10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2010; 6(2):129-132. doi:10.7150/ijbs.6.129 This issue Cite
Short Research Communication
Aberrant leukocyte infiltration: a direct trigger for breast tumor invasion and metastasis
1. Department of Gynecologic and Breast Pathology, Armed Forces Institute of Pathology and American Registry of Pathology, Washington DC, USA
2. Jilin University, Jilin, China
Received 2009-11-23; Accepted 2010-3-4; Published 2010-3-9
Abstract
Our previous studies revealed that leukocyte infiltration could trigger breast and prostate tumor invasion through physical disruption of tumor capsules. Our current study, involving multiple types of human tumors, further suggests that leukocyte infiltration also triggers metastasis through the following pathways : 1) the physical movement into the epithelium disrupts inter-cellular junctions and surface adhesion molecules, which cause the disassociation of tumor cells from tumor cores, 2) some of these tumor cells subsequently form tight junctions with the plasma membranes of leukocytes creating tumor cell-leukocyte chimeras (TLCs), and 3) the leukocytes of TLCs impart migratory capacity to associated tumor cell partners. Our findings suggest a novel pathway for tumor cell dissemination from primary sites and journey to new sites.
Keywords: Tumor invasion, Tumor metastasis, Leukocyte, Tumor stem cells, Intercellular junction, Tumor cell-leukocyte chimeras.
The epithelium, which is the origin of over 85% of breast malignancies, is physically separated from the stroma by the myoepithelial cell layer and basement membrane [1]. The epithelium is normally devoid of lymphatic ducts and blood vessels, and thus, totally relies on the stroma for its essential needs. The epithelial cells are normally held in place by intercellular junctions and surface adhesion molecules [2]. Due to these structural relationships, the disruption of these structures is a pre-requisite for tumor invasion into the stroma, which is believed to be a multistage process, progressing sequentially from normal to hyperplasia, to in situ, and to invasive stages [3]. Progression from an in situ to invasive stage is believed to be triggered by overproduction of proteolytic enzymes primarily by cancer cells [4,5]. Tumor metastasis is also believed to be a multistage process, requiring the tumor cells to dissociate from the primary site, enter the circulation, migrate to distant sites, and proliferate (6). The mechanism of tumor metastasis, however, remains as a subject of debate.
Recently, there has been a great deal of interest in the role of immune cells in tumor invasion and metastasis, and several models, including paracrine loop signaling, tumor-educated macrophages, immune cell-based mediation, and cancer cell-leukocyte fusion [7-11], have been proposed to explain how immune cells could facilitate metastasis. Collectively, these models suggest that immune cells facilitate tumor invasion and metastasis through the following mechanisms: (1) macrophages enhance tumor cell migration through secretion of chemotactic and chemokinetic factors, which promote angiogenesis and fibrillogenesis, allowing tumor cells track along collagen fibers to blood vessels [7,8], (2) T-lymphocytes indirectly promote invasion and metastasis by directly regulating the phenotype and effector function of tumor associated CD11b(+)Gr1(-)F4/80(+) macrophages [9,10], and (3) macrophages ingest tumor cells, resulting in the fusion of genetic materials of two cell types that creates a hybrid phenotype [11]. Each of these models is supported by laboratory findings or clinical data, suggesting that metastasis is likely to occur through multiple mechanisms. However, it is not currently possible to discern the mechanistic origin of a given metastatic lesion. In addition, two critical issues remain: (1) how metastasis-initiating cells are disseminated from their primary sites, and (2) how disseminated tumor cells escape from the immune-surveillance during their journey to new sites. More importantly, the specific molecule and unique morphological feature of pending or early metastatic lesions remain elusive, making it difficult for early detection and intervention of tumor metastasis.
Since the loss of the myoepithelial cell layer is the primary pathological criterion for the diagnosis of invasive breast lesions (1), our recent studies have attempted to identify early alterations of this layer. Of over 8,000 ducts and acini examined from over 400 cases, our study revealed that about 10% of patients harbored a high frequency of focal disruptions in the myoepithelial cell layers of ducts or acini with pre-invasive tumors. Ducts and acini with a focally disrupted myoepithelial cell layer had a significantly higher frequency of leukocyte infiltration than their counterparts with non-disrupted layers, 97.4% versus 22.2%, and a vast majority of infiltrated leukocytes were located at or near the disruptions [12-15]. Epithelial cells overlaying disruptions had a significantly higher frequency of ER negativity, elevated proliferation, aberrant DNA structural alterations, and expression of invasion and metastasis-related genes than their counterparts within the same duct but distant from disruptions [12-15]. Our findings have led to a novel hypothesis that breast tumor invasion is triggered by focal myoepithelial cell degeneration induced leukocyte (mainly CD8 (+) and CD 4(+) lymphocytes) infiltration that results in focal disruptions in the myoepithelial cell layer, which selectively favor monoclonal proliferation and stromal invasion of tumor progenitors overlying these disruptions [16].
