized by hyperthyroidism, hypothyroidism, and/or thyroiditis [106-108]. Compared to anti-PD-L1 monotherapy or anti-CTLA-4 monotherapy, the incidence of thyroid dysfunction appears to be higher with anti-PD-1 therapy and combined ipilimumab-nivolumab therapy [102]. A meta-analysis of anti-CTLA-4, anti-PD-1/PD-L1 treatments that included 37 randomized controlled studies found that 194 of 7531 patients developed hyperthyroidism and 472 of 7551 patients developed hypothyroidism [102]. Morganstein et al. conducted the biggest single-center study on thyroid dysfunction associated with ICIs. Their study confirmed the relatively high frequency of thyroid dysfunction in ICI-treated patients, and revealed that this condition was most common in patients who received the ipilimumab-nivolumab combination therapy [109]. According to case series reports, the detection of thyroid dysfunction associated with ICI occurred within a median range of 18 to 123 days from the start of ICIs treatment [110-112]. PAI is a rare irAEs relate to ICIs treatment and only a few cases of ICI-related PAI have been reported [113-116]. Although rare, ICIs-associated DM can be a life-threatening irAEs. The overwhelming majority of reported cases of diabetes mellitus associated with ICIs are linked to anti-PD-1 therapy. However, there have been a few reported instances of diabetes associated with anti-PD-L1 therapy as well [117-119]. Besides, anti-CTLA-4 monotherapy seems to have an exceptionally low incidence of leading to ICIs-associated DM [120].
The exact pathogenesis of ICIs-associated endocrinopathies is unclear. The incidence of ICI-associated endocrinopathies increases with dose and varies according to ICI therapy. Earlier prediction can improve patient survival and it is especially important to identify predictive markers. According to Nakamura et al., there is a positive correlation between absolute eosinophil counts >240/µL and relative eosinophil counts >3.2%, and the onset of endocrine irAEs [121]. Both in vitro experiments and studies using mouse models have demonstrated that antibody-dependent cell-mediated cytotoxicity (ADCC) and complement pathways play a role in the development of hypophysitis induced by ipilimumab [122, 123]. According to Tahir et al., alterations in the ploidy of autoantibodies against GNAL or ITM2B following ICIs treatment were linked to the subsequent occurrence of hypophysitis. The changes in anti-GNAL and anti-ITM2B autoantibodies were distinct from the values observed in patients who did not develop hypophysitis [104]. Previous studies have comprehensively analyzed indirect immunofluorescence (IIF) results and found significant differences in the distribution of cytoplasmic staining patterns between pituitary disorders, with granular cytoplasmic staining being highly predictive of pituitary autoimmunity. Moreover, in patients with positive IIF assays, purified IgG produced clearer signals, which improved the sensitivity and specificity of the assay [124]. High rate of antipituitary antibody (APA) positivity in hypophysitis induced by anti-CTLA-4 antibodies and the presence of anti-PD-1 therapy in hypophysitis is not yet known [125]. Antihypothalamic antibody (AHA) is frequently detected in patients receiving immunotherapy, suggesting its possible is a biomarker for early detection of pituitary disease associated with ICIs. In a cross-sectional study involving 54 cancer patients undergoing anti-PD-1 or anti-PD-L1 therapy and 50 healthy controls, there was a statistically significant rise in APA observed among these patients [126]. Furthermore, the impact of the host's germline on the response to ICI therapy is worth exploring, and human leukocyte antigen class I (HLA-I) serves as a valuable prognostic biomarker for this purpose [127]. CD8+ T cells play a major role in the antitumor activity of ICIs and are required to present tumor antigens via HLA-I [128]. One study evaluated HLA-Cw12, HLA-DR15, HLA-DQ7 confirmed that HLA-DQ7 is related to the development of ICI-associated isolated adrenocorticotropic hormone deficiency (IAD) in the absence of pituitary enlargement [129]. HLA-DR4 was present in 76% patients of insulin-dependent diabetes induced with ICIs [130, 131]. Besides, the class II trait is HLA HLA-DR4-DR53 and HLA-DR15 in ICI-induced thyroiditis patient [132].
Furthermore, elevated thyroperoxidase (TPO) antibodies and thyrotropin receptor antibody (TgAb) was found in many cases of thyroid dysfunction associated with ICIs [107, 112, 133, 134]. However, the role of thyroid autoantibodies in the pathogenesis of ICIs-associated thyroid dysfunction, as well as whether elevated levels raise the risk of such dysfunction, remains uncertain. Osorio et al. had reported that elevated thyroid autoantibody levels after ICIs treatment did not guarantee significant thyroid dysfunction [108]. However, Maekura et al. found that baseline levels of TPO Ab and TgAb may predict hypothyroidism due to nivolumab treatment of NSCLC. Out of the 64 patients who participated in this trial, 5 (7.8%) developed hypothyroidism following treatment with nivolumab. Patients who developed primary hypothyroidism were found to have significantly positive TPO antibody and TgAb levels [135]. Further studies are required to confirm whether increased levels of TPO antibodies and TgAbs at baseline are risk factors for ICIs-related thyroid dysfunction. A higher risk of developing atezolizumab-induced thyroid dysfunction, which is linked to improved survival rates, has been observed in individuals with elevated polygenic risk scores (PRS) for thyroid autoimmunity-associated genetic variants [136]. An analysis in the field of bioinformatics discovered that, in cases of hypothyroidism resulting from CTLA-4 treatment, genes such as ALB, MAPK1, SPP1, PPARG, and MIF are found to be overexpressed. On the other hand, in cases of hyperthyroidism caused by the same treatment, genes such as ALB, FCGR2B, CD44, LCN2, and CD74 are overexpressed. These genes may potentially serve as biomarkers to predict immunotherapy-induced thyroid dysfunction [137]. Although the exact mechanism of ICI-associated endocrinopathies remains unclear, these findings indicated that autoantibodies may be potential biomarkers for prediction and treatment of ICI induced endocrinopathies and deserve further investigation.
ICIs-associated Pneumonitis
The most prevalent manifestation of pulmonary toxicity in patients receiving ICIs is pneumonia [138]. The Society for Immunotherapy of Cancer's (SITC) Toxicity Management Working Group reports that targeted therapy of PD-1/PDL-1 and CTLA-4 is associated with pneumonitis in less than 5% of cases overall, with severe complications (grade three or higher) occurring in only 1-2% of patients [138]. In a meta-analysis of 20 RCTs investigating PD-1 inhibitors across different tumor types (including 12 melanoma studies, five NSCLC studies, and three RCC studies), which involved a total of 4496 unique patients, the occurrence of grade three or higher pneumonia was found to be between 0% to 4.3% and 0% to 10.6% for any grade of pneumonia. Four studies reported 1 to 3 pneumonia-related deaths (0.2% - 2.3%). Compared to any grade of pneumonia in melanoma, NSCLC had a significantly higher incidence of pneumonia (4.1% vs. 1.6%; P=0.002), as well as a higher incidence of grade ≥3 pneumonia (1.8% vs. 0.2%; P<0.001). Furthermore, the combination therapy group had a significantly higher incidence of morbidity (6.6% vs. 1.6%; P<0.001) and grade ≥3 pneumonia (1.5% vs. 0.2%; p=0.001) compared to the monotherapy group [139, 140]. Meta-analysis of published anti-PD-1/PD-L1 and anti-CTLA-4 trials assessing their incidence using over 16 million adverse drug reaction data from the WHO pharmacovigilance database and records from 7 academic institutions showed widespread central pneumonia with anti-PD-1/PD-L1 monotherapy (115 [35%]), compared with pneumonitis with ipilimumab monotherapy (15 [8%]) [141]. The risk of pneumonia is higher with anti-CTLA-4/anti-PD-1/PD-L1 combination therapy compared to ICI monotherapy [140, 142]. Extensive studies are required to identify the risk factors related to ICI-associated pneumonia (IAP), which should include investigating the association between smoking history and the risk of developing pneumonia, as well as exploring the role of the PD-1/PD-L1 pathway in the pathogenesis of pneumonia and the identification of predictive markers.
