Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2005; 1(4):126-134. doi:10.7150/ijbs.1.126 This issue Cite
Research Paper
Glycan analysis of the chicken synaptic plasma membrane glycoproteins - a major synaptic N-glycan carries the LewisX determinant
1. Brain and Behaviour Research Group, The Open University, Milton Keynes, MK7 6AA, UK
2. Department of Molecular Pharmacology, Glaxo SmithKline Research & Development Ltd., Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY, UK
3. Department of Biochemistry and Biophysics, Texas A&M University, College Station, TX-77843, USA
Received 2005-8-19; Accepted 2005-9-7; Published 2005-9-8
Abstract
The majority of synaptic plasma membrane components are glycosylated. It is now widely accepted that this post-translational modification is crucial during the establishment, maintenance and function of the nervous system. Despite its significance, structural information about the glycosylation of nervous system specific glycoproteins is very limited. In the present study the major glycan structures of the chicken synaptic plasma membrane (SPM) associated glycoprotein glycans were determined. N-glycans were released by hydrazinolysis, labelled with 2-aminobenzamide, treated with neuraminidase and subsequently fractionated by size exclusion chromatography. Individual fractions were characterized by the combination of high-pressure liquid chromatography, exoglycosidase treatment or reagent array analysis method (RAAM). In addition to oligomannose-type glycans, core-fucosylated complex glycans with biantennary bisecting glycans carrying the LewisX epitope were most abundant. The overall chicken glycan profile was strikingly similar to the rat brain glycan profile. The presence of the LewisX determinant in relatively large proportions suggests a tissue-specific function for these glycans.
Keywords: brain, chicken, synaptic plasma membrane, LewisX, bisecting GlcNAc
1. Introduction
Glycosylation is a common post-translational modification of proteins, lipids and a major constituent of proteoglycans. These modifications introduce an additional level of information that is interpreted by the surrounding molecules within and outside of the cell. The central nervous system is ideally suited for the study of glycosylation, since the establishment and extraordinary specificity of synapses is modulated by the glycans present on cell membrane glycoproteins, glycolipids and proteoglycans [1] , [2] , [3] .
A number of stage- and neuron-specific glycans have already been identified in the nervous system of different organisms. In the leech, mannose and galactose containing glyco-epitopes are expressed on specific subsets of neurons and function during different developmental stages [4] . They are required for the correct targeting of specific sensory afferents. Similarly, in the olfactory epithelium of the mouse, lactosamine containing carbohydrate markers have been identified through the use of a specific monoclonal antibody, 1B2. The glycans are selectively expressed on a subset of olfactory neurons and are required for the proper targeting of a subset of sensory axons [5] .
In addition to their developmental function, specific glycans have also been implicated in the functioning of the adult brain. Memory formation, for example, requires the modulation of synaptic connectivity and, as glycoproteins constitute a significant and functionally relevant proportion of synaptic membrane constituents, their potential role in memory consolidation has been studied in a variety of species. In the chick, as in mammals [6] , changes in fucose incorporation have been repeatedly described in many models of neural plasticity, including rearing in enriched environment [7] , transition to light after dark-rearing [8] and after successful acquisition of different learning tasks in a range of animals [9] , [10] . Induction of long-term potentiation (LTP) has also been shown to lead to increased incorporation of radiolabeled fucose [11] . 2-deoxygalactose, a galactose analogue that prevents the incorporation of fucose into the α1-2 position, is amnestic when administered at specific time points relative to training (30 min before or after, and 6-8 hours post-training, [12] , [13] ) indicating crucial time-points in the biochemical cascade of memory formation that are also likely to be related to certain aspects of fucose metabolism. The maintenance of LTP is also compromised in the presence of 2-deoxygalactose [14] . More recently, elegant in vitro studies by Hsieh-Wilson and colleagues [15] have further confirmed and extended the unique role of the fucose(α1-2)galactose epitope in neuronal plasticity.
The identity of some of the glycoproteins carrying the α1-2-fucosylated epitope has been determined in the rat hippocampus [16] . Although N-CAM 180, N-CAM 140, synaptic glycoproteins gp55/gp65, the NR1 subunit of the NMDA receptor, and N-cadherin all have shown radiolabeled fucose incorporation, only the latter three were found to carry the α1-2fucosylated epitope.
Given the importance of glycosylation during synaptic plasticity in the chick, we were interested in the glycosylation profile of synaptic plasma membrane glycoproteins from the region of the brain that shows the most significant changes in radiolabeled fucose incorporation, the IMHV (intermediate hyperstriatum ventrale). This study describes the isolation and characterization of the most abundant N-linked glycans from the chicken IMHVs. Comparison to published data on glycan structures from other species indicates the possibility of a brain-specific glycosylation that is conserved between avian and mammalian species.
2. Materials and Methods
Animals
Fertile eggs from Ross Chunky chickens were incubated on-site (Open University, Milton Keynes, UK) for 17 days in a commercial communal brooder maintained on an 8:16 h light/dark cycle at 37.5-37.9 °C. On day 18 of incubation the eggs were transferred to a hatching incubator held at 37-38 °C maintained on a 12:12 h light/dark cycle, where they hatched. Around 24 h after the first chick in each batch had hatched the chicks were transferred to a group cage and supplied ad libitum with chick starter crumbs and water.
