Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2009; 5(4):344-350. doi:10.7150/ijbs.5.344 This issue Cite
Research Paper
THE EXPRESSION OF APELIN AND ITS RECEPTOR APJ DURING DIFFERENT PHYSIOLOGICAL STAGES IN THE BOVINE OVARY
Physiology Weihenstephan, Technical University Munich, Freising, Germany
Received 2009-4-15; Accepted 2009-5-8; Published 2009-5-13
Abstract
Recent studies implicate that apelin and its receptor APJ may have important role for the modulation of angiogenesis. The aim of this study was to further characterise the regulation of apelin/APJ system in bovine ovary. Experiment 1: corpora lutea (CL) were assigned to the following stages: days 1-2, 3-4, 5-7, 8-12, 13-16, >18 (after regression) of oestrous cycle and of gravidity (month <3, 3-5, 6-7 and >8). Experiment 2: Follicles during maturation were divided into granulosa cells (GC) and theca interna (TI) and were examined separately. Classification of follicles occurred by follicle size and oestradiol-17β (E2) concentration in the follicular fluid (FF) (<0.5 ng/ml, 0.5-5 ng/ml; 5-40 ng/ml; 40-180 ng/ml; >180 ng/ml). Real-time RT-PCR (qPCR) was applied to investigate mRNA expression of examined factors. In general, the expression level of apelin during the oestrous cycle was significantly higher compared to the one during pregnancy. Apelin mRNA levels were always high during the cycle with a tendency of decrease after CL regression. The APJ mRNA in the CL was significantly up regulated on days 5-7 and 8-12 followed by a decrease on days 13-16, and further on days >18. The expression of APJ does not show any significant regulation in the CL throughout pregnancy. The expression of apelin and APJ was not statistically regulated in GC, but was significantly up regulated in follicles with an E2 concentration of more than 5 ng/ml and showed an increase according to growth and maturation of follicles. In conclusion, our data suggest that apelin/APJ system is involved in the mechanism regulating angiogenesis during follicle maturation as well as during CL formation and function in the bovine ovary.
Keywords: apelin, angiogenesis, bovine ovary, follicle, corpus luteum
INTRODUCTION
Apelin is a novel bioactive peptide and the endogenous ligand for APJ, an angiotensin-1-like receptor. It recently has been isolated from the bovine stomach [1-3]. Before the isolation of apelin, the APJ receptor was referred to as an orphaned G-protein-coupled receptor because its endogenous ligand was unidentified. Rat, mouse, cow, and human apelin cDNA have yet been characterized [2-4]. The apelin peptide is produced through processing from the C-terminal portion in the pre-proprotein consisting of 77 amino acid residues and exists in multiple molecular forms [5]. Subsequent studies have found that endogenous apelin exists in multiple molecular forms with different biological activities [6]. Apelin mRNA and immunoreactive apelin were detected in various tissues and organs such as stomach, brain, heart, lung, uterus and ovary [3-5,7]. Moreover, in rats, apelin is localized within the endothelia of small arteries in many organs such as liver, spleen, lung, pancreas and adipose tissues [8,9]. It is already postulated that the apelin signalling pathway plays a role in the central and peripheral regulation of the cardiovascular system, such as blood pressure and blood flow [5], in water and food intake, and possibly in immune function [6]. What is more, detailed evaluation of APJ and apelin expression patterns in embryogenesis, and in the developing retina, suggested the hypothesis that autocrine signalling of this pathway in endothelial cells (EC) provides a mechanism for regulating new blood vessel growth, or angiogenesis [10,11]. Activation of this pathway in cultured EC has been shown to promote migration and proliferation, and blood vessel growth-promoting functions of apelin have been demonstrated in the Matrigel plug assay in the mouse and chick chorioallantoic membrane assay [11].
