10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2009; 5(3):293-297. doi:10.7150/ijbs.5.293 This issue Cite
Short Research Communication
Characterization of persistent TTX-R Na+ currents in physiological concentration of sodium in rat visceral afferents
1. Department of Pharmacology, Harbin Medical University, Harbin, China
2. Department of Biomedical Engineering, Indiana University Purdue University Indianapolis, Indianapolis, USA
Received 2009-1-31; Accepted 2009-3-30; Published 2009-4-3
Abstract
Persistent tetrodotoxin-resistant (TTX-R) Na+ (Nav1.9/SCN11A) currents are not normally recorded in vagal afferent neurons (VANs) with 50 mM of extracellular Na+ although the functional expression of this current was observed in the presence of PGE2 or forskolin. However, it is uncertain whether this current can be seen under physiological condition (150 mM Na+). Using the whole-cell patch-clamp technique, we showed that persistent TTX-R Na+ currents were expressed in 9 out of 38 VANs bathed in 150 mM Na+. The current density, but not the whole-cell capacitance, was significantly enhanced in the VANs expressing Nav1.9. Persistent TTX-R Na+ channels were activated at a more hyperpolarized membrane potential near -60 mV, compared with TTX-sensitive (TTX-S at -40 mV) and TTX-R Na+ channels (at -20 mV). This indicates that persistent TTX-R Na+ channels provide a wider activation window than TTX-S and TTX-R Na channels to up-regulate neuronal excitability. These results suggest that the persistent TTX-R Na+ currents may be involved in the neuronal excitability by setting a lower pressure-discharge threshold and higher discharge frequency of VANs, especially the unique subset and gender-specific distribution of myelinated Ah-type VANs, including Ah-type aortic baroreceptor neurons, identified in our previous study.
Keywords: Sodium, Ion channel, Tetrodotoxin, Visceral afferent, Patch technique
1. INTRODUCTION
Extensive studies have demonstrated that persistent tetrodotoxin-resistant (TTX-R) sodium (Na+) channels are widely expressed in the peripheral nerve system and ascribed to the Nav1.9 sodium channel that encodes within SCN11A gene. Recent evidence suggests that the Nav1.9 subtype is implicated in inflammatory [1] and pain modulation [2] through up-regulation of the neuronal excitability in DRG neurons. Visceral afferent neurons (Vagal afferent neurons, VANs) located within nodose ganglia, such as aortic baroreceptor neurons (ABNs), not only sense the blood pressure change but also receive the sensory input related to the cardiac pain. Our previous results demonstrated that persistent TTX-R Na+ currents were not observed in VANs with extracellular solution contained 50 mM Na+ but it was up-regulated by cyclic AMP-dependent pathway [3], suggesting that persistent TTX-R Na+ currents might also impact on the regulation of neuronal excitability of visceral afferents under certain physiological or pathophysiological circumstances. Due to the technical reasons, such as loosing control of space clamp and having a difficulty to record a larger currents, 50 mM even lower Na+ concentration is usually applied in the extracellular recording solution [4,5], which is far lower than that in physiological extracellular fluid (~150 mM Na+). Based upon the voltage-dependent profiles of persistent TTX-R Na+ channel, if the neurons functionally express persistent TTX-R Na+ currents in extracellular recording solution contained 150 mM Na+, it is very likely that persistent TTX-R Na+ currents may play a crucial role in the modulation of neuronal excitability of unique subset of myelinated Ah-type VANs including baroreceptor neurons that is mainly seen in females with lower pressure-discharge threshold [6,7], which then elevates the parasympathetic drive and concomitant reduction in the activity of sympathetic pathway [8,9] through baroreflex mechanism. Furthermore, this lower tonic autonomic support of blood pressure could contribute to the lower resting blood pressure levels of premenopausal women [10,11]. Our current finding is a novel ion channel mechanism of neuronal control of circulation that can expands our understanding why the blood pressure in female is lower than that in males at similar age.
2. MATERIALS AND METHODS
Primary culture of adult NGNs
In order to achieve high resolution and high quality recordings of whole cell Na+ currents under the voltage-clamp configuration, either gender SD rats (200-300 g) were selected for this study and nodose neurons were enzymatically dispersed by incubating nodose ganglia with 10 Units/ml of Papain (Worthington Biochemical Co, Lakewood, NJ) at 37 °C for 20 min, and followed by 5 mg/ml of type II Collagenase (Worthington Biochemical Co, Lakewood, NJ) and 2.5 mg/ml of Dispase (Roche, Indianapolis, USA) at 37°C for another 30 min. The details of the procedures of cell-culture were described previously [12,13]. Experimental protocols used in this experiment have previously been approved by the Institutional Animal Care and Use Committee of School of Medical Science, Indiana University and Harbin Medical University.