Tissue preparation for glycan analysis
Synaptosomal membranes were prepared from 36 one day-old-chicks. IMHV regions were manually dissected on ice [17] and homogenised, with 7 strokes, in 9 volumes (w/v) of ice-cold 0.32M sucrose containing 10 mM HEPES pH 7.0 and protease inhibitors (Complete™ Protease Inhibitor Cocktail Tablets, Roche) in a glass homogeniser. The homogenate was centrifuged at 1000g for 15 min. The supernatant from this centrifugation step was further centrifuged at 11500 rpm in a Beckman JA-1 rotor. The resulting pellet, which contains the crude synaptosomal membrane fraction, was used for further analysis. Glycoproteins were prepared according to Finne and Krusius [18] omitting the protein digestion step. Left and right hemispheres were treated separately during charged glycan analysis. However as the IMHV represents only a very small part of the total brain volume, the left and right hemispheres were pooled for structural glycan analysis.
Glycan release and labelling
SPM glycoproteins (3 mg) were transferred to GlycoPrep 1000 reactor vials (Oxford GlycoSciences, Abingdon, Oxon, UK), lyophilised and subjected to automated hydrazinolysis in a GlycoPrep 1000 under conditions which result in the simultaneous release of N- and O-linked glycans [19] . The resulting glycan pools were filtered through 0.5 μm PTFE filters, dried and subsequently labelled with 2-aminobenzamide (Signal Labelling Kit, Oxford GlycoSciences Ltd) to facilitate their detection during fractionation.
HPLC
High pH anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) was performed with an HPLC system comprising a Dionex advanced gradient pump, a Dionex eluant-degas module, a Gilson 231 sample injector, a Gilson 401 Dilutor and a Dionex ED 40 electrochemical detector. A Dionex CarboPac PA-1 column coupled with an AminoPac guard column was used for the chromatography of monosaccharides.
Monosaccharide compositional analysis
Monosaccharides were released from glycoproteins by acid hydrolysis. Samples were hydrolysed in 6M trifluoroacetic acid (Sigma) solution at 100 °C for 4 h, dried, reconstituted in water and directly analysed by HPAEC-PAD on CarboPac PA-1 equipped with an AminoPac guard column (Dionex). Conditions were as follows: 18 mM isocratic NaOH for 20 min followed by 200mM NaOH for 10 min and then the column was re-equilibrated with 18 mM NaOH for 20 min. The flow rate was maintained at 1ml/min. Individual components were identified and quantified using a mixture of monosaccharides containing 1 nanomol each of fucose, galactosamine, glucosamine, galactose, glucose and mannose (Dionex).
For sialic acid analysis glycoproteins were hydrolysed in 0.1M HCl at 80 °C for 1 h, dried down from MilliQ water 3 times, and finally reconstituted in water prior to injection onto CarboPac PA1 column. The following gradient was applied at a flow rate of 1ml/min: 100 mM NaOH/50 mM NaOAc for 5 min, 100 mM NaOH/50-180mM NaOAc gradient for 20 min, 100mM NaOH/180 mM NaOAc for 5 min, and then the column was re-equilibrated with 100mM NaOH/50mM NaOAc for 20 min.
2-AB labelled glycan analysis
Charged glycans were separated by weak anion exchange chromatography on GlycoSepC using 20% acetonitrile (buffer A, Romil Ltd.) and 20% acetonitrile in 200 mM ammonium formate pH 4.5 (buffer B) as eluants. After 100% A for 5 min a gradient of 0-100% B was applied over 35 min followed by 100% B for the next 5 min. The column was subsequently re-equilibrated to initial conditions for 15 min. The total run time was 60 min at a flow rate of 0.3 ml/min.
Normal phase HPLC was performed using a GlycoSepN column with 50 mM ammonium formate, pH 4.4 (solvent A) and 100 % acetonitrile (solvent B) eluants. The following gradient was applied: initial conditions 35% A at 0.4 ml/min, then a linear gradient of 35-53% A over 72 min, followed by 53-100% A over the next 3 min. The column was washed with 100% A at 1.0 ml/min for 5 min before re-equilibration in the initial solvent system. The collected fractions were desalted by repeated evaporation from water, since ammonium formate is volatile. Each run was referenced to an external 2AB-labelled dextran hydrolysate standard run on the same column. This enabled oligosaccharide retention to be expressed in terms of glucose units (NPGU).
P4 gel permeation chromatography of neutral glycans was carried out using a RAAM 2000 GlycoSequencer (Oxford GlycoSciences Ltd). Each run was calibrated using a dextran hydrolysate internal standard. Oligosaccharide elution was expressed in glucose units (P4GU), which is a measure of hydrodynamic volume.
Alkaline phosphatase treatment
The desialylated glycan pool was digested with 2U of alkaline phosphatase (E. coli, type III, Sigma) in 100 mM Tris-HCl buffer, pH 8 for 2 h at 37°C. Samples were desalted on GlycoClean H cartridges (Oxford GlycoSciences) and subjected to desulphation.
Desulphation
For desulphation, samples were dried under nitrogen in 1 ml ReactiVials (Pierce), taken up in 500 μl of dry 50mM methanolic-HCl and left at room temperature for 18 h. The reaction mixture was evaporated to dryness under vacuum. The residue was re-N-acetylated by the addition of 500 μl saturated sodium bicarbonate solution, and two aliquots of 20μl of acetic anhydride (Fluka) separated by a 10 min interval. The reaction was allowed to proceed at room temperature for 50 min, and then the oligosaccharides were desalted on 500μl of Dowex AG50 x 12 (H+ form) resin, eluted in water and concentrated by rotary evaporation.