A retrospective study included 430 patients with anti-PD-1 antibodies in combination with or without chemotherapy or anti-angiogenic therapy showed a prevalence of 15.6% for IAP and 3.7% for severe IAP. Patients with IAP had low eosinophil percentages at endpoint (Eend)/ baseline (Ebas) values (p = 0.001), especially for severe IAP (p = 0.036). In addition, Eend/Ebas values performed well in the diagnosis of severe IAP (AUC:0.800, p<0.001), indicating that eosinophil percentage correlates with the diagnosis, prediction and prognosis of IAP [143]. A meta-analysis of 17 RCTs with 2758 patients revealed that individuals with NSCLC and interstitial lung disease (ILD) had a higher likelihood of developing pneumonia after immunotherapy. Subgroup analysis indicated that ILD, radiation pneumonitis, and interstitial lung abnormalities were predictors of IAP, along with the neutrophil lymphocyte ratio (NLR) and the actual eosinophil acidic granulocyte count, which may have potential predictive value for IAP [144]. Continuous monitoring of NLR trends may be indicative of the onset and severity of pneumonitis as well as the prognosis, according to these findings [145]. The presence of homozygosity at one or more HLA-I loci was associated with a decreased likelihood of developing pneumonia or experiencing toxicity of grade 3 or higher [146]. Both baseline and dynamic levels of IL-10 in the plasma are significantly and autonomously related to an increased risk of pneumonia, and may serve as useful clinical markers to monitor pneumonitis in patients undergoing ICIs therapy [147].
Cardiovascular toxicity
The most common cardiovascular irAEs in ICIs therapy is myocarditis, which initially surfaced in case reports, as well as in phase II and III clinical trials of ICIs, and subsequently in case series [148-152]. The incidence rate of myocarditis presented range from 0.5 to 1.7% [10]. In comparison to other irAEs, myocarditis exhibits the highest fatality rate at 39.7% [153, 154]. In a study of 964 patients treated with ICIs, Mahmood and colleagues discovered that the incidence of myocarditis was 1.14%, with a median onset of 34 days [155]. Pharmacovigilance reports indicate that myocarditis was detected in 0.06% of patients treated with single nivolumab therapy, whereas in patients treated with a combination of ipilimumab-nivolumab therapy, the incidence was 0.27% [151]. However, myocarditis is more frequent than initially reported in early ICIs clinical trials [156]. According to a recent review of the WHO database, the higher incidence of ICIs-related myocarditis reported in recent multicenter registries may more accurately reflect the true incidence [157]. In a recent randomized trial comparing nivolumab to combined relatlimab and nivolumab, where an incidence rate of myocarditis is 0.6% for single nivolumab and 1.7% for combination relatlimab and nivolumab [10]. In addition to myocarditis, other cardiovascular irAEs are increasingly being reported, such as vasculitis pericardial disease, arrhythmia, acute coronary syndrome, thrombotic events, and left ventricular (LV) dysfunction, without evidence of myocarditis [158-161].
Although the incidence of ICIs-associated myocarditis is relatively low, its lethality is high; therefore, early diagnosis of myocarditis, even in the subclinical phase without clear clinical signs and symptoms, enables timely intervention [162]. And biomarkers as the best diagnostic tool can predict early (e.g., administered at baseline) or monitor early (e.g., administered continuously during chemotherapy) the onset of myocarditis. Nearly all patients with ICIs-related myocarditis have been shown to exhibit elevated hypersensitivity troponin levels, as per several studies [163]. Persisting inflammation of the myocardium is suggested by elevated high troponin levels [164]. A strong association between troponin levels and the risk of major adverse cardiac events, including cardiovascular death and shock, has been observed in patients with ICIs-associated myocarditis.
Johnson and colleagues' report indicates that the myocarditis linked with ICIs involves the infiltration of CD4+ and CD8+ T cells, along with CD68+ macrophages, into the myocardium and conduction system. Notably, the presence of other immune cells, such as B cells, was not observed [151]. TNF-α, a cytokine with pro-inflammatory properties, has been shown to induce acute and chronic hemodynamic changes and left ventricular dysfunction. Overexpression of TNF-α in cardiomyocytes can lead to cardiomyopathy and myocarditis [165]. Pro-inflammatory cytokines, such as IL-1B, IL-2, and IL-6, have negative inotropic and cytotoxic effects on cardiomyocytes [166-168]. The lack of histological analysis makes it uncertain whether classical myocarditis, characterized by myocardial immune infiltration and subsequent myocardial cell death, occurs in cytokine release syndrome. As cancer immunotherapy advances, it becomes more crucial to comprehend the impact of pro-inflammatory cytokines on the cardiovascular system [151, 153, 169]. However, the few clinical trials in which baseline troponin was measured to predict myocarditis after ICIs initiation did not demonstrate good predictive power or specificity [170]. In one of the largest prospective studies conducted to date via Waliany et al. 11.2% of the 214 patients included were found to have positive high-sensitivity troponin i values (≥55 ng/L) compared to a 1.4% incidence of myocarditis (3 cases) in 2021. Thus, in most cases, troponin I positivity was attributed to cardiovascular causes other than myocarditis [171]. Zhang et al. found that cardiac biomarkers, including mean cardiac troponin T and NT-proBNP, were significantly elevated after treatment with ICI, peaked at about one month, and then gradually decreased [172]. Therefore, specific troponin thresholds for ICI-associated myocarditis need to be validated by larger clinical trials, and the effectiveness of troponin-based screening methods in obtaining timely diagnosis and risk of inappropriate treatment interruption still needs to be proven [170, 173]. Similarly, in cases of ICIs-related myocarditis, serum creatine kinase (CK), creatine kinase isoenzyme (CK-MB) and N-terminal pro-B type natriuretic peptide (NT-proBNP) were elevated, but specificity was as low as that of troponin [174, 175]. In their study, Boughdad and colleagues enrolled patients who were clinically suspected of having associated myocarditis and discovered that the majority of those who were tested (six out of seven) showed elevated levels of serum inflammatory cytokines such as IL-6, as well as chemokines CXCL9, CXCL10, and CXCL13. In the study, it was observed that four out of five patients with myocarditis displayed an imbalance between Th1 and Th2 immune cells, which resulted in significant inflammatory responses from Th1, Th1/Th17, and Th17 CD4 memory T-cells. Additionally, four to five patients had a notable increase in non-classical monocytes and reduced levels of CD31. Further investigation is necessary to determine if the aforementioned inflammatory cytokines can serve as indicators for immunotherapy-associated myocarditis [176]. Zhu et al. used time-of-flight mass spectrometry flow cytometry to investigate peripheral blood mononuclear cells of 52 patients who underwent immunotherapy. Their findings demonstrated a significant rise in clonally cytotoxic Temra CD8+ cells in the bloodstream of eight patients with ICI myocarditis, accompanied by distinct transcriptional alterations in these expanded effector CD8+ cells. Notably, the chemokines CCL5/CCL4/CCL4L2 were found to be up-regulated, and could be promising targets for diagnosis and treatment to lower the occurrence of life-threatening cardiac iRAEs in ICI-treated cancer patients [177]. Zhang et al. found that cardiac biomarkers, including mean cardiac troponin T and NT-proBNP, were significantly elevated after treatment with ICIs, peaked at about 1 month, and then gradually decreased [172]. In summary, ICIs may cause various adverse cardiovascular events (ACEs) at a higher rate than in patients receiving only conventional chemotherapy. Regular electrocardiography, echocardiography and cardiac biomarkers can help to detect ACE caused by ICIs early and treat it timely [172].
Neurological irAEs
Among the 3,763 patients with advanced melanoma who received nivolumab with or without ipilimumab in the global pharmacovigilance and epidemiology database, only a small proportion experienced neurological irAEs (NirAEs). Specifically, 35 patients (0.93%) developed a total of 43 serious neurological irAEs, which included neuropathy (n = 22), noninfectious meningitis (n = 5), encephalitis (n = 6), and others [178]. The most common NirAEs is neuromuscular disease (approximately 5.5%) [179], with the most prevalent neuromuscular syndrome being myositis, which occurs in nearly 3% of patients treated with anti-PD-1/PD-L1. In contrast, central nervous system (CNS) involvement is notably rare, with a frequency of only 0.5%, and usually presents as encephalitis, meningitis, vasculitis, myelitis, and cranial neuropathy [180]. Other studies have reported several neurological irAEs, including peripheral neuropathy (1.3%), myasthenia gravis (1.2%), myelitis (0.8%), meningitis (0.4%), encephalitis (0.3%), and Guillain-Barre syndrome (<0.1%) [181, 182]. Despite the prevalence of evidence, most of the evidence collected is from small case reports or case series. Most events are mild and accompanied by nonspecific symptoms, such as headache; <1% of patients have a grade 3 or higher irAEs [181]. The collective occurrence rates are less than 4% for anti-CTLA-4 antibody therapy, approximately 6% for anti-PD-1 antibody therapy, and around 12% for a combination of both anti-CTLA-4/PD-1 antibody therapy [181]. In contrast, relapsed/refractory large B-cell lymphoma (R/R LBCL) patients who received CD19-directed chimeric antigen receptor T-cell therapy (CAR-T) experienced a notably higher occurrence of immune effector cell-related neurotoxicity syndrome (ICANS). Of the patients, 25 (56%) developed ICANS, with 18 (72%) experiencing severe ICANS (CTCAE grade 3-4). The median duration of ICANS was 5 days (range, 3-11). However, neither the advancement of ICANS nor its treatment correlated with worse CR, PFS, or OS [183].