Besides hormonal effects, angiogenesis, changes in blood flow and extra cellular matrix remodelling are important processes which are associated with the development, function and regression of the bovine corpus luteum (CL) [7]. The bovine CL is a transient organ that secretes progesterone (P4), a prerequisite for the establishment and maintenance of pregnancy [7]. The CL undergoes drastic changes in its function and structure during the oestrous cycle. Active angiogenesis is necessary for the fast growth of the CL. Therefore angiogenesis and P4 synthesis occur during CL development and are kept up during maintenance of CL function. Furthermore locally produced growth factors may have important modulator roles in final ovarian follicular growth. In cattle, ovarian follicular development is characterised by two or three consecutive follicular waves per oestrous cycle [12,13]. Each wave involves the recruitment of a cohort of follicles and the selection of a dominant follicle, which continues to grow and mature to the preovulatory stage while others in the wave undergo atresia. A complex regulatory system must exist to determine which follicles are selected [14]. That angiogenesis, the formation of new capillaries to a dense network, may play an important role in the selection process was suggested by previous studies that demonstrated that the selected follicle possesses a more elaborate microvasculature than other follicles [15]. The participation of angiogenic factors, for instance vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (FGF2) in bovine follicles during final growth was aim of several investigation in the past [7,14,16] and Kidoya et al. [17] have recently shown in vitro, VEGF and FGF increase APJ and apelin expression in endothelial cells (EC) respectively.
Given their role in angiogenesis apelin and its receptor APJ are hypothesized to be involved in CL formation, function and regression during oestrus cycle and gravidity and in maturation and selection of developing follicles. Therefore, in the present study, local mRNA expression and the possible role o the apelin/APJ system in the bovine ovary was examined by q-RT-PCR.
MATERIAL AND METHODS
Experiment 1: Collection of CL during the oestrous cycle and pregnancy
The CL of the cows were collected at the local slaughterhouse within 10-20 min of slaughter. The stage of oestrous cycle was determined by examining macroscopically the size, colour, consistency, connective tissue and mucus of the ovaries and uteri as previously described [18]. CL were assigned to the following stages; days 1-2, 3-4, 5-7, 8-12, 13-16, >18 (after regression) of oestrous cycle and of pregnancy; month 1-2, 3-4, 6-7, >8 (the crown-rump length of the fetus was measured to evaluate the month of pregnancy). Luteal tissue was frozen in liquid nitrogen and stored at -80°C until RNA extraction.
Experiment 2: Collection of follicles during final follicular growth
Entire reproductive tracts from cows were collected at a local slaughterhouse within 10-20 min after slaughter and were transported on ice to the laboratory. The stage of the oestrous cycle was defined by macroscopic observation of the ovaries (color, consistency, corpus luteum stage, number and size of follicles) and the uterus (color, consistency and mucus). Only follicles which appeared healthy (i.e. well vascularized and having transparent follicular wall and fluid) and whose diameter was >5 mm were used. Large follicles (>14 mm) were collected only after CL regression, with signs of mucus production in the uterus and cervix and were assumed to be preovulatory. For the RNA extraction the follicles were dissected from the ovary. The surrounding tissue (theca externa) was removed with forceps under a stereo microscope as previously described [19]. After aspiration of FF, the follicles were bisected and their inside wall was gently scraped and flushed with Ringer's solution (Fresenius, Wendel, Germany) to remove the granulosa cells (GC). The GC in the FF as well as in the flushing solution were centrifuged at 2000 g for 10 min at 4°C. The theca interna tissue (TI) and GC pellet were snap frozen in liquid nitrogen and stored at -80°C until RNA isolation. The FF was stored at -20°C until determination of P4 and E2.
Since healthy follicles have relatively constant P4 levels in FF, only follicles with P4 below 100 ng/ml FF were used for the evaluation, to exclude atretic follicles. The follicles were classified according to the E2 content in FF as follows; (i) <0·5; (ii) 0·5-5; (iii) 5-40; (iv) 40-180; and (v) >180 ng/ml FF. The corresponding size of follicles were in the range of (i) 5-7 mm; (ii) 8-10 mm; (iii) 10-13 mm; (iv) 12-14 mm and (v) >14 mm.