Exoglycosidase digestions
All enzymes used were from Oxford GlycoSciences Ltd. Digestion conditions were as follows: 50 U/ml jack bean α-mannosidase in 100 mM sodium acetate buffer, pH 5.0 containing 2 mM Zn2+ ions; 1-2 U/ml Arthrobacter ureafaciens sialidase in 100 mM sodium acetate pH 5.0; 0.2 U/ml Newcastle Disease Virus sialidase in 50 mM sodium acetate pH 5.5; 125 mU/ml Bacteriodes fragilis endo-β-galactosidase in 50 mM sodium acetate pH 5.8 containing 250 μg/ml BSA and 1mM NaCl; 8-10 mU/ml (for β1,2 specificity) or 100 mU/ml (β1-2,3,4,6 specific) Streptococcus pneumoniae β-N-acetylhexosaminidase in 100 mM sodium citrate/phosphate buffer, pH 6.0; 0.2 U/ml almond meal fucosidase in 50 mM sodium acetate pH 5.0 and 0.2 U/ml bovine kidney fucosidase in 100 mM sodium citrate pH 6.0. All digests were incubated at 37 °C for 18h.
SDS-PAGE and Western blotting
SDS-PAGE was performed according to Laemmli [20] using 6-12% gradient gels (Nowex). After electrophoresis the proteins were transferred to PVDF Immobilon P membranes (0.45 μm, Millipore) in a transfer buffer containing 12.5 mM Tris, 96 mM glycine and 15% (v/v) methanol. Non-specific binding sites were blocked with 5% (w/v) bovine serum albumin in Tris-buffered saline (TBS) and the blots washed 3 times 20 minutes in TBS/0.05% (w/v) Tween. The primary monoclonal antibodies against CD15 (mouse IgG, Immunotech) and sialyl-LewisX (mouse IgM, Immunotech) were applied at 5 μg/ml concentration and incubated overnight at 4 °C with gentle shaking in TBS/0.5% (w/v) BSA. The blots were washed again 3 times 20 minutes in TBS/0.05% Tween/0.5% BSA. Secondary antibodies conjugated with alkaline phosphatase against IgM or IgG (Sigma) were diluted 1: 30 000 and the blots incubated for 3 h at room temperature. After removal of the unbound antibodies by rinsing 3 times 20 minutes in washing solution, the blots were visualised by the addition of insoluble alkaline phosphatase substrate BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate nitro blue, Sigma).
3. Results
Glycan analysis
Monosaccharide analysis was performed on the glycoprotein pool prepared from left and right intermediate hyperstriatum ventrale (IMHV) synaptic plasma membranes (SPMs). Table 1. summarizes the identity and ratio of individual monosaccharides released by acid hydrolysis from the SPM glycoprotein pool. Although the amount of glucose is likely to be overestimated (since the homogenisation buffer contained high amounts of sucrose), the relative ratios of individual monosaccharides were very similar to previously published results from adult rat brain [21] .
Monosaccharide composition of the glycoprotein fraction prepared from left and right chicken IMHV synaptic plasma membranes
| Monosaccharide | Left IMHVs | Right IMHV | ||
|---|---|---|---|---|
| nmol/mg dry material | molar ratio (Man=3) | nmol/mg dry material | molar ratio (Man=3) | |
| Fucose | 9.5 | 1.5 | 9.4 | 1.5 |
| Galactosamine | 3.0 | 0.5 | 3.1 | 0.5 |
| Glucosamine | 27.5 | 4.4 | 28.9 | 4.5 |
| Galactose | 14.0 | 2.2 | 15.5 | 2.4 |
| Glucose | 9.9 | 1.6 | 10.2 | 1.6 |
| Mannose | 18.9 | 3 | 19.3 | 3 |
| Neu5Ac | 5.4 | 0.9 | 3.9 | 0.6 |
Charged glycan analysis
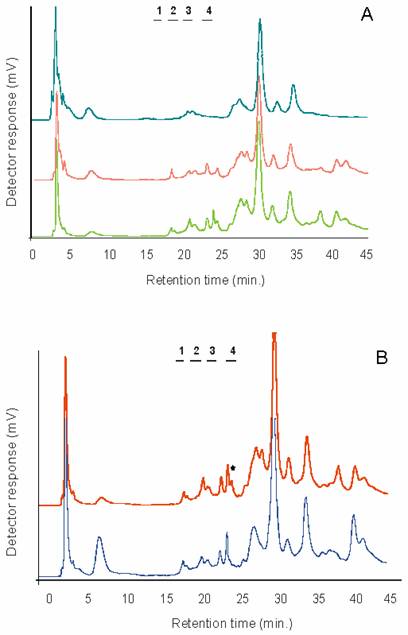
The presence and distribution of α2-3/8 and α2-6 linked sialic acids was determined using two sialidases. Digestion with the Newcastle Disease Virus sialidase (recognising α2-3/8 linked sialic acids) was followed by Arthrobacter ureafaciens sialidase (for removal of α2-6 linked sialic acids, after removal of α2-3/8 linked residues). Figure 1a shows the weak anion-exchange profile of 2-AB labelled glycans on GlycoSepC prior to and after treatment with the sialidases. It is not clear whether the sialidase sensitive peaks in the highly negatively charged region represent polysialylated species, as they most preferentially disappeared after A. ureafaciens sialidase treatment, suggesting α2-6, rather than α2-8 linkages.
A relatively large proportion of negatively charged glycan species was still present after the removal of sialic acid residues. These could be due to phosphate or sulphate residues, as they have been previously reported for the mouse brain tissue in similar proportions [22] . Most of the residual peaks were sensitive to alkaline phosphatase (AP) digestion and peaks remaining after AP digestion were further eliminated by mild methanolysis (data not shown) indicating the presence of sulphated glycans.
Direct comparison of the GlycoSepC chromatograms from the two hemispheres indicated some differences between the left and right IMVH regions (Figure 1b). An α2-6 sialylated peak present in the left IMVH profile was absent from the right IMVH profile, and this observation was constant through different batches. Other differences between the hemispheres were also apparent especially in the more negatively charged region, however the identity of those peaks was not further investigated in this study. Differences between the hemispheres may not be restricted to glycoprotein glycans in the chick, as similar observations were noted for glycosphingolipids [23] .