Although the incidence of NirAEs is low, the potential severity and consequent interruption of cancer treatment that they can entail makes early detection particularly essential [184]. Studies have shown that elevated levels of IL17A are correlated with NirAEs[185]. Elevated CK levels are commonly observed in patients diagnosed with neuromyositis optica, particularly in those who receive a combination of ICIs. Although some studies suggest that CK levels are higher in individuals with severe disease as opposed to mild disease, there is no clear association between CK levels and symptom severity [186-188]. Oligoclonal bands, polycythemia and hyperproteinemia in cerebrospinal fluid are nonspecific inflammatory alterations that are usually found in patients with immune-mediated encephalitis [189]. There have been reports indicating that heightened levels of adenosine deaminase (ADA) can serve as a valuable diagnostic marker for ICIs-associated CNS toxicity [190]. In summary, these predictive markers may serve as a predictive and therapeutic target, but relatively poor specificity and further research is necessary to substantiate this hypothesis.
Rheumatic irAEs
Although the literature on rheumatic irAEs (Rh-irAEs) in patients treated with ICIs is growing rapidly [191, 192], the incidence has not been well appreciated, especially in some cases where Rh-irAEs resembles various rheumatic diseases, Such as arthritis, Sicca and Sjogren's s (SS), systemic lupus erythematosus (SLE), myositis, vasculitis, and polymyalgia rheumatic (PMR) [193-197]. The European League Against Rheumatism (EULAR) led by screening 630 references and including 22 retrospective and prospective studies found that the prevalence of Rh-irAEs ranging from 1.5% to 22% in the real world, suggests that Rh-irAEs is underreported in clinical trials [198-202]. A systematic review consisting of 33 clinical trials, three observational studies, and 16 case reports or series on rheumatic and musculoskeletal irAEs found that the occurrence rates of arthralgia ranged from 1% to 43%, while the prevalence rates for myalgia were between 2% to 20%. These results underscore notable variations in symptom reporting and diagnosis of rheumatic irAEs [191]. The reasons for this discrepancy may be multifaceted. Firstly cancer-related adverse events in clinical trials are reported using multiple mutually exclusive musculoskeletal symptom codes. For example, arthritis may be coded as arthritis, arthralgia, joint swelling, or pain in the extremities [203]. Second, many clinical trials do not report Rh-irAEs or partially report only highly and/or frequently occurring adverse events (i.e., in 10% of patients). After ICI treatment, patients may develop arthritis or arthralgia, with incidence rates of 3-9% for anti-CTLA-4, 7-11% for anti-PD-1/PD-L1, and 11% for combination therapy [204, 205]. According to a prospective observational study conducted at a single center involving 524 patients treated with ICI, 35 (6.6%) of the participants developed Rh-irAEs. The Rh-irAEs included inflammatory arthritis (3.8%), non-inflammatory musculoskeletal disorders (2.8%), as well as specific conditions like rheumatoid arthritis, PMR, or psoriatic arthritis [206]. The most significant Canadian multicenter cohort study to date, consisting of 117 patients with Rh-irAEs, revealed that the most prevalent Rh-irAEs was symmetrical polyarthritis. The cohort also reported other Rh-irAEs, including non-inflammatory musculoskeletal symptoms, PMR, and myositis [207]. A retrospective study conducted at a single center and involving 264 patients with different types of cancer who received ICI, revealed that 16.3% (n=43) of these patients developed at least one Rh-irAEs. Interestingly, patients who developed Rh-irAEs had a significantly higher median overall survival of 132 weeks as compared to those who did not develop Rh-irAEs, whose median overall survival was 42.7 weeks, with a P value of less than 0.01. This significant difference was observed even after adjusting for various factors in a multivariate analysis, P < 0.05), indicating that Rh-irAE development in ICI therapy is linked with a better prognosis [208]. Therefore, it is crucial to detect Rh-irAEs early to enable prompt and optimal management, considering the long-term response potential of these patients.
Current information on the incidence of Rh-irAEs is found in reports of observational studies. There are few studies investigating possible underlying immunopathogenic mechanisms. Until now, the majority of reports on Rh-irAEs have not identified typical autoantibodies, traditional HLA correlations, or any other biomarkers of unexplained illness. However, there are some markers that have demonstrated the ability to predict Rh-irAEs. Disease activity in antinuclear cytoplasmic antibody (ANCA)-related vasculitis and SLE could be linked to T-cell exhaustion, as an instance [209]. In addition, in rheumatoid arthritis patients who test positive for anti-cyclic citrullinated peptide (ACPA), ICI treatment predisposes them to acute exacerbations, especially those receiving anti-PD-1 therapy [210]. Elevated levels of inflammatory cytokines such as IL17 and TNF-α have been observed in rheumatic irAEs, and inhibitors targeting these cytokines can be utilized for managing these adverse effects. However, whether these inflammatory cytokines can be used as predictive markers in this regard is still controversial [211, 212], further research in this area is urgently needed.
Conclusion
The treatment landscape for diverse solid tumors and hematological malignancies has been transformed by cancer immunotherapy, such as ICIs and CAR-T, resulting in increased benefits for a larger number of individuals with cancer. However, an increasing number of adverse reactions caused by ICIs have also been reported. As the clinical use of immunotherapy continues to rise, it becomes crucial to recognize and manage its distinct toxicities. The most demanding issue in clinical practice is presently how to maximize the therapeutic benefits of ICIs while minimizing the issues arising from irAEs. Clinicians should fully understand the diversity and severity of adverse reactions related to immunotherapy drugs, improve their ability to diagnose and treat them early, pay attention to medication details, and make these drugs work better to bring more clinical benefits to patients. Although publications on potential predictive irAEs biomarkers are also rapidly increasing, unfortunately, there are currently no clinically validated and effective precise biomarkers except for some markers with potential predictive ability but low specificity. Hence, it is imperative to conduct extensive and comprehensive prospective clinical studies in the future, along with interdisciplinary collaborations, to delve deeper into the pathogenesis and disease patterns of these irAEs. This would aid in developing appropriate grading criteria, optimal treatment measures, and identifying more specific predictive biomarkers. Additionally, enhancing physicians' proficiency in diagnostic and management skills and formulating relevant treatment guidelines would assist clinicians in improving their early recognition, diagnosis, and treatment of ICIs-related adverse reactions.
Abbreviations
ICIs: Immune checkpoint inhibitors;
irAEs: immune-related adverse events;
NSCLC: non-small cell lung cancer;
PD-1: programmed cell death 1;
PD-L1: programmed cell death ligand 1;
CTLA-4: cytotoxic T lymphocyte-associated protein 4;
LAG-3: lymphocyte-activated gene-3;
FDA: Food and Drug Administration;
NMPA: National Medical Products Administration;
TCR: T-cell receptor;
MSI-H: MicroSatellite Instability-High;
dMMR: Mismatch repair deficiency;
RCTs: randomized clinical trials;
BP: bullous pemphigoid;
TRAE: treatment-related adverse event;
RCC: renal cell carcinoma;
MM: metastatic melanoma;
IECs: intestinal epithelial cells;
IELs: intraepithelial lymphocytes;
CIC: combined immunotherapy for cancer;
GCSF: granulocyte colony-stimulating factor;
ALT: Alanine aminotransferase;
AST: Aspartate aminotransferase;
AILD: autoimmune liver disease;
ANA: antituberculous antibody;
AMA: antimitochondrial antibody;
PAI: primary adrenal insufficiency;
DM: diabetes mellitus;
ADCC: antibody-dependent cell-mediated cytotoxicity;
IIF: indirect immunofluorescence;
APA: antipituitary antibody;
AHA: Antihypothalamic antibody;
HLA-I: human leukocyte antigen class I;
IAD: isolated adrenocorticotropic hormone deficiency;
TPO: thyroperoxidase;
TgAb: thyrotropin receptor antibody;
CK: creatine kinase;
CK-MB: creatine kinase isoenzyme;
NT-proBNP: N-terminal pro-B type natriuretic peptide;
CNS: central nervous system;
CAR-T: chimeric antigen receptor T-cell;
ICANS: immune effector cell-associated neurotoxicity syndrome;
ADA: adenosine deaminase;
SS: Sicca and Sjogren's s;
SLE: systemic lupus erythematosus;
PMR: polymyalgia rheumatic;
EULAR: European League Against Rheumatism;
ANCA: antinuclear cytoplasmic antibody;
ACPA: anti-cyclic citrullinated peptide.