Hormone determination
Concentrations of P4 and E2 were determined directly in the FF with an enzyme immunoassay using the second antibody technique [20,21]. We used as enzyme solution progesterone-6β-hydroxy-hemisuccinate-horseradish Peroxidase (HRP) or oestradiol-17β-6-carboxy-methyloxim-HRP. Each polyclonal antibody was raised in a rabbit against progesterone-7α-carboxyethylthioether-BSA or for E2 against oestradiol-17β-6-carboxymethyloxim-BSA. The effective dose for 50% inhibition (ED50) of the assay was 6 ng/ml for P4 and 3.5 pg/ml for E2. The FF was diluted accordingly. The intraassay variations were 4-5% (P4) and 6-7% (E2) and the interassay variations 8-9% (P4) and 9-10% (E2) [14].
Total RNA extraction and quality determination
Small Slices of deep frozen (-80°C) CL and follicle cells were cut and weighted. Total RNA from the follicles and CL were isolated using the NucleoSpin RNA kit (Macherey-Nagel, Dueren, Germany). The RNA was dissolved in RNase-free water and spectroscopically quantified at 260 nm. Aliquots were subjected to 1% denaturing agarose gel electrophoresis and ethidium bromide staining to verify the quantity and quality of RNA.
The purity of RNA was verified by optical density (OD) absorption ratio OD260 nm/OD280 nm between 1.8 and 2.0. Degradation of the RNA was measured with the Agilent 2100 Bioanalyzer (Agilent Technologies, Deutschland GmbH,Waldbronn, Germany) in conjunction with the RNA 6000 Nano Assay according to the manufacturer´s instructions. The Bioanalyzer enables the standardisation of RNA quality control. RNA samples were electrophoretically separated on a microfabricated chip and subsequently detected with laser induced fluoresence induction. Each chip contains an interconnected set of microchannels that is used for separation of nucleic acid fragments based on their size as they are driven through the chip electrophoretically. The RNA 6000 ladder standard is used as a reference for data analysis. The software compares the unknown samples to the ladder fragments to determine its concentration and to identify the ribosomal RNA peaks of the unknown sample (Bioanalyzer service). The Bioanalyzer electropherogram of total RNA shows two distinct ribosomal peaks corresponding to either 18S and 28S for eukaryotic RNA and a relatively flat baseline between the 5S and 18S ribosomal peaks. The automatically calculated RNA integrity number (RIN) allows classification of total RNA based on a numbering system from 1 to 10, with 1 being the most degraded profile and 10 being the most intact [22].
RNA Reverse Transcription
Constant amounts of 1 mg of total RNA were reverse transcribed to cDNA using the following master mix: 26 µl Rnase-free water, 12 µl 5x Buffer (Promega, Mannheim, Germany), 3 µl Random Primers (50 mM) (Invitrogen, Carlsbad, Germany), 3 µl dNTPs (10 mM) (Fermentas, St. Leon-Rot, Germany) and 200 U of MMLV Reverse Transcriptase (Promega, Mannheim, Germany) according to the manufacturer's instructions as previously described [14].
Primers
The primers used for real-time PCR were as follows: apelin (106 bp) forward, 5'-AAGGCACCATCCGATACCTG-3' and reverse , 5'-ATGGGACCCTTGTGGGAGA-3'; APJ (100 bp) forward, 5'-TCTGGGCCACCTACACCTAT-3' and reverse , 5'-ACGCTGGCGTACATGTTG-3' [7,23] and histone (232 bp) forward, 5'- ACTGCTACAAAAGCCGCTC-3' and reverse, 5'-ACTGCCTCCTGCAAAGCAC-3' [24].