A) Sialidase treatment of the 2-AB labelled N- and O-linked glycans from chick IMHV (left hemisphere). Bottom trace: untreated sample, middle trace: after Newcastle Disease Virus sialidase treatment (specific for alpha2-3/8 linked terminal sialic acids), and top chromatogram: after Arthrobacter ureafaciens sialidase digestions (removes terminal alpha2-3/6/8 linked sialic acids). Numbers indicate the elution position of mono-, di- tri- and tetrasialylated standards derived from bovine fetuin. B) Comparison of the 2-AB labelled N-and O-linked glycan profiles separated on GlycoSepC (weak anion-exchange chromatography) from the left and right IMHVs. Bottom trace: right IMHV, top trace: left IMHV. Note the peak marked with an asterisk in the sialidase sensitive area of the chromatogram is only present in the left IMHV. Peak eluting around 6 minutes is 2-aminobenzamide
Neutral glycan analysis
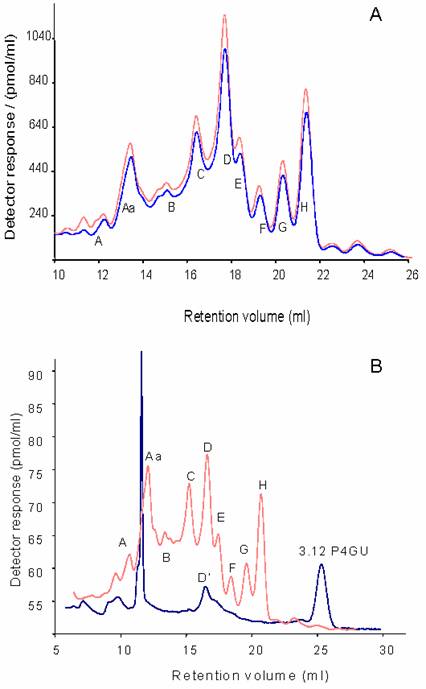
The P4 gel filtration profiles of the neutral glycans were very similar, indicating that the major glycan constituents are the same between the two hemispheres (Figure 2a). In this study neutral glycans represent the pool of naturally occurring neutral glycans and glycans arising after desialylation, dephosphorylation and desulphation. Since relatively large amounts of pure glycans are required for structural analysis, the left and right-hemisphere samples were pooled.
Dextran hydrolysate was used to calibrate the chromatograms in glucose units (P4GU, measure of hydrodynamic volume). Nine major oligosaccharide peaks were resolved (hydrodynamic volumes in parenthesis): A (>20 P4GU), Aa (19.3-16.5 P4GU), B (14.4 P4GU), C (12.1 P4GU), D (10.5 P4GU), E (9.5 P4GU), F (8.5 P4GU), G (7.7 P4GU) and H (6.9 P4GU) as shown on Figure 2a. These profiles are very similar to the glycan profiles obtained from delipidated adult rat [24] and murine [22] brain derived glycoprotein samples.
From their hydrodynamic volumes it was predicted that Peaks D, E, F, G and H were oligomannosidic glycans containing 9,8,7,6 and 5 mannose residues, respectively. These peaks were therefore pooled, digested with jack bean α-mannosidase, which is specific for α1-2/3/6-linked mannosidic bonds -and hence recognises oligomannose type structures-, and then rechromatographed on the P4 column (Figure 2b). After digestion, peaks D, E, F, G and H collapsed to a single peak corresponding to Manβ1-4GlcNAcβ1-4GlcNAc-2AB (3.1 P4GU) confirming their identity as oligomannosidic glycans with 9,8,7,6 and 5 mannose residues, respectively. Peak D however contained an additional component, D', which was resistant to α-mannosidase digestion (Figure 2b).
A) P4 gel-permeation chromatography profiles of the neutral 2-AB labelled N- and O-linked glycans from left (top trace) and right IMHVs. B) Peaks D, E, F, G and H were pooled and digested with jack bean mannosidase. The rechromatographed digest (bottom line) indicates that the majority of the glycans were of the oligomannose type, as they were converted to a smaller structure eluting around 25 ml (3.12 P4GU). Peak D also contained a mannosidase resistant component, denoted D'. Large peak around 12 ml is an artefact.
Normal phase HPLC
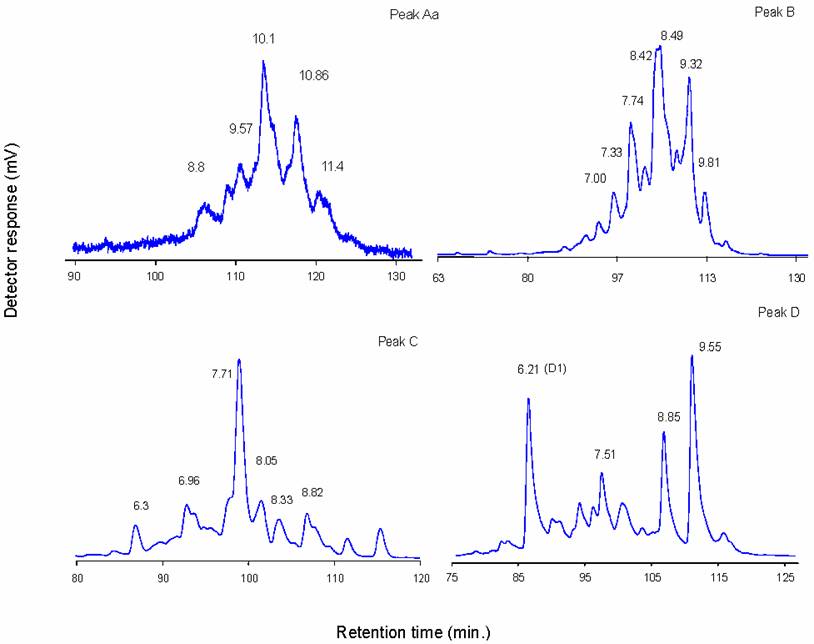
Judging from their P4GU values, peaks A, Aa, B, C and D' appear to be complex type glycans. Fractions corresponding to individual peaks were pooled for analysis by normal phase HPLC (Figure 3). In normal phase HPLC elution is dependent upon hydrophilic interactions between the polar functional groups of the column matrix and hydroxyl residues of oligosaccharides. As can be seen in Figure 3, the individual P4 peaks could be resolved into a number of subcomponents. Depending on the composition and complexity of the glycan, several hundred picomoles of pure glycans are required for enzymatic sequence analysis, and there was only sufficient material in peak C, 7.71 NPGU subcomponent and in peak D, 6.21 NPGU subcomponent to permit further structural analysis.