Supplementary Material
Supplementary figure.
Acknowledgements
Funding
This work was supported by Shandong Province Medical and Health Science and Technology Development Plan Project (No. 202203030713). Science and Technology Program of Yantai affiliated Hospital of Binzhou Medical University (No. YTFY2022KYQD06).
Author contributions
Miao YD: Conceptualization, Formal analysis, Funding acquisition, Methodology, Software, writing - original draft, Writing - review & editing.
Quan WX: Data curation, Formal analysis, Methodology, Writing - original draft.
Tang XL: Data curation, Formal analysis, Writing - original draft.
Shi WW: Data curation, Investigation.
Li Q: Formal analysis, Methodology.
Li RJ: Methodology, Software.
Wang JT: Methodology, Software.
Gan J: Methodology.
Dong X: Data curation.
Hao L: Data curation.
Luan WY: Investigation.
Zhang F: Conceptualization, Project administration, Writing - review & editing.
All authors reviewed the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature immunology. 2002;3:991-8
2. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1-10
3. Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. The New England journal of medicine. 2018;378:158-68
4. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA: a cancer journal for clinicians. 2020;70:86-104
5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12:252-64
6. Sobhani N, Tardiel-Cyril DR, Davtyan A, Generali D, Roudi R, Li Y. CTLA-4 in Regulatory T Cells for Cancer Immunotherapy. Cancers (Basel). 2021 13
7. Shibru B, Fey K, Fricke S, Blaudszun AR, Fürst F, Weise M. et al. Detection of Immune Checkpoint Receptors - A Current Challenge in Clinical Flow Cytometry. Front Immunol. 2021;12:694055
8. Kon E, Benhar I. Immune checkpoint inhibitor combinations: Current efforts and important aspects for success. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2019;45:13-29
9. Triebel F. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends in immunology. 2003;24:619-22
10. Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E. et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. The New England journal of medicine. 2022;386:24-34
11. Maruhashi T, Sugiura D, Okazaki IM, Shimizu K, Maeda TK, Ikubo J. et al. Binding of LAG-3 to stable peptide-MHC class II limits T cell function and suppresses autoimmunity and anti-cancer immunity. Immunity. 2022;55:912-24.e8
12. Wang J, Sanmamed MF, Datar I, Su TT, Ji L, Sun J. et al. Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell. 2019;176:334-47.e12
13. Lecocq Q, Keyaerts M, Devoogdt N, Breckpot K. The Next-Generation Immune Checkpoint LAG-3 and Its Therapeutic Potential in Oncology: Third Time's a Charm. Int J Mol Sci. 2020 22
14. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA. et al. Immune-related adverse events of checkpoint inhibitors. Nature reviews Disease primers. 2020;6:38
15. Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L. et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ (Clinical research ed). 2018;360:k793
16. Kalinich M, Murphy W, Wongvibulsin S, Pahalyants V, Yu KH, Lu C. et al. Prediction of severe immune-related adverse events requiring hospital admission in patients on immune checkpoint inhibitors: study of a population level insurance claims database from the USA. Journal for immunotherapy of cancer. 2021 9
17. Shoushtari AN, Friedman CF, Navid-Azarbaijani P, Postow MA, Callahan MK, Momtaz P. et al. Measuring Toxic Effects and Time to Treatment Failure for Nivolumab Plus Ipilimumab in Melanoma. JAMA oncology. 2018;4:98-101
18. Xin Z, You L, Na F, Li J, Chen M, Song J. et al. Immunogenetic variations predict immune-related adverse events for PD-1/PD-L1 inhibitors. European journal of cancer (Oxford, England: 1990). 2023;184:124-36
19. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE. et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373:1627-39
20. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E. et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373:123-35
21. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L. et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. The New England journal of medicine. 2015;372:2521-32
22. Miao YD, Quan W, Dong X, Gan J, Ji CF, Wang JT. et al. A bibliometric analysis of ferroptosis, necroptosis, pyroptosis, and cuproptosis in cancer from 2012 to 2022. Cell Death Discov. 2023;9:129
23. Sibaud V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. American journal of clinical dermatology. 2018;19:345-61
24. Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front Pharmacol. 2017;8:49
25. Curry JL, Tetzlaff MT, Nagarajan P, Drucker C, Diab A, Hymes SR. et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. Journal of cutaneous pathology. 2017;44:158-76
26. Tarhini A. Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: the underlying mechanisms and clinical management. Scientifica. 2013;2013:857519
27. Belum VR, Benhuri B, Postow MA, Hellmann MD, Lesokhin AM, Segal NH. et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. European journal of cancer (Oxford, England: 1990). 2016;60:12-25
28. Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME. et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Annals of oncology: official journal of the European Society for Medical Oncology. 2015;26:2375-91
29. Gu J, Shi L, Jiang X, Wen J, Zheng X, Cai H. et al. Severe immune-related adverse events of immune checkpoint inhibitors for advanced non-small cell lung cancer: a network meta-analysis of randomized clinical trials. Cancer immunology, immunotherapy: CII. 2022;71:2239-54
30. Zhang W, Gu J, Bian C, Huang G. Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors for Advanced Non-small Cell Lung Cancer: A Network Meta-Analysis of Randomized Clinical Trials. Front Pharmacol. 2021;12:686876
31. Patil PD, Fernandez AP, Velcheti V, Tarhini A, Funchain P, Rini B. et al. Cases from the irAE Tumor Board: A Multidisciplinary Approach to a Patient Treated with Immune Checkpoint Blockade Who Presented with a New Rash. The oncologist. 2019;24:4-8
32. Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI. et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:773-81
33. Min Lee CK, Li S, Tran DC, Zhu GA, Kim J, Kwong BY. et al. Characterization of dermatitis after PD-1/PD-L1 inhibitor therapy and association with multiple oncologic outcomes: A retrospective case-control study. Journal of the American Academy of Dermatology. 2018;79:1047-52
34. Fujimura T, Kakizaki A, Furudate S, Kambayashi Y, Aiba S. Tumor-associated macrophages in skin: How to treat their heterogeneity and plasticity. Journal of dermatological science. 2016;83:167-73
35. Yamaguchi Y, Ono J, Masuoka M, Ohta S, Izuhara K, Ikezawa Z. et al. Serum periostin levels are correlated with progressive skin sclerosis in patients with systemic sclerosis. The British journal of dermatology. 2013;168:717-25
36. Furudate S, Fujimura T, Kakizaki A, Kambayashi Y, Asano M, Watabe A. et al. The possible interaction between periostin expressed by cancer stroma and tumor-associated macrophages in developing mycosis fungoides. Experimental dermatology. 2016;25:107-12
37. Fujimura T, Sato Y, Tanita K, Kambayashi Y, Otsuka A, Fujisawa Y. et al. Serum levels of soluble CD163 and CXCL5 may be predictive markers for immune-related adverse events in patients with advanced melanoma treated with nivolumab: a pilot study. Oncotarget. 2018;9:15542-51
38. Chennamadhavuni A, Abushahin L, Jin N, Presley CJ, Manne A. Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors. Front Immunol. 2022;13:779691
39. Hasan Ali O, Bomze D, Ring SS, Berner F, Fässler M, Diem S. et al. BP180-specific IgG is associated with skin adverse events, therapy response, and overall survival in non-small cell lung cancer patients treated with checkpoint inhibitors. Journal of the American Academy of Dermatology. 2020;82:854-61
40. Wang J, Hu X, Jiang W, Zhou W, Tang M, Wu C. et al. Analysis of the clinical characteristics of pembrolizumab-induced bullous pemphigoid. Front Oncol. 2023;13:1095694
41. Hashimoto T, Ohzono A, Teye K, Numata S, Hiroyasu S, Tsuruta D. et al. Detection of IgE autoantibodies to BP180 and BP230 and their relationship to clinical features in bullous pemphigoid. The British journal of dermatology. 2017;177:141-51