Quantitative RT-PCR
A master mix of the following reaction components was prepared: 6.4 µl water, 1.2 µl MgCl2 (4 mM), 0.2 µl forward primer (0.2 mM), 0.2 µl reverse primer (0.2 mM), and 1.0 µl LightCycler Fast Start DNA Master SYBR Green I (Roche Diagnostics, Mannheim, Germany). The master mix (9 µl) was added to the strip tubes and 1 µl PCR template containing 16.66 ng (CL) or 8.33 ng (GC and TI) reverse transcribed total RNA was added. The following general real-time PCR protocol was employed for all investigated factors: denaturation for 10 min at 95°C, 40 cycles of a three segmented amplification and quantification program (denaturation for 10 sec at 95°C, annealing for 10 sec at the primer specific temperature, elongation for 15 sec at 72°C), a melting step by slow heating from 60 to 99°C with a rate of 0.58°C/sec and continuous fluorescence measurement, and a final cooling down to 40°C. Crossing point (cp) values were acquired by using the second derivative maximum method of the Rotor-Gene 6 software (Corbett Research, Mortlake, Australia). Real-time PCR efficiencies were determined by amplification of a standardized dilution series, and slopes were calculated using Rotor-Gene 6 software (Corbett Research, Mortlake, Australia). The specificity of the desired products in bovine CL was documented using a high resolution gel electrophoresis and analysis of the melting temperature, which is product specific.
Statistical Analyses
The statistical significance of differences in mRNA expressions of the examined factors was assessed by one-way ANOVA followed by the Holm-Sidak as a multiple comparison test. Differences were considered significant if P<0.05. All experimental data are shown as 40 minus the mean of cp ± SEM.
RESULTS
RNA Integrity
RNA quality was assessed using the Agilent Bioanalyzer 2100. The RNA samples were examined randomly and showed good RNA quality with a mean RIN of 7.86 ± 0.31, which is perfectly suitable for PCR analysis.
Gene expression analysis
The mRNA expression of the housekeeping gene histone was not statistically regulated. Thus it is assumed that equal amounts of mRNA were used in each sample. In order to obtain the mRNA expression differences, the cp (crossing points) was not subtracted from a control group, but from the value 40, so that a high “40-cp” value indicated a high gene expression level and vice versa.
The mRNA expression of apelin and APJ in bovine CL during luteal phase and pregnancy
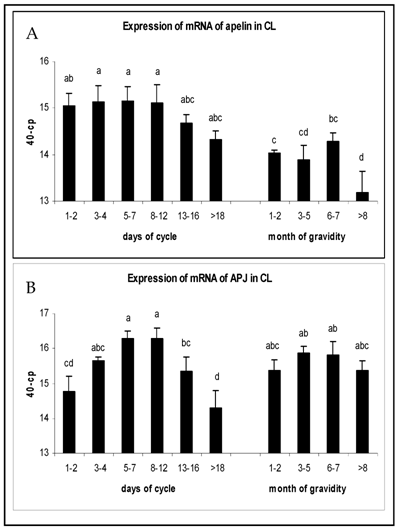
The expression of mRNA in CL tissue during the luteal phase and pregnancy is shown in Fig. 1. Apelin mRNA expression in early (days 1-7) and mid-luteal stages (days 8-12) maintained high levels and were statistically higher than during pregnancy (P<0.05). On days 13-16 a tendency of decrease could be observed, that dropped even further during regression of CL (Fig.1A). The APJ receptor mRNA expression increased in early luteal stages to reach the highest level at mid-luteal phase (P<0.05), followed by a significant decrease afterwards. There were no significant regulations in APJ expression during pregnancy and mRNA levels were comparable to those in mid-luteal phase (Fig. 1B).
Expression of mRNA of (A) apelin and (B) APJ in the CL of oestrus cycle and gravidity. Results are presented by 40 minus mean of crossing points (cp) ± SEM (n=6-7). Different superscripts denote statistically different values (P<0.05).