Individual P4 peak profiles on GlycoSepN. Numbers indicate normal-phase glucose unit values (NPGU)
P4 Peak D
It has been shown above (Figure 2b) that peak D contained an oligomannosidic glycan, and an oligosaccharide that was resistant to jack bean α-mannosidase digestion. Normal-phase HPLC of peak D resolved it into 4 major subcomponents (Figure 3d). Two of these peaks (8.85 and 9.55 NPGU) were sensitive to jack bean α-mannosidase digestion resulting in a NPGU shift corresponding to the loss of 7 and 8 mannose residues, respectively. Thus these peaks correspond to Man9GlcNAc2 and Man8GlcNAc2 structures and must have come from an overlap with Peak E during fractionation.
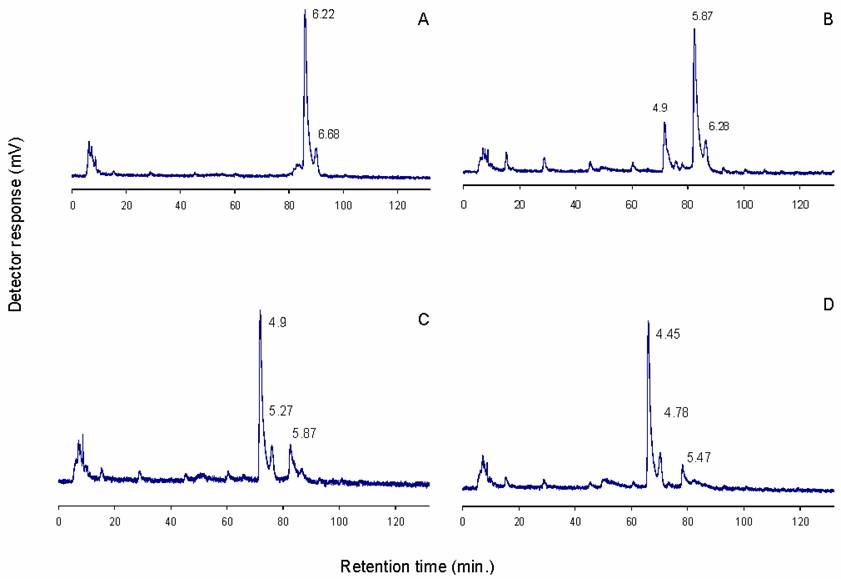
The identity of the 6.21 NPGU (D1) peak was further investigated by sequential exoglycosidase digestion followed by normal phase HPLC of each digest (Figure 4). Initial digestion was performed with 0.01 U/ml S. pneumoniae β-hexosaminidase. At this concentration the enzyme will cleave only terminal ß1-2 GlcNAc residues with two provisos. Firstly, the β(1-2)GlcNAc is not released if it is attached to a mannose which is also substituted at the C6 position, and secondly, if a bisecting GlcNAc is also present, only β(1-2)GlcNAc on the Manα(1-3) arm is cleaved. As shown in Figure 4b, a shift in elution of 0.3 NPGU corresponding to the loss of a single GlcNAc residue was observed. At higher enzyme concentration (0.1 U/ml) the fine specificity of the enzyme is lost and all terminal GlcNAc residues are removed (Figure 4c). Digestion with 0.1 U/ml of enzyme resulted in a 1.3 NPGU shift corresponding to a loss of 3 GlcNAc residues. These two digestions indicate that D1 is a biantennary complex glycan containing three terminal GlcNAc residues, one of which is bisecting.
Further digestion with bovine kidney fucosidase (specificity for α-2,3,4,6 linked fucose) shifted the elution position by 0.45 NPGU units (Figure 4d), which corresponds to a core fucose residue. The results of this sequence of exoglycosidase digestions suggest that D1 is a core fucosylated agalacto biantennary complex glycan (Figure 5).