Electrophysiological recordings
The whole-cell patch recordings were performed using Axon 200B or MultiClamp 700A amplifier (Axon Instruments). Borosilicate glass pipettes (Sutter) are pulled and polished down to 1-1.5 MΩ in normal saline. Following the formation of a giga-ohm seal the pipette capacitance was compensated. Upon going whole-cell the total cell capacitance (30-50 pF) and electrode access resistance (3-5 MΩ) were also compensated and generally to within 60-80%. The recording pipettes were filled with (mM) 7.0 NaF, 140.0 NMDG, 2.0 TEA-Cl, 2.0 MgCl2, 10.0 HEPES and the pH was adjusted to 7.25 using a measured quantity of 1N Pipes. Just prior to recording, Bapta-Na 4.0 mM, CaCl2 0.25 mM, Mg-ATP 2.0 mM (Sigma, St Louis, MO), and Na-GTP 2.0 mM (Sigma, St Louis, MO) were added to the pipette solution, and [Ca2+]i was finally buffered to 100 nM. The bath solution contains: (mM) 50 or 150.0 NaCl, 10.0 MgCl2, 10.0 HEPES, 25.0 glucose, the pH was adjusted to 7.30-7.35 using a measured quantity of 1N NaOH. Osmolarity of extracellular and intracellular solutions were adjusted to 310-315 and 290-295, respectively, using D-Manitol (Sigma, St Louis, MO). All patch experiments were recorded at room temperature (22-23 °C).
Data acquisition
All data are low pass filtered to 10 KHz and digitized at 50 KHz. Data collection and preliminary analysis are carried out using pCLAMP 10.2 and the Digidata 1440A (Axon Instruments) operating on a PC platform. In voltage clamp recordings, standard activation protocols are sufficient for electrophysiological and pharmacological classification of Na+ channel subtypes. Voltage clamp recordings generally involve standard activation protocols with calculation of cord conductance as a function of membrane voltage being a standard measure of the voltage-dependent properties of the whole cell Na+ currents.
Activation protocol
For both TTX-S and TTX-R Na+ currents, the holding potential is -80 mV; step depolarization is from -70 to +40 mV with 5 mV increments; the sweep last 60 ms with 1 sec between sweeps.
Data analysis
In the present experiments, all results were expressed as mean ± SD. t-Test was applied for statistic analysis. P value less than 0.05 was considered significant.
3. RESULTS AND DISCUSSION
Functional expression of TTX-S, TTX-R, and persistent TTX-R Na+ currents
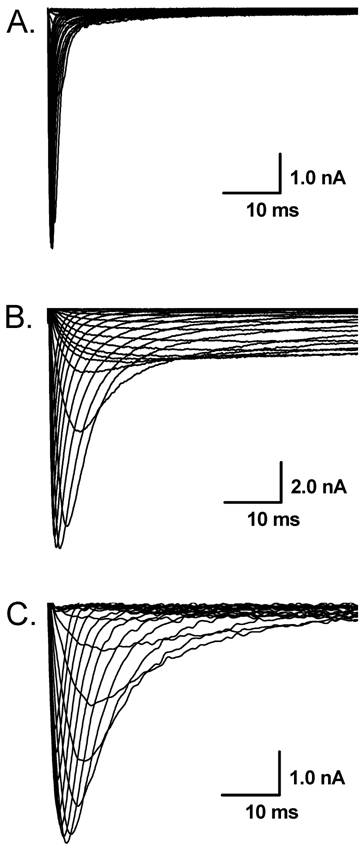
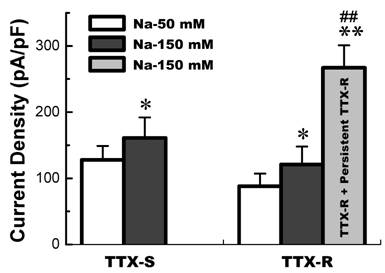
Whole-cell Na+ current were recorded in different extracellular concentration of Na+. In 50 mM of Na+ recording medium (data not shown), 41 VANs were observed. 7 of them functionally expressed TTX-S Na+ currents and the rests were functionally expressed both TTX-S and TTX-R Na+ currents. Those VANs only expressed TTX-S Na+ currents are traditionally classified into myelinated A-types, whereas those VANs expressed both TTX-S and TTX-R Na+ channels were generally classified into unmyelinated C-types [6]. However, recent study showed that a unique subset of myelinated Ah-type VANs with lower discharge threshold and higher repetitive discharge frequency were observed to not only express TTX-S but also TTX-R Na+ channels [6,7]. None of tested VANs showed persistent TTX-R Na+ currents. These data are consistent with our previous report [3]. Interestingly, as we increased extracellular Na+ concentration up to physiological concentration (150 mM Na+), 8 (~21%) out of 38 VANs only expressed TTX-S Na+ currents (Fig. 1A), 21 VANs expressed both TTX-S and TTX-R Na+ currents (Fig. 1B), and 9 (~23%) out of total 38 VANs functionally expressed persistent TTX-R Na+ currents except for TTX-S and TTX-R (Fig. 1C) without any treatment. The current density of either TTX-S Na+ currents in traditional A-types and TTX-R Na+ currents in traditional C-type VANs were increased by about 25% in the extracellular solution contained 150 mM of Na+ compared with that recorded from the recording medium with low extracellular Na+ (50 mM Na+), whereas the current density of VANs co-expressed persistent TTX-R Na+ currents were at least twice larger than that of C-types expressing TTX-S and TTX-R Na+ currents (Fig. 2). These results implied that the VANs co-expressed persistent TTX-R Na+ currents could have a lower discharge threshold, faster depolarization rate, and higher discharge frequency. Our recent report showed a perfect match that a subset of myelinated Ah-type VANs did possessed lower firing threshold and faster up-stroke velocity with significantly higher action potential firing frequency compared with C-type unmyelinated VANs [7].