42. Chanprapaph K, Ounsakul V, Pruettivorawongse D, Thadanipon K. Anti-BP180 and anti-BP230 enzyme-linked immunosorbent assays for diagnosis and disease activity tracking of bullous pemphigoid: A prospective cohort study. Asian Pacific journal of allergy and immunology. 2021;39:272-8
43. Johnson D, Patel AB, Uemura MI, Trinh VA, Jackson N, Zobniw CM. et al. IL17A Blockade Successfully Treated Psoriasiform Dermatologic Toxicity from Immunotherapy. Cancer immunology research. 2019;7:860-5
44. Marthey L, Mateus C, Mussini C, Nachury M, Nancey S, Grange F. et al. Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. Journal of Crohn's & colitis. 2016;10:395-401
45. Paz-Ares LG, Ciuleanu TE, Pluzanski A, Lee JS, Gainor JF, Otterson GA. et al. Safety of First-Line Nivolumab Plus Ipilimumab in Patients With Metastatic NSCLC: A Pooled Analysis of CheckMate 227, CheckMate 568, and CheckMate 817. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2023;18:79-92
46. Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L. et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet (London, England). 2017;390:1853-62
47. Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE. et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24:2283-9
48. Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Alimentary pharmacology & therapeutics. 2015;42:406-17
49. Yamada K, Sawada T, Nakamura M, Yamamura T, Maeda K, Ishikawa E. et al. Clinical characteristics of gastrointestinal immune-related adverse events of immune checkpoint inhibitors and their association with survival. World J Gastroenterol. 2021;27:7190-206
50. Pitt JM, Vétizou M, Waldschmitt N, Kroemer G, Chamaillard M, Boneca IG. et al. Fine-Tuning Cancer Immunotherapy: Optimizing the Gut Microbiome. Cancer Res. 2016;76:4602-7
51. Khan Z, Hammer C, Guardino E, Chandler GS, Albert ML. Mechanisms of immune-related adverse events associated with immune checkpoint blockade: using germline genetics to develop a personalized approach. Genome medicine. 2019;11:39
52. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121-41
53. Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C. et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Annals of oncology: official journal of the European Society for Medical Oncology. 2019;30:2012
54. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science (New York, NY). 2018;359:91-7
55. Liu W, Ma F, Sun B, Liu Y, Tang H, Luo J. et al. Intestinal Microbiome Associated With Immune-Related Adverse Events for Patients Treated With Anti-PD-1 Inhibitors, a Real-World Study. Front Immunol. 2021;12:756872
56. Sakurai T, De Velasco MA, Sakai K, Nagai T, Nishiyama H, Hashimoto K. et al. Integrative analysis of gut microbiome and host transcriptomes reveals associations between treatment outcomes and immunotherapy-induced colitis. Mol Oncol. 2022;16:1493-507
57. Yoon HS, Cho CH, Yun MS, Jang SJ, You HJ, Kim JH. et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nature microbiology. 2021;6:563-73
58. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science (New York, NY). 2018;359:97-103
59. Liu T, Xiong Q, Li L, Hu Y. Intestinal microbiota predicts lung cancer patients at risk of immune-related diarrhea. Immunotherapy. 2019;11:385-96
60. Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620-5
61. Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science (New York, NY). 2015;350:1079-84
62. Miller PL, Carson TL. Mechanisms and microbial influences on CTLA-4 and PD-1-based immunotherapy in the treatment of cancer: a narrative review. Gut pathogens. 2020;12:43
63. Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nature reviews Cancer. 2017;17:271-85
64. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science (New York, NY). 2015;350:1084-9
65. Wang F, Yin Q, Chen L, Davis MM. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proceedings of the National Academy of Sciences of the United States of America. 2018;115:157-61
66. Wang T, Zheng N, Luo Q, Jiang L, He B, Yuan X. et al. Probiotics Lactobacillus reuteri Abrogates Immune Checkpoint Blockade-Associated Colitis by Inhibiting Group 3 Innate Lymphoid Cells. Front Immunol. 2019;10:1235
67. Mu Q, Tavella VJ, Luo XM. Role of Lactobacillus reuteri in Human Health and Diseases. Frontiers in microbiology. 2018;9:757
68. Soularue E, Lepage P, Colombel JF, Coutzac C, Faleck D, Marthey L. et al. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut. 2018;67:2056-67
69. Tyan K, Baginska J, Brainard M, Giobbie-Hurder A, Severgnini M, Manos M. et al. Cytokine changes during immune-related adverse events and corticosteroid treatment in melanoma patients receiving immune checkpoint inhibitors. Cancer immunology, immunotherapy: CII. 2021;70:2209-21
70. Meshkibaf S, Martins AJ, Henry GT, Kim SO. Protective role of G-CSF in dextran sulfate sodium-induced acute colitis through generating gut-homing macrophages. Cytokine. 2016;78:69-78
71. Shahabi V, Berman D, Chasalow SD, Wang L, Tsuchihashi Z, Hu B. et al. Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events. J Transl Med. 2013;11:75
72. Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C. et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology. 2022;33:1217-38
73. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer treatment reviews. 2016;44:51-60
74. Suzman DL, Pelosof L, Rosenberg A, Avigan MI. Hepatotoxicity of immune checkpoint inhibitors: An evolving picture of risk associated with a vital class of immunotherapy agents. Liver international: official journal of the International Association for the Study of the Liver. 2018;38:976-87
75. Wang W, Lie P, Guo M, He J. Risk of hepatotoxicity in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis of published data. Int J Cancer. 2017;141:1018-28
76. EASL Clinical Practice Guidelines. Drug-induced liver injury. Journal of hepatology. 2019;70:1222-61
77. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD. et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine. 2015;373:23-34
78. Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D. et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. The New England journal of medicine. 2015;372:2006-17
79. Kitagataya T, Suda G, Nagashima K, Katsurada T, Yamamoto K, Kimura M. et al. Prevalence, clinical course, and predictive factors of immune checkpoint inhibitor monotherapy-associated hepatitis in Japan. J Gastroenterol Hepatol. 2020;35:1782-8
80. Lin LL, Lin GF, Yang F, Chen XQ. A systematic review and meta-analysis of immune-mediated liver dysfunction in non-small cell lung cancer. International immunopharmacology. 2020;83:106537
81. Riveiro-Barciela M, Barreira-Díaz A, Callejo-Pérez A, Muñoz-Couselo E, Díaz-Mejía N, Díaz-González Á. et al. Retreatment With Immune Checkpoint Inhibitors After a Severe Immune-Related Hepatitis: Results From a Prospective Multicenter Study. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2023;21:732-40
82. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J. et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. The Lancet Oncology. 2021;22:198-211
83. Herbst RS, Arkenau HT, Santana-Davila R, Calvo E, Paz-Ares L, Cassier PA. et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. The Lancet Oncology. 2019;20:1109-23
84. Gudd CLC, Au L, Triantafyllou E, Shum B, Liu T, Nathwani R. et al. Activation and transcriptional profile of monocytes and CD8(+) T cells are altered in checkpoint inhibitor-related hepatitis. Journal of hepatology. 2021;75:177-89
85. Soon CF, Zhang S, Suneetha PV, Antunes DA, Manns MP, Raha S. et al. Hepatitis E Virus (HEV)-Specific T Cell Receptor Cross-Recognition: Implications for Immunotherapy. Front Immunol. 2019;10:2076
86. Llewellyn HP, Arat S, Gao J, Wen J, Xia S, Kalabat D. et al. T cells and monocyte-derived myeloid cells mediate immunotherapy-related hepatitis in a mouse model. Journal of hepatology. 2021;75:1083-95
87. Zhang J, Zhang Y, Wang Q, Li C, Deng H, Si C. et al. Interleukin-35 in immune-related diseases: protection or destruction. Immunology. 2019;157:13-20
88. Li X, Liu X, Tian L, Chen Y. Cytokine-Mediated Immunopathogenesis of Hepatitis B Virus Infections. Clinical reviews in allergy & immunology. 2016;50:41-54
89. Hercun J, Vincent C, Bilodeau M, Lapierre P. Immune-Mediated Hepatitis During Immune Checkpoint Inhibitor cancer Immunotherapy: Lessons From Autoimmune Hepatitis and Liver Immunology. Front Immunol. 2022;13:907591