The mRNA expression of apelin and APJ in follicles during finale follicular growth
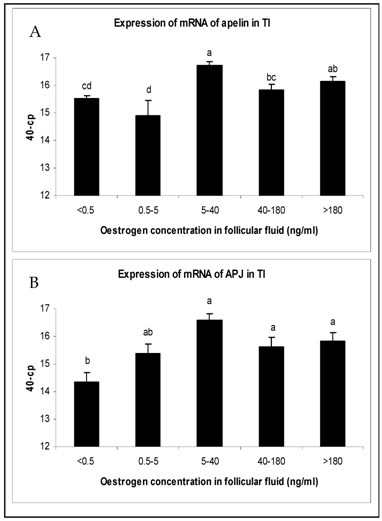
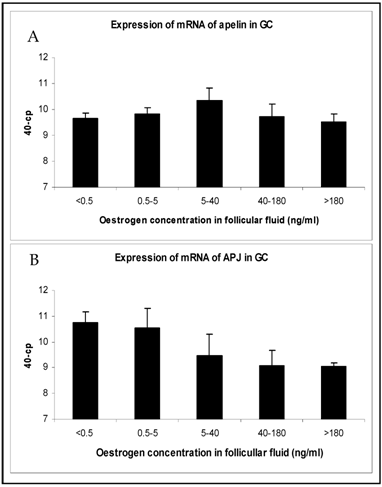
The changes in mRNA expression for apelin and APJ in TI and GC of growing follicles are shown in Fig. 2 and Fig. 3. Apelin expression in TI of follicles with an E2 concentration from 5 to >180 ng/ml were significantly up regulated (Fig. 2A). The mRNA expression of its receptor APJ also increased significantly from the smallest follicles to those with increased E2 concentrations over 5 ng/ml in their FF (Fig. 2B). Apelin as well as APJ expression in GC showed no statistically significant regulations. For apelin mRNA constant expression levels (Fig. 3A) and for APJ a slight decrease from small to matured follicles could be observed (Fig. 3B).
Expression of mRNA of (A) apelin and (B) APJ in the theka interna (TI) in follicles of different stages of follicle development and maturation. Results are presented by 40 minus mean of crossing points (cp) ± SEM (n=7-12). Different superscripts denote statistically different values (P<0.05). Classification of follicles occurred by follicle size and oestradiol-17β concentration in the FF (<0.5 ng/ml, 0.5-5 ng/ml; 5-40 ng/ml; 40-180 ng/ml; >180 ng/ml).
Expression of mRNA of (A) apelin and (B) APJ in granulosa cells (GC) in follicles of different stages of follicle development and maturation. Results are presented by 40 minus mean of crossing points (cp) ± SEM (n=7-12). Classification of follicles occurred by follicle size and oestradiol-17β concentration in the FF (<0.5 ng/ml, 0.5-5 ng/ml; 5-40 ng/ml; 40-180 ng/ml; >180 ng/ml).
DISCUSSION
Apelin and APJ have been investigated in many organs and tissues including the brain, heart, lung, kidney, uterus and ovary using PCR and immunohistochemistry [3-5, 25-27]. The objective of the present study was to demonstrate the expression of apelin and APJ in detail in bovine CL obtained from different stages of the oestrous cycle and during pregnancy - and to demonstrate that apelin and its receptor are expressed clearly in bovine follicles during final growth to preovulatory follicles. We evaluated their expression in separated bovine GC and TI.