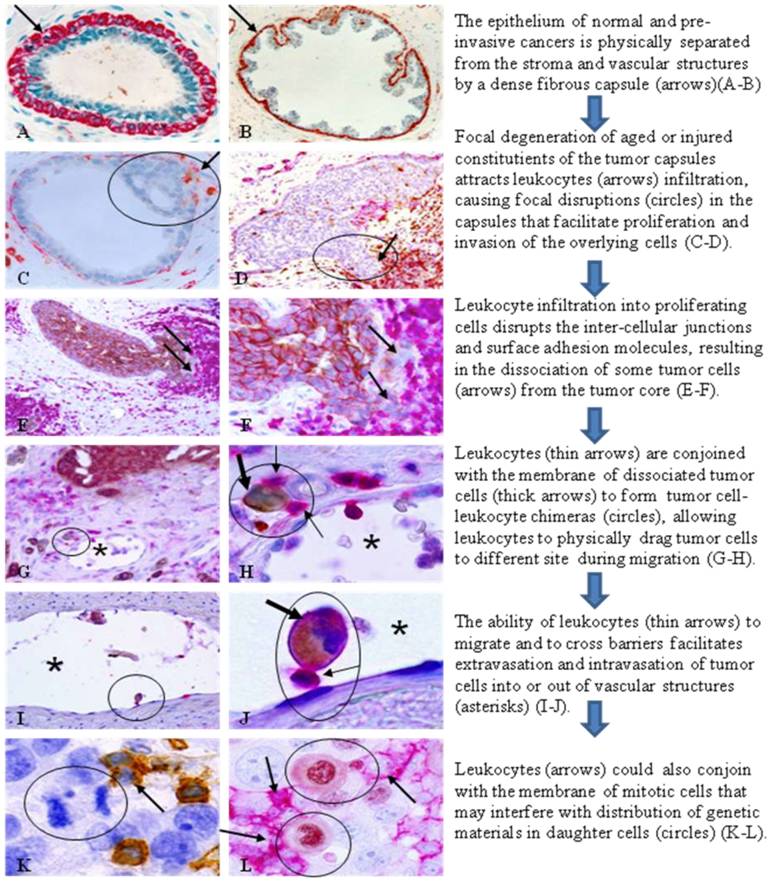
Our more recent studies have further shown that aberrant leukocyte infiltration may also represent a trigger factor for tumor metastasis through the pathways depicted in Fig 1.
This hypothesis fundamentally differs from existing theories of tumor metastasis [7-11]. Based on this hypothesis, leukocytes (mainly T-lymphocytes) could directly trigger tumor invasion and metastasis through three correlated pathways: (1) physical disruptions of the inter-cellular junctions and surface adhesion molecules, causing tumor cell dissociation from primary sites, (2) formation of tight junctions with the plasma membrane of tumor cells, allowing leukocytes to physically drag their partners to distant sites during their migration, and (3) formation of tight junctions with the plasma membranes of mitotic cells, interfering with the cell division process. Our recent studies with confocal microscope have confirmed the tight plasma membrane junctions between leukocytes and tumor cells [17]. The mechanism for the formation of tight membrane junctions between leukocytes and tumor cells is unknown, but may involve the formation of microvesicles by the tumor cells. Microvesicles released from the membranes of tumor cells contain proteins that may act as self-epitopes [18,19], which stimulate production of corresponding auto-antibodies, or activate a subset of leukocytes. Prior to their release, these microvesicles are small particles embedded within the tumor cell plasma membrane, and could potentially function as receptors for the leukocytes, leading to the formation of TLCs [18, 19]. It is also possible that these dissociated tumor cells may belong to a population of tumor stem cells, which has unique expression of plasma membrane-related proteins that function as receptors for the leukocytes.
This hypothesis presents a unique explanation for the initial dissemination of tumor cells from their primary sites and their subsequent journey to new sites. If confirmed, it could have several significant implications. Scientifically, it could lead to a new direction to acquire more effective approaches for detection, intervention, and prevention of invasion and metastasis. Clinically, it may have a number of beneficial applications, including: (1) double immunostaining to assess the extent of focal myoepithelial cell layer disruptions and leukocyte infiltration of in situ tumors may facilitate differentiation between those at high or low risk to progress, (2) a quantitative assessment of myoepithelial cell degeneration-related molecules, or the number of leukocyte subtypes preferentially associated with degeneration-related changes in blood samples may be used as a screen tool to indentify the individuals at a greater risk to develop invasive or metastatic lesions, and (3) the development of therapeutic agents to target the specific leukocyte subtypes that are physically associated with epithelial cells may provide a more effective means to treat and prevent tumor invasion and metastasis. In addition, this hypothesis may be applicable to all epithelium-derived tumors, as leukocytes are widely distributed in abundance in almost all human tissues.