90. Nakako S, Nakashima Y, Okamura H, Tani Y, Ueda T, Makuuchi Y. et al. Delayed immune-related neutropenia with hepatitis by pembrolizumab. Immunotherapy. 2022;14:101-5
91. Johncilla M, Misdraji J, Pratt DS, Agoston AT, Lauwers GY, Srivastava A. et al. Ipilimumab-associated Hepatitis: Clinicopathologic Characterization in a Series of 11 Cases. The American journal of surgical pathology. 2015;39:1075-84
92. An M, Wang W, Zhang J, Till BG, Zhao L, Huang H. et al. Association of hepatitis B virus DNA levels with overall survival for advanced hepatitis B virus-related hepatocellular carcinoma under immune checkpoint inhibitor therapy. Cancer immunology, immunotherapy: CII. 2023;72:385-95
93. Alkrekshi A, Tamaskar I. Safety of Immune Checkpoint Inhibitors in Patients with Cancer and Hepatitis C Virus Infection. The oncologist. 2021;26:e827-e30
94. Cancado ELR, Goldbaum-Crescente J, Terrabuio DRB. HLA-related genetic susceptibility in autoimmune hepatitis according to autoantibody profile. Front Immunol. 2022;13:1032591
95. Wölffer M, Battke F, Schulze M, Feldhahn M, Flatz L, Martus P. et al. Biomarkers Associated with Immune-Related Adverse Events under Checkpoint Inhibitors in Metastatic Melanoma. Cancers (Basel). 2022 14
96. Purde MT, Niederer R, Wagner NB, Diem S, Berner F, Hasan Ali O. et al. Presence of autoantibodies in serum does not impact the occurrence of immune checkpoint inhibitor-induced hepatitis in a prospective cohort of cancer patients. Journal of cancer research and clinical oncology. 2022;148:647-56
97. Hagiwara S, Watanabe T, Kudo M, Minaga K, Komeda Y, Kamata K. et al. Clinicopathological analysis of hepatic immune-related adverse events in comparison with autoimmune hepatitis and graft-versus host disease. Sci Rep. 2021;11:9242
98. Coukos A, Vionnet J, Obeid M, Bouchaab H, Peters S, Latifyan S. et al. Systematic comparison with autoimmune liver disease identifies specific histological features of immune checkpoint inhibitor-related adverse events. Journal for immunotherapy of cancer. 2022 10
99. Brown ZJ, Heinrich B, Greten TF. Mouse models of hepatocellular carcinoma: an overview and highlights for immunotherapy research. Nature reviews Gastroenterology & hepatology. 2018;15:536-54
100. Mishra A, Upadhyay PK, Nagarajan P. Immunotherapy in Liver Diseases: A Balance Between Immunity and Tolerance. Current drug metabolism. 2016
101. Kondo Y, Akahira J, Morosawa T, Toi Y, Endo A, Satio H. et al. Anti-nuclear antibody and a granuloma could be biomarkers for iCIs-related hepatitis by anti-PD-1 treatment. Sci Rep. 2022;12:3669
102. Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE. et al. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA oncology. 2018;4:173-82
103. Ferrari SM, Fallahi P, Elia G, Ragusa F, Ruffilli I, Patrizio A. et al. Autoimmune Endocrine Dysfunctions Associated with Cancer Immunotherapies. Int J Mol Sci. 2019 20
104. Tahir SA, Gao J, Miura Y, Blando J, Tidwell RSS, Zhao H. et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proceedings of the National Academy of Sciences of the United States of America. 2019;116:22246-51
105. Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nature reviews Endocrinology. 2017;13:195-207
106. O'Malley G, Lee HJ, Parekh S, Galsky MD, Smith CB, Friedlander P. et al. RAPID EVOLUTION OF THYROID DYSFUNCTION IN PATIENTS TREATED WITH NIVOLUMAB. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2017;23:1223-31
107. Orlov S, Salari F, Kashat L, Walfish PG. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. The Journal of clinical endocrinology and metabolism. 2015;100:1738-41
108. Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D. et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Annals of oncology: official journal of the European Society for Medical Oncology. 2017;28:583-9
109. Morganstein DL, Lai Z, Spain L, Diem S, Levine D, Mace C. et al. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clinical endocrinology. 2017;86:614-20
110. Yamauchi I, Sakane Y, Fukuda Y, Fujii T, Taura D, Hirata M. et al. Clinical Features of Nivolumab-Induced Thyroiditis: A Case Series Study. Thyroid: official journal of the American Thyroid Association. 2017;27:894-901
111. Guaraldi F, La Selva R, Samà MT, D'Angelo V, Gori D, Fava P. et al. Characterization and implications of thyroid dysfunction induced by immune checkpoint inhibitors in real-life clinical practice: a long-term prospective study from a referral institution. Journal of endocrinological investigation. 2018;41:549-56
112. Scott ES, Long GV, Guminski A, Clifton-Bligh RJ, Menzies AM, Tsang VH. The spectrum, incidence, kinetics and management of endocrinopathies with immune checkpoint inhibitors for metastatic melanoma. European journal of endocrinology. 2018;178:173-80
113. Trainer H, Hulse P, Higham CE, Trainer P, Lorigan P. Hyponatraemia secondary to nivolumab-induced primary adrenal failure. Endocrinology, diabetes & metabolism case reports. 2016. 2016
114. Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL. et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. Journal of immunotherapy (Hagerstown, Md: 1997). 2007;30:825-30
115. Galliazzo S, Morando F, Sartorato P, Bortolin M, De Menis E. A Case of Cancer-Associated Hyponatraemia: Primary Adrenal Insufficiency Secondary to Nivolumab. Endocrine, metabolic & immune disorders drug targets. 2022;22:363-6
116. Grouthier V, Lebrun-Vignes B, Moey M, Johnson DB, Moslehi JJ, Salem JE. et al. Immune Checkpoint Inhibitor-Associated Primary Adrenal Insufficiency: WHO VigiBase Report Analysis. The oncologist. 2020;25:696-701
117. Patti R, Malhotra S, Sinha A, Singh P, Marcelin M, Saxena A. Atezolizumab-Induced New Onset Diabetes Mellitus With Ketoacidosis. American journal of therapeutics. 2018;25:e565-e8
118. Way J, Drakaki A, Drexler A, Freeby M. Anti-PD-L1 therapy and the onset of diabetes mellitus with positive pancreatic autoantibodies. BMJ case reports. 2017. 2017
119. Hickmott L, De La Peña H, Turner H, Ahmed F, Protheroe A, Grossman A. et al. Anti-PD-L1 atezolizumab-Induced Autoimmune Diabetes: a Case Report and Review of the Literature. Targeted oncology. 2017;12:235-41
120. Yamazaki N, Kiyohara Y, Uhara H, Fukushima S, Uchi H, Shibagaki N. et al. Phase II study of ipilimumab monotherapy in Japanese patients with advanced melanoma. Cancer chemotherapy and pharmacology. 2015;76:997-1004
121. Nakamura Y, Tanaka R, Maruyama H, Ishitsuka Y, Okiyama N, Watanabe R. et al. Correlation between blood cell count and outcome of melanoma patients treated with anti-PD-1 antibodies. Japanese journal of clinical oncology. 2019;49:431-7
122. Romano E, Kusio-Kobialka M, Foukas PG, Baumgaertner P, Meyer C, Ballabeni P. et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:6140-5
123. Laurent S, Queirolo P, Boero S, Salvi S, Piccioli P, Boccardo S. et al. The engagement of CTLA-4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-α production. J Transl Med. 2013;11:108
124. Ricciuti A, De Remigis A, Landek-Salgado MA, De Vincentiis L, Guaraldi F, Lupi I. et al. Detection of pituitary antibodies by immunofluorescence: approach and results in patients with pituitary diseases. The Journal of clinical endocrinology and metabolism. 2014;99:1758-66
125. Iwama S, Arima H. Anti-pituitary antibodies as a marker of autoimmunity in pituitary glands. Endocrine journal. 2020;67:1077-83
126. Bellastella G, Carbone C, Scappaticcio L, Cirillo P, Troiani T, Morgillo F. et al. Hypothalamic-Pituitary Autoimmunity in Patients Treated with Anti-PD-1 and Anti-PD-L1 Antibodies. Cancers (Basel). 2021 13
127. Wang S, Fang Y, Jiang N, Xing S, Li Q, Chen R. et al. Comprehensive Genomic Profiling of Rare Tumors in China: Routes to Immunotherapy. Front Immunol. 2021;12:631483
128. Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V. et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science (New York, NY). 2018;359:582-7
129. Kobayashi T, Iwama S, Sugiyama D, Yasuda Y, Okuji T, Ito M. et al. Anti-pituitary antibodies and susceptible human leukocyte antigen alleles as predictive biomarkers for pituitary dysfunction induced by immune checkpoint inhibitors. Journal for immunotherapy of cancer. 2021 9
130. Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA. et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes. 2018;67:1471-80
131. Lo Preiato V, Salvagni S, Ricci C, Ardizzoni A, Pagotto U, Pelusi C. Diabetes mellitus induced by immune checkpoint inhibitors: type 1 diabetes variant or new clinical entity? Review of the literature. Reviews in endocrine & metabolic disorders. 2021;22:337-49