After angiogenesis the CL becomes one of the most highly vascularized organs and receives the greatest rate of blood flow [28]. Our findings that apelin mRNA maintained high levels in early and mid-luteal stages, where angiogenesis takes place to ensure the fast growth of the CL and its supply are in coincidence with those of Shirasuna et al. [7]. Recent studies have shown the angiogenic effect of apelin when working cooperatively with the prominent angiogenic factors VEGF or FGF [29,31]. The presence of a localized source of apelin induced vascular development and angiogenic branching whose effects were abolished in APJ-deficient frog embryos in vivo [11]. Moreover, it was shown that apelin can stimulate the proliferation and migration of EC in mouse [28]. Both, apelin and APJ showed a decline at the end of the luteal phase and dropped steeply during CL regression. At this point of time no angiogenesis is needed any more due to the beginning degradation of CL tissue and vessels [32]. The co-expression of mRNA of apelin and APJ strongly suggest that apelin may have some function as a local regulator in the bovine CL. Therefore, our findings suggest that the apelin/APJ system may be an autocrine and/or paracrine factor, and may have physiological roles in the vascular establishment, maturation, and maintenance mainly in the CL during oestrous cycle. The possible role during pregnancy is not strong convincing. For both, apelin and APJ mRNA expression an increase could be observed in TI from growing follicles with increasing E2 concentrations. The strong expression of apelin and its receptor APJ in the mature follicles raises the possibility that these factors may play a role in angiogenesis that accompanies follicular growth and selection. Previous work in primate follicles has shown that the density of the microvascular network of the selected follicle is at least double that of follicles of lesser maturity. This increased capillary density resulted in a greater delivery of gonadotropic hormones to the selected follicle in vivo [15]. The process of follicle selection requires a mechanism by which a single follicle continues to survive in the presence of gonadotropin concentrations which are insufficient to support the growth of other follicles [29]. Our finding that apelin and APJ are expressed and strongly regulated in TI underline these assumptions, suggesting that the apelin/APJ system may act as a chemo attractant for sprouting endothelial cells. In addition, Shimizu et al. [23] also reported that APJ is expressed in bovine granulosa cells, and progesterone stimulated the expression of APJ in granulosa cells. The expression of apelin and APJ in mature follicles suggests that the apelin/APJ system could play an important role during follicle selection and dominance in the cow.
In conclusion, our results demonstrate the distinct up-regulation during development of bovine follicles of mRNA of the apelin/APJ system. The results are consistent with the hypothesis that these factors may have angiogenic effect and may be involved in the proliferation of capillaries that accompanies the selection of the preovulatory follicle, resulting in an increased supply of nutrients and precursors, and therefore supporting growth of the dominant follicle. Moreover apelin and APJ showed statistically regulated expression levels during CL formation and function. That led to the assumption that this system may be associated with vascular function in the bovine ovary.
Acknowledgements
We greatly acknowledge the support of the work by the German Research Foundation (DFG).
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exits.
References
1. Edinger AL, Hoffman TL, Sharron M, Lee B, Yi Y, Choe W, Kolson DL, Mitrovic B, Zhou Y, Faulds D, Collman RG, Hesselgesser J, Horuk R, Doms RW. An orphan seven-transmembrane domain receptor expressed widely in the brain functions as a coreceptor for human immunodeficiency virus type 1 and simian immunodeficiency virus. J. Virol. 1998;72(10):7934-7940
2. Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998;251(2):471-476
3. Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S, Nishizawa N, Kitada C, Onda H, Nishimura O, Fujino M. Molecular properties of apelin: tissue distribution and receptor binding. Biochim. Biophys. Acta. 2001;1538(2-3):162-171
4. Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, Kitada C, Nishizawa N, Murosaki S, Kurokawa T, Onda H, Tatemoto K, Fujino M. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim. Biophys. Acta. 1999;1452(1):25-35
5. De Falco M, De Luca L, Onori N, Cavallotti I, Artigiano F, Esposito V, De Luca B, Laforgia V, Groeger AM, De Luca A. Apelin expression in normal human tissues. In Vivo. 2002;16(5):333-336
6. Masri B, Knibiehler B, Audigier Y. Apelin signalling: a promis- ing pathway from cloning to pharmacology. Cell Signal. 2005;17(4):415-426
7. Shirasuna K, Shimizu T, Sayama K, Asahi T, Sasaki M, Berisha B, Schams D, Miyamoto A. Expression and localisation of apelin and is receptor APJ in the bovien corpus luteum during the estrus cycle and prostaglandin F2α-induced luteolysis. Reproduction. 2008;135(4):519-525
8. Tatemoto K, Takayama K, Zou M-X, Kumaki I, Zhang W, Kumano K, Fujimiya M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 2001;99(2-3):87-92
9. De Falco M, Fedele V, Russo T, Virgilo F, Sciarrillo R, Leone S, Laforgia V, De Luca A. Distribution of apelin, the endogenous ligand of the APJ receptor, in the lizard Podarcis sicula. J. Mol. Histol. 2004;35(5):521-527
10. Lee DK, George SR, O'Dowd BF. Unravelling the roles of the apelin system: prospective therapeutic applications in heart failure and obesity. Trends Pharmacol. Sci. 2006;27(4):190-194
11. Cox CM, D'Agostino SL, Miller MK, Heimark RL, Krieg PA. Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev. Biol. 2006;296(1):177-189
12. Savio D, Keenan L, Boland MP & Roche JF. Pattern of growth of dominant follicles during the oestrous cycle of heifers. J. Reprod. Fertil. 1988;83(2):663-671
13. Sirois J und Fortune JE. Ovarian follicular dynamics during the estrous cycle in heifers monitored by real-time ultrasonography. Biol. Reprod. 1988;39(2):308-317
14. Berisha B, Schams D, Kosmann M, Amselgruber W, Einspanier R. Expression and localisation of vascular endothelial growth factor and basic fibroblast growth factor during the final growth of bovine ovarian follicles. J. Endocrinol. 2000;167(3):371-382
15. Zeleznik AJ, Schuler HM, Reichert LE Jr. Gonadotropin-binding sites in the rhesus monkey ovary: role of the vasculature in the selective distribution of human chorionic gonadotropin to the preovulatory follicle. Endocrinology. 1981;109(2):356-362
16. Berisha B, Steffl M, Welter H, Kliem H, Meyer HH, Schams D, Amselgruber W. Effect of the luteinising hormone surge on regulation of vascular endothelial growth factor and extracellular matrix-degrading proteinases and their inhibitors in bovine follicles. Reprod Fertil Dev. 2008;20(2):258-268
17. Kidoya H, Ueno M, Yamada Y, Mochizuki N, Nakata M, Yano T, Fujii R, Takakura N. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 2008;27(3):522-534
18. Berisha B, Schams D, Kosmann M, Amselgruber W, Einspanier R. Expression and tissue concentratiuon of vascular endothelial growth factor, its receptor, and localisation in the bvine corpus luteum during estrous cycle and pregnancy. Biol. Reprod. 2000;63(4):1106-1114
19. Berisha B, Sinowatz F, Schams D. Expression and localization of fibroblast growth factor (FGF) family members during the final growth of bovine ovarian follicles. Mol Reprod Dev. 2004;67(2):162-71
20. Prakash BS, Meyer HH, Schallenberger E, van de Wiel DF. Development of a sensitive enzyme immunoassay (EIA) for progesterone determination in unextracted bovine plasma using the second antibody technique. J. Steroid Biochem. 1987;28(6):623-627
21. Meyer HH, Sauerwein H & Mutayoba BM. Immunoaffinity chromatography and a biotin-streptavidin amplified enzyme immunoassay for sensitive and specific estimation of estradiol-17β. J. Steroid Biochem. 1990;35(2):263-269