Sequential sequencing of D1: a) untreated sample; b) 0.01 U/ml S.pneum. hexosaminidase; c) 0.1 U/ml S.pneum. hexosaminidase; d) 0.1 U/ml S. pneum. hexosaminidase and bovine kidney fucosidase. Numbers indicate NPGU values
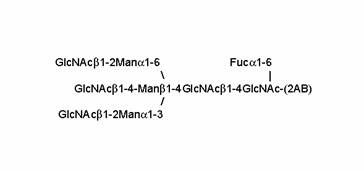
Proposed structure of peak D1
P4 Peak C
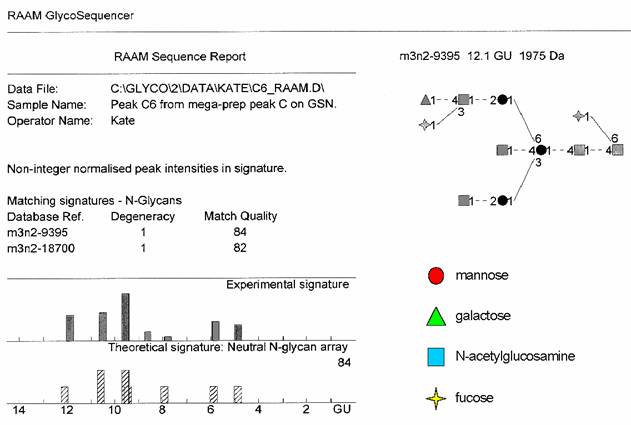
There was sufficient material isolated from the 7.7 NPGU subcomponent of P4 Peak C to enable its sequence to be determined by the RAAM procedure [25] . Briefly, this entails dividing the oligosaccharide into nine equal aliquots, which are digested with defined mixtures of exoglycosidases, each producing a stop-point fragment that cannot be digested further. The digests are pooled and the mixture of oligosaccharides is fractionated by P4 gel permeation chromatography. Proprietary software (Glycolink and Eve, Oxford GlycoSciences Ltd) reconstructs the sequence of the original glycan based on measured values of the hydrodynamic volumes of the stop-point fragments. The software assumes a certain hydrodynamic volume contribution for each monosaccharide component irrespective of the actual structure and conformation of the glycan, therefore the theoretical and observed signatures may be slightly different. RAAM determined the sequence of the 7.7 NPGU component of P4 Peak C to be a core fucosylated bisecting biantennary complex glycan containing the LewisX determinant on the α1-6 arm (Figure 6).
The result and RAAM report for peak C
Western blotting with anti-CD15 antibody
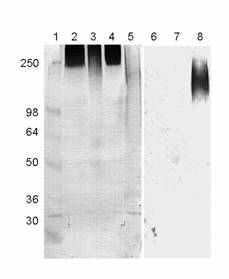
The presence of the LewisX determinant (also known as CD15) was further corroborated by Western blotting with a monoclonal antibody against CD15 using crude synaptosomal fractions prepared from IMHVs. Most of the reactive bands were of high molecular weight, which, judging from their streaked appearance, are likely to represent heavily glycosylated glycoproteins or proteoglycans (Figure 7).
The sialylated epitope (sialyl-LewisX) was apparently absent, as an antibody specific for this structure did not recognise any of the glycoproteins from IMHV P2 fractions or whole chicken brain homogenate (Figure 7).
Western blots with monoclonal antibodies recognizing the LewisX (2-5) and sialyl-LewisX (6-8) epitopes. 1. Mw standards (kDa); 2. IMHV P2 fraction; 3. same digested with Arthrobacter ureafaciens sialidase; 4. blank digest; 5. control HL60 cell lysate; 6. IMHV P2 fraction; 7. whole brain homogenate; 8. control HL60 cell lysate known to express sialyl-LewisX
4. Discussion
The aim of the present study was to characterise the major N-glycans of the SPM associated glycoproteins from a chick brain region where the most pronounced changes occur after passive avoidance learning task. The IMHV region of the brain has been identified by a number of groups as being important for early learning [26] , [27] .
Although the sample material available from these very small regions of the brain limited the structural characterisation of the complex-type N-linked glycans, the results obtained provided interesting new insights.
The charged glycan profile of the 2-AB labelled N- and O-linked glycans was dominated by highly charged structures, which were resistant to sialidase treatment. The majority of these peaks disappeared after alkaline phosphatase digestion, which removes terminal monophosphate residues, and mild methanolysis, which removes sulphate residues. Sialidase digestion of the total glycan pool indicated the presence of mono-, di-, tri-, tetra- and possibly polysialylated structures. Some of these were present in only one hemisphere. The ratio of α2-6 and α2-3 sialylated structures was approximately equal. This is in contrast to the whole brain tissue from the adult rat, where a greater proportion of sialylated structures were observed, and also, sialic acid α2-3 linked to galactose was more abundant than α2-6 linked sialic acid [28] , [29] .
Unlike the charged glycans of the chick synaptic membrane glycoproteins, the neutral glycan profile showed many similarities to those of rat, mouse and human brain tissues, and a striking resemblance to rat synaptic plasma membrane glycoprotein glycans [30] . Oligomannosidic structures with 5-9 mannose residues were the most abundant structures, further corroborating previous observations about the high abundance of these least processed glycans in nervous tissue and particularly in synaptic regions where Con A-binding glycoproteins are enriched in NMDA and AMPA sub-units of glutamate receptors [30] .
The most abundant complex-type N-linked neutral glycans described in the present study were found to be identical with the most abundant rat [24] mouse and human [31] brain specific structures, despite possible developmental differences due to the young age of the chicks in the current study and the substantial species differences. There is also some evidence that the bisecting biantennary core-fucosylated structure (Peak D1) is among the most abundant brain specific glycans in the mouse [32] . On at least two human glycoproteins synthesised in the brain and secreted to the cerebrospinal fluid, the β-trace protein [33] and asialo-transferrin [34] the main neutral glycan structure also corresponded to the structure of peak D1. Despite its apparently conserved nature in the central nervous system, and high expression levels in the hippocampus as detected by in situ hybridisation [35] , the biological significance of this or other glycans with bisecting GlcNAc is unknown. Mice lacking the bisecting β1-4 GlcNAc residues appear to be viable, fertile and without any phenotypic abnormality including the brain [36] , although the expression of truncated, inactive GlcNAC-TIII transferases induces novel neurological phenotypes [35] .