132. Kotwal A, Gustafson MP, Bornschlegl S, Kottschade L, Delivanis DA, Dietz AB. et al. Immune Checkpoint Inhibitor-Induced Thyroiditis Is Associated with Increased Intrathyroidal T Lymphocyte Subpopulations. Thyroid: official journal of the American Thyroid Association. 2020;30:1440-50
133. Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S. et al. Pembrolizumab-Induced Thyroiditis: Comprehensive Clinical Review and Insights Into Underlying Involved Mechanisms. The Journal of clinical endocrinology and metabolism. 2017;102:2770-80
134. de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B. et al. Incidence of Thyroid-Related Adverse Events in Melanoma Patients Treated With Pembrolizumab. The Journal of clinical endocrinology and metabolism. 2016;101:4431-9
135. Maekura T, Naito M, Tahara M, Ikegami N, Kimura Y, Sonobe S. et al. Predictive Factors of Nivolumab-induced Hypothyroidism in Patients with Non-small Cell Lung Cancer. In vivo (Athens, Greece). 2017;31:1035-9
136. Khan Z, Hammer C, Carroll J, Di Nucci F, Acosta SL, Maiya V. et al. Genetic variation associated with thyroid autoimmunity shapes the systemic immune response to PD-1 checkpoint blockade. Nat Commun. 2021;12:3355
137. Zhang Y, Garofano F, Wu X, Schmid M, Krawitz P, Essler M. et al. Integrative analysis of key candidate genes and signaling pathways in autoimmune thyroid dysfunction related to anti-CTLA-4 therapy by bioinformatics. Investigational new drugs. 2020;38:1717-29
138. Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R. et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. Journal for immunotherapy of cancer. 2017;5:95
139. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA oncology. 2016;2:1607-16
140. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J. et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017;35:709-17
141. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F. et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA oncology. 2018;4:1721-8
142. Wu J, Hong D, Zhang X, Lu X, Miao J. PD-1 inhibitors increase the incidence and risk of pneumonitis in cancer patients in a dose-independent manner: a meta-analysis. Sci Rep. 2017;7:44173
143. Li Y, Jia X, Du Y, Mao Z, Zhang Y, Shen Y. et al. Eosinophil as a biomarker for diagnosis, prediction, and prognosis evaluation of severe checkpoint inhibitor pneumonitis. Front Oncol. 2022;12:827199
144. Chen X, Li Z, Wang X, Zhou J, Wei Q, Jiang R. Association of pre-existing lung interstitial changes with immune-related pneumonitis in patients with non-small lung cancer receiving immunotherapy. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2022;30:6515-24
145. Matsukane R, Watanabe H, Minami H, Hata K, Suetsugu K, Tsuji T. et al. Continuous monitoring of neutrophils to lymphocytes ratio for estimating the onset, severity, and subsequent prognosis of immune related adverse events. Sci Rep. 2021;11:1324
146. Abed A, Law N, Calapre L, Lo J, Bhat V, Bowyer S. et al. Human leucocyte antigen genotype association with the development of immune-related adverse events in patients with non-small cell lung cancer treated with single agent immunotherapy. European journal of cancer (Oxford, England: 1990). 2022;172:98-106
147. Wang H, Zhou F, Zhao C, Cheng L, Zhou C, Qiao M. et al. Interleukin-10 Is a Promising Marker for Immune-Related Adverse Events in Patients With Non-Small Cell Lung Cancer Receiving Immunotherapy. Front Immunol. 2022;13:840313
148. Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine. 2015;373:1270-1
149. Läubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. Journal for immunotherapy of cancer. 2015;3:11
150. Geisler BP, Raad RA, Esaian D, Sharon E, Schwartz DR. Apical ballooning and cardiomyopathy in a melanoma patient treated with ipilimumab: a case of takotsubo-like syndrome. Journal for immunotherapy of cancer. 2015;3:4
151. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y. et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. The New England journal of medicine. 2016;375:1749-55
152. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. 2019; 93: 280.
153. Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG. et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovascular research. 2019;115:854-68
154. Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A. et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. The Lancet Oncology. 2018;19:1579-89
155. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM. et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. Journal of the American College of Cardiology. 2018;71:1755-64
156. Zhang L, Reynolds KL, Lyon AR, Palaskas N, Neilan TG. The Evolving Immunotherapy Landscape and the Epidemiology, Diagnosis, and Management of Cardiotoxicity: JACC: CardioOncology Primer. JACC CardioOncology. 2021;3:35-47
157. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet (London, England). 2018;391:933
158. Gong J, Drobni ZD, Zafar A, Quinaglia T, Hartmann S, Gilman HK. et al. Pericardial disease in patients treated with immune checkpoint inhibitors. Journal for immunotherapy of cancer. 2021 9
159. Gong J, Drobni ZD, Alvi RM, Murphy SP, Sullivan RJ, Hartmann SE. et al. Immune checkpoint inhibitors for cancer and venous thromboembolic events. European journal of cancer (Oxford, England: 1990). 2021;158:99-110
160. D'Souza M, Nielsen D, Svane IM, Iversen K, Rasmussen PV, Madelaire C. et al. The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study. European heart journal. 2021;42:1621-31
161. Baik AH, Oluwole OO, Johnson DB, Shah N, Salem JE, Tsai KK. et al. Mechanisms of Cardiovascular Toxicities Associated With Immunotherapies. Circulation research. 2021;128:1780-801
162. Semeraro GC, Cipolla CM, Cardinale DM. Role of Cardiac Biomarkers in Cancer Patients. Cancers (Basel). 2021 13
163. Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. The Lancet Oncology. 2018;19:e447-e58
164. Chen DY, Huang WK, Chien-Chia Wu V, Chang WC, Chen JS, Chuang CK. et al. Cardiovascular toxicity of immune checkpoint inhibitors in cancer patients: A review when cardiology meets immuno-oncology. Journal of the Formosan Medical Association = Taiwan yi zhi. 2020;119:1461-75
165. Franco F, Thomas GD, Giroir B, Bryant D, Bullock MC, Chwialkowski MC. et al. Magnetic resonance imaging and invasive evaluation of development of heart failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1999;99:448-54
166. Comini L, Pasini E, Bachetti T, Dreano M, Garotta G, Ferrari R. Acute haemodynamic effects of IL-6 treatment in vivo: involvement of vagus nerve in NO-mediated negative inotropism. Cytokine. 2005;30:236-42
167. Ferdinandy P, Danial H, Ambrus I, Rothery RA, Schulz R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circulation research. 2000;87:241-7
168. Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science (New York, NY). 1992;257:387-9
169. Atallah-Yunes SA, Kadado AJ, Kaufman GP, Hernandez-Montfort J. Immune checkpoint inhibitor therapy and myocarditis: a systematic review of reported cases. Journal of cancer research and clinical oncology. 2019;145:1527-57
170. Delombaerde D, Vervloet D, Franssen C, Croes L, Gremonprez F, Prenen H. et al. Clinical implications of isolated troponinemia following immune checkpoint inhibitor therapy. ESMO open. 2021;6:100216
171. Waliany S, Neal JW, Reddy S, Wakelee H, Shah SA, Srinivas S. et al. Myocarditis Surveillance with High-Sensitivity Troponin I During Cancer Treatment with Immune Checkpoint Inhibitors. JACC CardioOncology. 2021;3:137-9
172. Zhang C, Chen Z, Mo C, Gao D, Zhu Y, Qin S. et al. Real-world cardiovascular toxicity of immune checkpoint inhibitors in cancer patients: a retrospective controlled cohort study. Am J Cancer Res. 2021;11:6074-85
173. Sarocchi M, Grossi F, Arboscello E, Bellodi A, Genova C, Dal Bello MG. et al. Serial Troponin for Early Detection of Nivolumab Cardiotoxicity in Advanced Non-Small Cell Lung Cancer Patients. The oncologist. 2018;23:936-42
174. Martinez-Calle N, Rodriguez-Otero P, Villar S, Mejías L, Melero I, Prosper F. et al. Anti-PD1 associated fulminant myocarditis after a single pembrolizumab dose: the role of occult pre-existing autoimmunity. Haematologica. 2018;103:e318-e21