22. Berisha B, Bridger P, Toth A, Kliem H, Meyer HH, Schams D, Pfarrer C. Expression and localization of gap junctional connexins 26 and 43 in bovine periovulatory follicles and in corpus luteum during different functional stages of oestrous cycle and pregnancy. Reprod Domest Anim. 2009;44(2):295-302
23. Shimizu T, Kosaka N, Murayama C, Tetsuka M, Miyamoto A Apelin, APJ receptor expression in granulosa, theca cells during different stages of follicular development in the bovine ovary. Involvement of apoptosis and hormonal regulation. Anim Reprod Sci. 2009 [Epub ahead of print]
24. Kliem H, Berisha B, Meyer HH, Schams D. Regulatory changes of apoptotic factors in the bovine corpus luteum after induced luteolysis. Mol Reprod Dev. 2009;76(3):220-30
25. O'Carrol AM, Selby TL, Palkovits M, Lolait SJ. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and preipheral tissues. Biochim. Biopys. Acta. 2000;1492(1):72-80
26. Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY, Lawrie KW, Hervieu G, Riley G, Bolaky JE. Pharmological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J. Neurochem. 2003;84(5):1162-1172
27. Kleinz MJ, Skepper JN, Davenport AP. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul. Pept. 2005;126(3):233-240
28. Wiltbank MC, Dysko RC, Gallagher KP, Keyes PL. Relationship between blood flow and steroidgenesis in the rabbit corpus luteum. J. Reprod. Fertil. 1988;84(2):513-520
29. Kasai A, Shintani N, Oda M, Kakuda M, Hashimoto H, Matsuda T, Hinuma S, Baba A. Apelin is a novel angiogenic factor in retinal endothelial cells. Biochem. Biophys. Res. Commun. 2004;325(2):395-400
30. Zeleznik AJ, Kubik CJ. Ovarian responses in macaques to pulsatile infusion of follicle-stimulating hormone (FSH) and luteinizing hormone: increased sensitivity of the maturing follicle to FSH. Endocrinology. 1986;119(5):2025-2032
31. Kojima Y, Quertermous T. Apelin-APJ signaling in retinal angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2008;28(10):1687-1688
32. Schams D und Berisha B, Neuvians T, Amselgruber W, Kraetzl WD. Real-time changes of the local vasoactive peptide systems (angiotensin, endothelin) in the bovine corpus luteum after induced luteal regression. Mole. Reprod. Dev. 2003;65(1):57-66
Author biography
Bajram Berisha, PD Dr. habil., heads the Reproduction Unit at the Chair of Physiology in Weihenstephan, Technische Universitaet Muenchen. He is a senior scientist with a long research experience in the area of Reproduction Biology. Mr. Berisha has a special interest in elucidating the molecular mechanisms of locally produced factors on the physiology of reproductive tract and especially on ovarian function. His research work is concentrated in the very important steps in the regulation of final follicle growth and especially of angiogenesis during follicle development, periovulation as well as corpus luteum formation and function. Currently he focuses on the action of locally produced angiogenic and lymphangiogenic factors in ovary during different physiological stages.
Stefanie Schilffarth, Dr. med. vet., is junior scientist at the Chair of Physiology in Weihenstephan, Technische Universitaet Muenchen. After her academic studies at the Ludwig-Maximilians-Unversitaet Muenchen she earned her doctorate in the Reproduction Unit of PD Dr. Berisha at the Technische Universitaet Muenchen. Currently she focuses on the analyses of angiogenic, anti-angiogenic and lymphangiogenic factors during different stages of ovarian function.
![]() Correspondence to: PD Dr. Bajram Berisha, Chair of Physiology, Centre of Life and Food Sciences, Weihenstephan, Technische Universität München, Germany, Weihenstephaner Berg 3., 85354 Freising-Weihenstephan. Tel.: +49 (0) 8161-71 3510; Fax: +49 (0) 8161-71 4204; E-mail: berishatum.de. http://www.wzw.tum.de/fml/physio/
Correspondence to: PD Dr. Bajram Berisha, Chair of Physiology, Centre of Life and Food Sciences, Weihenstephan, Technische Universität München, Germany, Weihenstephaner Berg 3., 85354 Freising-Weihenstephan. Tel.: +49 (0) 8161-71 3510; Fax: +49 (0) 8161-71 4204; E-mail: berishatum.de. http://www.wzw.tum.de/fml/physio/

 Global reach, higher impact
Global reach, higher impact