Another interesting feature of the neutral glycan profile was the relative abundance of the LewisX epitope, Galβ1-4[Fucα1-3]GlcNAc-; as in peak C. A wide range of recognition and adhesive phenomena have been attributed to LewisX and its sialylated homologue, sialyl-LewisX. LewisX is required for the normal compaction and subsequent embryogenesis of the mouse embryo [37] , [38] and is thought to play a role during early neuronal development [39] , [40] . Its characterization, in this study, in the synaptic plasma membrane glycoproteins of the chick would be consistent with a plausible modulatory role for this epitope in synaptic plasticity. Again this was one of the most abundant of the complex neutral N-glycans observed in all the mammalian brains studied so far (rat, mouse and human brain, [31] , [24] ), further substantiating cross-species conservation in neural tissue.
Fucose(α1-2)galactose was not observed as a major carbohydrate epitope in the present study. This also reflects results from studies with rat and mouse brain tissue [24] , [22] . Therefore it is likely that the increased incorporation of radioactive fucose seen both in vivo and in vitro models of neuronal plasticity reflects fucose in several different linkages, including fucose(α1-2)galactose.
In summary, we have identified the major N-linked glycans of the chick brain SPM glycoproteins specifically in a region known to be important for cellular and functional plasticity. Our data suggest the highly conserved nature of some neutral glycans (oligomannosidic structures, LewisX, core-fucose, bisecting GlcNAc) in the brain. It will be of interest to decipher the specific –and possibly conserved- roles these glycans play in the establishment and functioning of the nervous system.
Conflict of interest
The authors have declared that no conflict of interest exists.
Abbreviations
2-AB: 2-aminobenzamide; AP: alkaline phosphatase; BSA: bovine serum albumine; GlcNAc: N-acetylglucosamine; HEPES: 2-[4-(2-Hydroxyethyl)-1-piperazinyl]ethanesulfonic acid; HPAEC-PAD: high-pH-anion exchange chromatography with pulsed amperometric detection; HPLC: high-performance liquid chromatography; IMHV: intermediate hyperstriatum ventrale; LNnT: lacto-N-neotetraose; NPGU: normal phase glucose unit; LTP: long-term potentiation; P4GU: P4 glucose unit; PAGE: polyacrylamide gel electrophoresis; PTFE: polytetrafluoroethylene; RAAM: reagent array analysis method; SDS: sodium dodecyl sulphate; SPM: synaptic plasma membrane.
References
1. Jessell TM, Hynes MA, Dodd J. Carbohydrates and carbohydrate-binding proteins in the nervous system. Annu Rev Neurosci. 1990 ;13:227-55
2. Kleene R, Schachner M. Glycans and neural cell interactions. Nat Rev Neurosci. 2004 ;5:195-208
3. Lee JS, Chien CB. When sugars guide axons: insights from heparan sulphate proteoglycan mutants. Nat Rev Genet. 2004 ;5:923-35
4. Tai MH, Zipser B. Sequential steps of carbohydrate signaling mediate sensory afferent differentiation. J Neurocytol. 2002 ;31:743-54
5. Henion TR, Raitcheva D, Grosholz R, Biellmann F, Skarnes WC, Hennet T, Schwarting GA. Beta1,3-N-acetylglucosaminyltransferase 1 glycosylation is required for axon pathfinding by olfactory sensory neurons. J Neurosci. 2005 ;25:1894-903
6. Matthies H. In search of cellular mechanisms of memory. Prog Neurobiol. 1989 ;32:277-349
7. Damstra T, Entingh D, Wilson JE, Glassman E. Incorporation of 3H-L-fucose into brain glycoproteins during environmental stimulation following intracranial, intravenous, or subcutaneous injections. Behav Biol. 1975 ;13:121-6
8. Burgoyne RD, Rose SP. Increased incorporation of [3H]fucose into synaptic membranes of the visual cortex after first exposure to light of dark-reared rats. Biochem Soc Trans. 1978 ;6:1025-6
9. Popov N, Schulzeck S, Pohle W, Matthies H. Changes in the incorporation of [3H]fucose into rat hippocampus after acquisition of a brightness discrimination reaction. An electrophoretic study. Neuroscience. 1980 ;5:161-7
10. Bullock S, Rose SPR, Zamani R. Characterization and regional localization of presynaptic and postsynaptic glycoproteins of the chick forebrain showing changed fucose incorporation following passive-avoidance training. J Neurochem. 1992 ;58:2145-2154
11. Angenstein F, Matthies H Jr, Staeck S, Reymann KG, Staak S. The maintenance of hippocampal long-term potentiation is paralleled by a dopamine-dependent increase in glycoprotein fucosylation. Neurochem Int. 1992 ;21:403-8
12. Scholey AB, Rose SP, Zamani MR, Bock E, Schachner M. A role for the neural cell adhesion molecule in a late, consolidating phase of glycoprotein synthesis six hours following passive avoidance training of the young chick. Neuroscience. 1993 ;55:499-509
13. Rose SP, Jork R. Long-term memory formation in chicks is blocked by 2-deoxygalactose, a fucose analog. Behav and Neural Biol. 1987 ;48:246-58
14. Krug M, Jork R, Reymann K, Wagner M, Matthies H. The amnesic substance 2-deoxy-D-galactose suppresses the maintenance of hippocampal LTP. Brain Res. 1991 ;540:237-42
15. Kalovidouris SA, Gama CI, Lee LW, Hsieh-Wilson LC. A role for fucose alpha(1-2) galactose carbohydrates in neuronal growth. J Am Chem Soc. 2005 ;127:1340-1