175. Sławiński G, Wrona A, Dąbrowska-Kugacka A, Raczak G, Lewicka E. Immune Checkpoint Inhibitors and Cardiac Toxicity in Patients Treated for Non-Small Lung Cancer: A Review. Int J Mol Sci. 2020 21
176. Boughdad S, Latifyan S, Fenwick C, Bouchaab H, Suffiotti M, Moslehi JJ. et al. (68)Ga-DOTATOC PET/CT to detect immune checkpoint inhibitor-related myocarditis. Journal for immunotherapy of cancer. 2021 9
177. Zhu H, Galdos FX, Lee D, Waliany S, Huang YV, Ryan J. et al. Identification of Pathogenic Immune Cell Subsets Associated With Checkpoint Inhibitor-Induced Myocarditis. Circulation. 2022;146:316-35
178. Larkin J, Chmielowski B, Lao CD, Hodi FS, Sharfman W, Weber J. et al. Neurologic Serious Adverse Events Associated with Nivolumab Plus Ipilimumab or Nivolumab Alone in Advanced Melanoma, Including a Case Series of Encephalitis. The oncologist. 2017;22:709-18
179. Xu M, Nie Y, Yang Y, Lu YT, Su Q. Risk of Neurological Toxicities Following the Use of Different Immune Checkpoint Inhibitor Regimens in Solid Tumors: A Systematic Review and Meta-analysis. The neurologist. 2019;24:75-83
180. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X. et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA oncology. 2019;5:1008-19
181. Cuzzubbo S, Javeri F, Tissier M, Roumi A, Barlog C, Doridam J. et al. Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. European journal of cancer (Oxford, England: 1990). 2017;73:1-8
182. Sato K, Mano T, Iwata A, Toda T. Neurological and related adverse events in immune checkpoint inhibitors: a pharmacovigilance study from the Japanese Adverse Drug Event Report database. Journal of neuro-oncology. 2019;145:1-9
183. Holtzman NG, Xie H, Bentzen S, Kesari V, Bukhari A, El Chaer F. et al. Immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy for lymphoma: predictive biomarkers and clinical outcomes. Neuro-oncology. 2021;23:112-21
184. Albarrán V, Chamorro J, Rosero DI, Saavedra C, Soria A, Carrato A. et al. Neurologic Toxicity of Immune Checkpoint Inhibitors: A Review of Literature. Front Pharmacol. 2022;13:774170
185. Mazzarella L, Giugliano S, D'Amico P, Belli C, Duso BA, Rescigno M. et al. Evidence for interleukin 17 involvement in severe immune-related neuroendocrine toxicity. European journal of cancer (Oxford, England: 1990). 2020;141:218-24
186. Moreira A, Loquai C, Pföhler C, Kähler KC, Knauss S, Heppt MV. et al. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. European journal of cancer (Oxford, England: 1990). 2019;106:12-23
187. Seki M, Uruha A, Ohnuki Y, Kamada S, Noda T, Onda A. et al. Inflammatory myopathy associated with PD-1 inhibitors. Journal of autoimmunity. 2019;100:105-13
188. Mei H, Wen W, Fang K, Xiong Y, Liu W, Wang J. et al. Immune checkpoint inhibitor-induced myocarditis and myositis in liver cancer patients: A case report and literature review. Front Oncol. 2022;12:1088659
189. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T. et al. A clinical approach to diagnosis of autoimmune encephalitis. The Lancet Neurology. 2016;15:391-404
190. Müller-Jensen L, Zierold S, Versluis JM, Boehmerle W, Huehnchen P, Endres M. et al. Characteristics of immune checkpoint inhibitor-induced encephalitis and comparison with HSV-1 and anti-LGI1 encephalitis: A retrospective multicentre cohort study. European journal of cancer (Oxford, England: 1990). 2022;175:224-35
191. Cappelli LC, Gutierrez AK, Bingham CO 3rd, Shah AA. Rheumatic and Musculoskeletal Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors: A Systematic Review of the Literature. Arthritis care & research. 2017;69:1751-63
192. Suarez-Almazor ME, Kim ST, Abdel-Wahab N, Diab A. Review: Immune-Related Adverse Events With Use of Checkpoint Inhibitors for Immunotherapy of Cancer. Arthritis & rheumatology (Hoboken, NJ). 2017;69:687-99
193. Ghosh N, Bass AR. Rheumatic Complications of Immune Checkpoint Inhibitors. The Medical clinics of North America. 2021;105:227-45
194. Shen P, Deng X, Hu Z, Chen Z, Huang Y, Wang K. et al. Rheumatic Manifestations and Diseases From Immune Checkpoint Inhibitors in Cancer Immunotherapy. Frontiers in medicine. 2021;8:762247
195. Calabrese LH, Calabrese C, Cappelli LC. Rheumatic immune-related adverse events from cancer immunotherapy. Nature reviews Rheumatology. 2018;14:569-79
196. Melissaropoulos K, Klavdianou K, Filippopoulou A, Kalofonou F, Kalofonos H, Daoussis D. Rheumatic Manifestations in Patients Treated with Immune Checkpoint Inhibitors. Int J Mol Sci. 2020 21
197. Mooradian MJ, Nasrallah M, Gainor JF, Reynolds KL, Cohen JV, Lawrence DP. et al. Musculoskeletal rheumatic complications of immune checkpoint inhibitor therapy: A single center experience. Seminars in arthritis and rheumatism. 2019;48:1127-32
198. Kostine M, Finckh A, Bingham CO, Visser K, Leipe J, Schulze-Koops H. et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Annals of the rheumatic diseases. 2021;80:36-48
199. Narváez J, Juarez-López P, J LL, Narváez JA, Palmero R, García Del Muro X. et al. Rheumatic immune-related adverse events in patients on anti-PD-1 inhibitors: Fasciitis with myositis syndrome as a new complication of immunotherapy. Autoimmunity reviews. 2018;17:1040-5
200. Richter MD, Crowson C, Kottschade LA, Finnes HD, Markovic SN, Thanarajasingam U. Rheumatic Syndromes Associated With Immune Checkpoint Inhibitors: A Single-Center Cohort of Sixty-One Patients. Arthritis & rheumatology (Hoboken, NJ). 2019;71:468-75
201. Amoroso V, Gallo F, Alberti A, Paloschi D, Ferrari Bravo W, Esposito A. et al. Immune-related adverse events as potential surrogates of immune checkpoint inhibitors' efficacy: a systematic review and meta-analysis of randomized studies. ESMO open. 2023;8:100787
202. Williams SG, Mollaeian A, Katz JD, Gupta S. Immune checkpoint inhibitor-induced inflammatory arthritis: identification and management. Expert review of clinical immunology. 2020;16:771-85
203. Cappelli LC, Shah AA, Bingham CO 3rd. Immune-Related Adverse Effects of Cancer Immunotherapy- Implications for Rheumatology. Rheumatic diseases clinics of North America. 2017;43:65-78
204. Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR American journal of roentgenology. 2011;197:W992-w1000
205. Arnaud-Coffin P, Maillet D, Gan HK, Stelmes JJ, You B, Dalle S. et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer. 2019;145:639-48
206. Kostine M, Rouxel L, Barnetche T, Veillon R, Martin F, Dutriaux C. et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Annals of the rheumatic diseases. 2018;77:393-8
207. Roberts J, Ennis D, Hudson M, Ye C, Saltman A, Himmel M. et al. Rheumatic immune-related adverse events associated with cancer immunotherapy: A nationwide multi-center cohort. Autoimmunity reviews. 2020;19:102595
208. Adda L, Batteux B, Saidak Z, Poulet C, Arnault JP, Chauffert B. et al. Rheumatic and musculoskeletal disorders induced by immune checkpoint inhibitors: Consequences on overall survival. Joint bone spine. 2021;88:105168
209. McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523:612-6
210. Belkhir R, Burel SL, Dunogeant L, Marabelle A, Hollebecque A, Besse B. et al. Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Annals of the rheumatic diseases. 2017;76:1747-50
211. Manson G, Norwood J, Marabelle A, Kohrt H, Houot R. Biomarkers associated with checkpoint inhibitors. Annals of oncology: official journal of the European Society for Medical Oncology. 2016;27:1199-206
212. Esfahani K, Miller WH Jr. Reversal of Autoimmune Toxicity and Loss of Tumor Response by Interleukin-17 Blockade. The New England journal of medicine. 2017;376:1989-91
Author contact
![]() Corresponding authors: Fang Zhang, Cancer Center, Yantai Affiliated Hospital of Binzhou Medical University, The 2nd Medical College of Binzhou Medical University, Yantai 264100, China. E-mail: zhangfang_0127edu.cn. Yan-Dong Miao, Cancer Center, Yantai Affiliated Hospital of Binzhou Medical University, The 2nd Medical College of Binzhou Medical University, Yantai 264100, China. E-mail: miaoyd_22edu.cn.
Corresponding authors: Fang Zhang, Cancer Center, Yantai Affiliated Hospital of Binzhou Medical University, The 2nd Medical College of Binzhou Medical University, Yantai 264100, China. E-mail: zhangfang_0127edu.cn. Yan-Dong Miao, Cancer Center, Yantai Affiliated Hospital of Binzhou Medical University, The 2nd Medical College of Binzhou Medical University, Yantai 264100, China. E-mail: miaoyd_22edu.cn.
Received 2023-8-22
Accepted 2023-11-25
Published 2024-1-1