Hypothesized mechanism and steps of leukocytes triggering tumor invasion and metastasis. Human breast and lung tissue sections were immunostained with the following biomarkers. A-B. smooth muscle actin (SMA; red). C-D: SMA (red) + leukocyte common antigen (LCA; brown). E-J: LCA (red)+cytokeratin (CK) AE 1/3 (brown). K: LCA (brown). L: LCA (red)+ Ki-67 (brown).
Acknowledgements
This study was supported in part by grants DAMD17-01-1-0129, DAMD17-01-1-0130, PC051308 from Congressionally Directed Medical Research Programs, grant BCTR0706983 from The Susan G. Komen Breast Cancer Foundation, and grant 2008-02 from The US Military Cancer Institute and Henry M. Jackson Foundation, and by grant 2006CB910505 from The Ministry of Chinese Science and Technology Department.
The opinions and assertions contained herein represent the personal views of the author and are not to be construed as official or as representing the views of the Department of the Army or the Department of Defense.
Conflict of Interest
The author has declared that no conflict of interest exists.
References
1. Guelstein VI, Tehypysheva TA, Ermilova VD, Liubimov AV. Myoepithelial and besement membrane antigens in benign and malignant human breast tumors. In J Cancer. 1993;53:269-77
2. Mărgineanu E, Cotrutz CE, Cotrutz C. Correlation between E-cadherin abnormal expressions in different types of cancer and the process of metastasis. Rev Med Chir Soc Med Nat Iasi. 2008;112(2):432-6
3. Beckmann MW, Niederacher D, Schnurch HG, Gusterson BA, Bender HG. Multistep carcinogenesis of breast cancer and tumour heterogeneity. J Mol Med. 1997;75:429-39
4. Goldfarb RH, Liotta LA. Proteolytic enzymes in cancer invasion and metastasis. Semin Thromb Hemost. 1986;12:294-307
5. Scherer RL, Mclntyre JO, Matrisian LM. Imaging matrix metalloproteinases in cancer. Cancer Metastasis Rev. 2008;27(4):6679-90
6. Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239-52
7. Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER. et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64(19):7022-9
8. Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84(3):623-30
9. DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27(1):11-8
10. DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhalkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91-102
11. Pawelek JM, Chakraborty AK. The cancer cell--leukocyte fusion theory of metastasis. Adv Cancer Res. 2008;101:397-444
12. Man YG, Tai L, Barner R, Vang R, Saenger JS, Shekitka KM. et al. Cell clusters overlying focally disrupted mammary myoepithelial cell layers and adjacent cells within the same duct display different immunohistochemical and genetic features: implications for tumor progression and invasion. Breast Cancer Res. 2003;5:R231-41
13. Yousefi M, Mattu R, Gao C, Man YG. Mammary ducts with and without focal myoepithelial cell layer disruptions show a different frequency of white blood cell infiltration and growth pattern: Implications for tumor progression and invasion. Appl Immunohistochem Mol Mor. 2005;13:30-7
14. Man YG, Zhang Y, Shen T, Vinh TN, Zeng X, Tauler J. et al. cDNA expression profiling identifies elevated expressions of tumor progression and invasion related genes in cell clusters of in situ breast tumors. Breast Cancer Res Treat. 2005;89:199-208
15. Zhang XC, Hashemi SS, Yousefi M, Gao CL, Sheng J, Mason J, Man YG. Atypical expression of c-erbB2 in cell clusters overlying focally disrupted breast myoepithelial cell layers: a potential sign for increasing cell motility and invasion. Int J Biol Sci. 2008;4:259-69
16. Man YG. Focal degeneration of aged or injured myoepithelial cells and the resultant auto-immunoreactions are trigger factors for breast tumor invasion. Medical Hypotheses. 2007;69(6):1340-57
17. Man YG, Mason J, Harley R, Kim YH, Zhu KM, Gardner WA. Causative impact and mechanism of leukocyte infiltration in tumor invasion and metastasis. Submitted.
18. Lima LG, Chammas R, Monteiro RQ, Moreira ME, Barcinski MA. Tumor-derived microvesticles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Letter. 2009;83(2):168-75
19. Bari R, Zhang YH, Zhang F, Wang NX, Stipp CS, Zheng JJ, Zhang XA. Transmembrane interactions are needed for KAI1/CD82-mediated suppression of cancer invasion and metastasis. Am J Pathol. 2009;174:647-60
Author contact
![]() Corresponding author: Yan-gao Man, MD., PhD. Director of Gynecologic and Breast Research Laboratory Department of Gynecologic and Breast Pathology Armed Forces Institute of Pathology and American Registry of Pathology 6825 16th Street, NW Washington DC 20306-6000 Tel: 202-782-1612; Fax: 202-782-3939; E-mail: manosd.mil
Corresponding author: Yan-gao Man, MD., PhD. Director of Gynecologic and Breast Research Laboratory Department of Gynecologic and Breast Pathology Armed Forces Institute of Pathology and American Registry of Pathology 6825 16th Street, NW Washington DC 20306-6000 Tel: 202-782-1612; Fax: 202-782-3939; E-mail: manosd.mil

 Global reach, higher impact
Global reach, higher impact