16. Smalla KH, Angenstein F, Richter K, Gundelfinger ED, Staak S. Identification of fucose alpha(1-2) galactose epitope-containing glycoproteins from rat hippocampus. Neuroreport. 1998 ;9:813-7
17. Horn G. Techniques for removing IMHV from the chick brain. In: (ed.) Andrew RJ. Neural and Behavioural Plasticity: The use of the domestic chick as a model. Oxford: Oxford University Press. 1991:570-
18. Finne J, Krusius T. Preparation and fractionation of glycopeptides. In: (ed.) Ginsburg V. Methods Enzymol. : Elsevier. 1982:269-277
19. Patel T, Bruce J, Merry A, Bigge C, Wormald M, Jaques A, Parekh R. Use of hydrazine to release in intact and unreduced form both N- and O-linked oligosaccharides form glycoproteins. Biochemistry. 1993 ;32:659-693
20. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 ;227:680-5
21. Zanetta J, Reeber A, Vincemdon G, Gombos G. Synaptosomal plasma membrane glycoproteins. II. Isolation of fucosyl-glycoproteins by affinity chromatography on the Ulex europeus lectin specific for L-fucose. Brain Res. 1977 ;138:317-328
22. Wing DR, Rademacher TW, Field MC, Dwek RA, Schmitz B, Thor G, Schachner M. Use of large-scale hydrazinolysis in the preparation of N-linked oligosaccharide libraries: application to brain tissue. Glycoconjugate J. 1992 ;9:293-301
23. Rizzo AM, Rossi F, Berra B. Glycosyltransferases in different brain regions during chick embryo development. Neurochem Res. 2002 ;27:815-21
24. Chen YJ, Wing DR, Guile GR, Dwek RA, Harvey DJ, Zamze S. Neutral N-glycans in adult rat brain tissue--complete characterisation reveals fucosylated hybrid and complex structures. Eur J Biochem. 1998 ;251:691-703
25. Edge CJ, Rademacher TW, Wormald MR, Parekh RB, Butters TD, Wing DR, Dwek RA. Fast sequencing of oligosaccharides: The reagent-array analysis method. Proc Natl Acad Sci USA. 1992 ;89:6338-6342
26. Bradley PM, Burns BD, King TM, Webb AC. Morphological and electrophysiological properties of neurons in an area of the chick brain involved in learning. Brain Res. 1996 ;727:125-32
27. Rose SPR, Stewart MG. Cellular correlates of stages of memory formation in the chick following passive avoidance training. Behav Brain Res. 1999 ;98:237-243
28. Krusius T, Finne J. Structural features of tissue glycoproteins. Fractionation and methylation analysis of glycopeptides derived from rat brain, kidney and liver. Eur J Biochem. 1977 ;78:369-379
29. Zamze S, Harvey DJ, Chen YJ, Guile GR, Dwek RA, Wing DR. Sialylated N-glycans in adult rat brain tissue--a widespread distribution of disialylated antennae in complex and hybrid structures. Eur J Biochem. 1998 ;258:243-270
30. Clark RAC, Gurd JW, Bissoon N, Tricaud N, Molnar E, Zamze SE, Dwek RA, McIlhinney RAJ, Wing DR. Identification of lectin-purified neural glycoproteins, GPs 180, 116, and 110, with NMDA and AMPA receptor subunits: Conservation of glycosylation at the synapse. J Neurochem. 1998 ;70:2594-2605
31. Albach C, Klein RA, Schmitz B. Do rodent and human brains have different N-glycosylation patterns? Biol Chem. 2001 ;382:187-94
32. Shimizu H, Ochiai K IK, Mikoshiba K, Hase S. Structures of N-linked Sugar Chains Expressed Mainly in Mouse Brain. J Biochem. 1993 ;114:334-338
33. Hoffmann A, Nimtz M, Wurster U, Conradt HS. Carbohydrate Structures of Beta-Trace Protein From Human Cerebrospinal-Fluid - Evidence For Brain-Type N-Glycosylation. J Neurochem. 1994 ;63:2185-2196
34. Hoffmann A, Nimtz M, Getzlaff R, Conradt HS. Brain-Type N-Glycosylation of Asialo-Transferrin From Human Cerebrospinal-Fluid. Febs Letters. 1995 ;359:164-168
35. Bhattacharyya R, Bhaumik M, Raju TS, Stanley P. Truncated, inactive N-acetylglucosaminyltransferase III (GlcNAc-TIII) induces neurological and other traits absent in mice that lack GlcNAc-TIII. J Biol Chem. 2002 ;277:26300-9
36. Priatel JJ, Sarkar M, Schachter H, Marth JD. Isolation, characterization and inactivation of the mouse Mgat3 gene: the bisecting N-acetylglucosamine in asparagine-linked oligosaccharides appears dispensable for viability and reproduction. Glycobiology. 1997 ;7:45-56
37. Bird JM, Kimber SJ. Oligosaccharides containing fucose linked alpha(1-3) and alpha(1-4) to N-acetylglucosamine cause decompaction of mouse morulae. Dev Biol. 1984 ;104:449-60
38. Solter D, Knowles BB. Monoclonal antibody defining a stage-specific mouse-embryonic antigen (SSEA-1). Proc. Natl. Acad.Sci. USA. 1978 ;75:5565-5569
39. Allendoerfer KL, Magnani JL, Patterson PH. FORSE-1, an antibody that labels regionally restricted subpopulations of progenitor cells in the embryonic central nervous system, recognizes the Le(x) carbohydrate on a proteoglycan and two glycolipid antigens. Mol Cell Neurosci. 1995 ;6:381-95
40. Streit A, Yuen CT, Loveless RW, Lawson AM, Finne J, Schmitz B, Feizi T, Stern CD. The Le(x) carbohydrate sequence is recognized by antibody to L5, a functional antigen in early neural development. Journal Of Neurochemistry. 1996 ;66:834-44
Author contact
![]() Corresponding address:
Corresponding address:
Kate Koles, PhD, Texas A&M University, Department of Biochemistry and Biophysics, 2128 TAMU, College Station, TX-77843, USA. e-mail: kolesedu. Tel: (979) 458 4637 Fax: (979) 862 4718

 Global reach, higher impact
Global reach, higher impact