The representative recordings of voltage-gated Na+ currents in the extracellular solution contained 150 mM Na+ in vagal afferent neurons (VANs) isolated from adult rats. A: Tetrodotoxin-sensitive (TTX-S) Na+ currents (Nav1.7) from a VAN in which TTX-S Na+ is the only Na+ channel expressed; B: Tetrodotoxin-resistant (TTX-R, Nav1.8) and persistent TTX-R Na+ currents (Nav1.9) in the presence of 1.0 µM TTX from the VAN that co-expresses Nav1.7, Nav1.8, and Nav1.9; C: TTX-R Na+ currents (Nav1.8) in the presence of 1.0 µM TTX from the VAN that co-expresses Nav1.7 and Nav1.8.
The current density of TTX-S and TTX-R Na+ channels functionally expressed in low extracellular Na+ (50 mM Na+) and physiological extracellular Na+ environment. TTX-S Na+ currents (n = 7 for 50 mM Na+ and n = 8 for 150 mM Na+) were recorded from the VAN expressed TTX-S Na+ channel only; TTX-R Na+ currents (n = 34 for 50 mM Na+ and n = 21 for 150 mM Na+) were recorded from the VAN co-expressed TTX-S and TTX-R Na+ channel; and persistent TTX-R Na+ currents (n = 9) were recorded from the VAN co-expressed TTX-S, TTX-R, and persistent TTX-R Na+. TTX-R Na+ currents were separated by 1.0 µM TTX applied. Data are expressed as mean ± SD, *P < 0.05 and **P < 0.01 vs TTX-S in 50 mM Na+, ## P < 0.01 vs TTX-R in 150 mM Na+.
Voltage-dependent properties of persistent TTX-R Na+ channels
In the present study, the voltage-dependent properties of Na+ channels were also analyzed and the average data showed that the voltage-dependent properties of TTX-S and TTX-R Na+ currents recorded in 150 mM extracellular Na+, such as current-voltage relationship (I-V curve, Fig. 3A) and voltage-dependent activation (Fig. 3B), were not changed significantly compared with those in 50 mM of extracellular Na+ (data not shown), which was consistent with our previous report [3]. As indicated in I-V curve created from those VANs co-expressed persistent TTX-R Na+ channels, the currents activated at more hyperpolarized potential near -60 mV (Fig. 3). The activation between -60 to -20 mV maintained no inactivation during 60 ms recording protocol and this portion of I-V curve absolutely differed from the same portion of either TTX-S or TTX-R Na+ currents. However, the activation from -20 mV to +40 mV was similar to that of TTX-R Na+ currents (Fig. 1B and Fig. 3). This early activation of persistent TTX-R Na+ may account for the lower firing threshold setting in subset of myelinated Ah-type VANs.
Comparison of normalized current-voltage (A.) relationship and voltage-dependent activation (B.). TTX-S, TTX-R, and persistent TTX-R Na+ currents were recorded in VANs expressed Nav1.7 only, Nav1.7 + Nav1.8, and Nav1.7 + Nav1.8 + Nav1.9, respectively. TTX-R Na+ currents were separated in the presence of 1.0 µM TTX.
