10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2018; 14(10):1256-1267. doi:10.7150/ijbs.27100 This issue Cite
Research Paper
TIMMDC1 Knockdown Inhibits Growth and Metastasis of Gastric Cancer Cells through Metabolic Inhibition and AKT/GSK3β/β-Catenin Signaling Pathway
1. Key Laboratory of Laboratory Medicine, Ministry of Education, and Zhejiang Provincial Key Laboratory of Medical Genetics, School of Laboratory Medicine and Life sciences, Wenzhou Medical University, Wenzhou, China, 325035
2. Laboratory Medicine College, Hangzhou Medical College, Hangzhou, Zhejiang 310053, P. R. China
Abstract
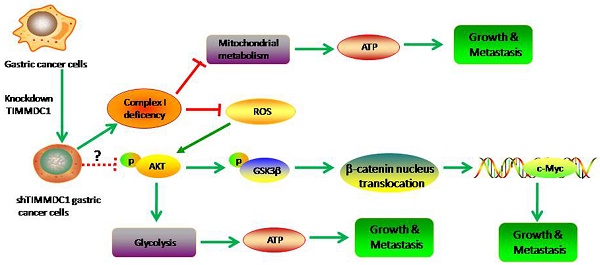
TIMMDC1 (C3orf1), a predicted 4-pass membrane protein, which locates in the mitochondrial inner membrane, has been demonstrated to have association with multiple member of mitochondrial complex I assembly factors and core mitochondrial complex I subunits. The expression level of TIMMDC1 in highly-metastatic tumor cells is higher than that in lowly- metastatic tumor cells. However, the role of TIMMDC1 in human gastric cancer progression is unclear. In this study, human gastric cancer cells SGC-7901 and BGC-823 cells were used, and TIMMDC1 was knockdown with small interfering RNA. The data showed that TIMMDC1 knockdown caused inhibitory effects on the cell proliferation in vitro and tumor progression in vivo. Knockdown of TIMMDC1 significantly and exclusively reduced the activity of mitochondrial complex I but not complex II~ IV, and caused an obvious inhibition in mitochondrial respiration and ATP-linked oxygen consumption. Besides, the glycolysis pathway was also attenuated by TIMMDC1 knockdown, and the ATP content in the group of shTIMMDC1 cells was significantly lower than that in the shCont cells. The expression levels of phosphoylated AKT(Ser473) and GSK-3β (Ser9), as well as the downstream protein β-catenin and c-Myc were also markedly reduced in the group of shTIMMDC1 cells. Taken together, these findings suggest that TIMMDC1 may play an important role in human gastric cancer development, and its underlying mechanism is not only associated with mitochondrial complex I inhibition and reduced mitochondrial respiration, but is also associated with reduced glycolysis activity and the AKT/GSK3β/β-catenin signaling pathways.
Keywords: Human gastric cancer cells, TIMMDC1, Complex, Mitochondrial respiration, Glycolysis, AKT

 Global reach, higher impact
Global reach, higher impact