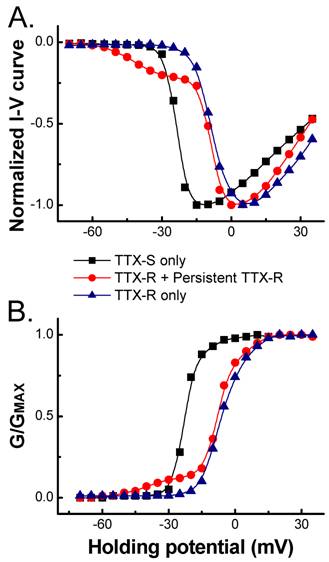
Because of the difference in activation among Na+ channels, the activation window could be significantly enlarged in those VANs co-expressed persistent Na+ channels compared with those not (Fig. 3). The larger activation window means that more Na+ channels are available at the resting stage [3], which results in the increase in neuronal excitability in VANs (Ah-type) co-expressed persistent TTX-R Na+ channel. This may be one of reasons why myelinated Ah-type VANs are always fire action potential repetitively with a significant lower firing threshold and faster depolarization [6,7].
Based upon the present experiment, we still could not figure out why the persistent Na+ channels are not functionally expressed in the extracellular recording solution contained 50 mM Na+ or less, however, our data, at least, suggest that it is very important for us to deal with isolated cell study physiologically.
4. CONCLUSION
These data suggest that the persistent TTX-R Na+ currents may play a key role in the enhancement of neuronal excitability by setting a lower pressure-discharge threshold and higher discharge frequency of VANs, especially the gender-specific unique subset of myelinated Ah-type VANs, including Ah-type aortic baroreceptor neurons.
AKNOWLEGEMENT
This project was supported by award (HL072012) from National Institute of Health to Dr. JHS and partially supported by research grants from State Health (2007-474) and State Education (11531111) Departments of Heilongjiang to Dr. GFQ.
CONFLICT OF INTERESTS
The authors have declared that no conflict of interest exists.
References
1. Maingret F, Coste B, Padilla F. et al. Inflammatory mediators increase Nav1.9 current and excitability in nociceptors through a coincident detection mechanism. J Gen Physiol. 2008;131:211-225
2. Ostman JA, Nassar MA, Wood JN. et al. GTP up-regulated persistent Na+ current and enhanced nociceptor excitability require Nav1.9. J Physiol. 2008;586:1077-1087
3. Li B, Schild JH. Persistent tetrodotoxin-resistant Na+ currents are activated by prostaglandin E2 via cyclic AMP-dependent pathway in C-type nodose neurons of adult rats. Biochem Biophys Res Commun. 2007;355:1064-1068
4. Qiao G, Li S, Yang B. et al. Inhibitory effects of artemisinin on voltage-gated ion channels in intact nodose ganglion neurons of adult rats. Basic Clin Pharmacol Toxicol. 2007;100:217-224
5. Qiao GF, Cheng ZF, Huo R. et al. GM1 ganglioside contributes to retain the neuronal conduction and neuronal excitability in visceral and baroreceptor afferents. J Neurochem. 2008;106:1637-1645
6. Li BY, Schild JH. Electrophysiological and pharmacological validation of vagal afferent fiber type of neurons enzymatically isolated from rat nodose ganglia. J Neurosci Methods. 2007;164:7-85
7. Li BY, Qiao GF, Feng B. et al. Electrophysiological and neuroanatomical evidence of sexual dimorphism in aortic baroreceptor and vagal afferents in rat. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1301-1310
8. Christou DD, Jones PP, Jordan J. et al. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111:494-498
9. Hogarth AJ, Burns J, Mackintosh AF. et al. Sympathetic nerve hyperactivity of essential hypertension is lower in postmenopausal women than man. J Hum Hypertens. 2008;22:544-549
10. Conte MR. Gender differences in the neurohumoral control of the cardiovascular system. Ital Heart J. 2003;4:367-370
11. Hinojosa-Laborde C, Chapa I, Lange D. et al. Gender differences in sympathetic nervous system regulation. Clin Exp Pharmacol Physiol. 1999;26:122-126
12. Li BY, Schild JH. Patch clamp electrophysiology in nodose ganglia of adult rats. J Neurosci Methods. 2002;115:157-167
13. Li BY, Schild JH. Comparisons of somatic action potential from dispersed and intact rat nodose ganglia using patch-clamp techniques. Acta Pharmacol Sin. 2002;23:481-489
Author contact
![]() Corresponding Author: Dr. B-Y Li, MD & PhD, Department of Pharmacology, Harbin Medical University, Harbin 150081, China; Phone: 86 451-8667-1354; E-mail: bailiedu
Corresponding Author: Dr. B-Y Li, MD & PhD, Department of Pharmacology, Harbin Medical University, Harbin 150081, China; Phone: 86 451-8667-1354; E-mail: bailiedu

 Global reach, higher impact
Global reach, higher impact