10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2010; 6(6):590-598. doi:10.7150/ijbs.6.590 This issue Cite
Communication
Debate on GMOs Health Risks after Statistical Findings in Regulatory Tests
1. CRIIGEN, 40 rue Monceau, 75008 Paris France
2. University of Rouen, LITIS EA 4108, 76821 Mont Saint-Aignan, France
3. University Paris-Sud, Bâtiment 360 91405 Orsay, France
4. University of Caen, Institute of Biology, Risk Pole MRSH CNRS, EA2608, Esplanade de la Paix 14032 Caen Cedex, France
Received 2010-7-5; Accepted 2010-9-24; Published 2010-10-5
Abstract
We summarize the major points of international debate on health risk studies for the main commercialized edible GMOs. These GMOs are soy, maize and oilseed rape designed to contain new pesticide residues since they have been modified to be herbicide-tolerant (mostly to Roundup) or to produce mutated Bt toxins. The debated alimentary chronic risks may come from unpredictable insertional mutagenesis effects, metabolic effects, or from the new pesticide residues. The most detailed regulatory tests on the GMOs are three-month long feeding trials of laboratory rats, which are biochemically assessed. The tests are not compulsory, and are not independently conducted. The test data and the corresponding results are kept in secret by the companies. Our previous analyses of regulatory raw data at these levels, taking the representative examples of three GM maize NK 603, MON 810, and MON 863 led us to conclude that hepatorenal toxicities were possible, and that longer testing was necessary. Our study was criticized by the company developing the GMOs in question and the regulatory bodies, mainly on the divergent biological interpretations of statistically significant biochemical and physiological effects. We present the scientific reasons for the crucially different biological interpretations and also highlight the shortcomings in the experimental protocols designed by the company. The debate implies an enormous responsibility towards public health and is essential due to nonexistent traceability or epidemiological studies in the GMO-producing countries.
Keywords: GMOs, Health risks, Pesticides, Regulatory toxicology, Animal tests
Introduction and Context
The debate on the safety of genetically modified organisms (GMOs) used for food and feed is still very lively throughout the world, more than 15 years after their first commercial release [3-5]. Huge social, economical, and political issues have been raised. Unfortunately, although some stakeholders claim that a history of safe use of GMOs can be upheld, there are no human or animal epidemiological studies to support such a claim as yet, in particular because of the lack of labeling and traceability in GMO-producing countries. As a matter of fact, 97% of edible GMOs among cultivated GMOs (soy, corn and oilseed rape or canola, excluding cotton) are grown in South and North America [6], where GMOs are not labeled. All these plants have been modified to tolerate and/or produce one or more pesticides [6], and contain therefore such residues at various levels [5]. Most are Roundup residues (it is a major herbicide used worldwide and tolerated by about 80% of GMOs). Other residues are from modified Bt insecticide toxins, which are directly synthesized by the GM plants from transgenes.
The debate on health risks is first of all based on theoretical considerations, and second on the knowledge derived from mammalian experiments fed on GMOs. The latter experiments are not systematically performed, and can be part of non-compulsory regulatory tests. The scientific question about edible GMOs health risks amounts to how they have been tested and interpreted, especially in mammals. Nutritional tests with weight, bone mass, and for instance milk or meat production are available, as well as acute toxicological tests with recombinant proteins, in vitro digestibility of transgenic proteins, and limited compositional analysis among other data. However, the possible chronic side effects of pesticide residues are not scientifically assessed, whereas these edible GMOs were modified in order to either tolerate or produce such residues in the first place. In addition, unpredictable metabolic effects, such as metabolic interferences, or direct or indirect insertional mutagenesis consequences cannot be excluded. All these possibilities have been summarized (Fig. 1). For instance, insertion of the transgene in varieties producing Cry1Ab toxin caused a complex recombination event, leading to the synthesis of new RNA products encoding unknown proteins [7], or/and to metabolic pathways variations which caused up to 50% changes in measured osmolytes and branched aminoacids [8]. The frequency of such events in comparison to classical hybridization is by nature unpredictable and new proteomic technologies have shown to be effective in evaluating the potential collateral effects due to insertional mutagenesis [9].
In order to analyze subchronic or chronic toxicological signs, it is more informative to focus on studies including numerous blood and organ parameters. Most of these are 90 day-long feeding regulatory trials on rats eating GM corn or soy. The raw data issued by the companies particularly attracted our interest. We obtained the said data by Court order and lawyers (since the data were previously kept secret). We recently published a second batch of new assessments [1, 2, 5]. We reviewed all of them, and they revealed significant statistical differences (~9%) which concentrate mostly on kidneys and livers, and are considered both by the companies and the official approval committees as irrelevant where the safety of GMOs is concerned [10].
In fact, behind this scientific controversy, what is at stake really is the commercialization (or not) of GMOs around the world, and overall, the rules of scientific assessment that could be modified. The present rules for GMO risk assessment are mainly based on the concept of substantial equivalence that was accepted by OECD in 1993 and then included in the FDA (Food and Drug Administration) regulation (Part IX : Foods Derived From New Plants Varieties) : « In most cases the substances expected to become components of food as a result of genetic modification will be the same as or substantially similar to substances commonly found in food such as proteins, fats and oils, and carbohydrates. » Such a concept is subjected to debate in the scientific community because it is based on a simplistic view of living cells. In particular, it overlooks all the interactions between genes, and the direct or indirect potential metabolic consequences of insertional mutagenesis. This implies that GMOs are insufficiently evaluated. We realize that the requirement for longer and more detailed regulatory tests would reduce the profitability of GMOs, but protecting mammalian and human health seems even more essential in our views.
Some official agencies authorizing GMOs consumption eventually decided not to take into account our published results [1, 2], and in particular the agencies defended Monsanto's opinions on their websites. Here, we review the arguments of the scientific debate for data interpretation:
It is well known that there were different opinions over the interpretations of the significant differences in the blood and organs of rats eating GMOs in comparison to controls, especially in our counter-analyses on the raw data of three toxicological tests carried out by Monsanto, the results of which we obtained by Court decision [1, 2]. These tests pertain to three GM corns owned by Monsanto: MON 863 and MON 810 which are continuously producing a rootworm and a corn-borer insecticide, respectively, and NK 603 Roundup-tolerant maize, which contains Roundup herbicide residues. Although the European legislation requests transparency of health and environmental impacts in regulatory tests, the raw data were first considered as confidential by biotech firms. It is also true for all other commercialized GMOs, especially those varieties producing or tolerating one or several pesticides, for which the data should be made public.
Proposed mode of actions of agricultural GMOs and/or associated pesticides on health. Almost all GMOs disseminated in the environment are plants, namely soy, maize, cotton, and oilseed rape (1995-2010). Their genetic and phenotypic modifications are only herbicide tolerance and / or insecticide production (modified Bt toxins) in more than 99% cases. Thus they can be described as pesticide plants. Consequently, two major health risks are described: (1) due to mid or long term side effects, brought by new pesticide residues in food or feed, and directly due to the new genetic characteristic. These residues can be from herbicide(s) absorbed by tolerance (Roundup residues in more than 90% herbicide-tolerant GMOs) in most cases, or from new modified insecticide Bt toxins, mutated or truncated in all insecticide-GMOs. (2) Insertional mutagenesis linked to the genetic modification, or post-genomic metabolic interferences or derivations. These are direct or indirect less specific effects independent from the toxicology assessment of the transgene product. These unexpected possible consequences cannot be approached by gross substantial equivalence studies without metabolomic analyses. They can be invisible on the plant phenotype, but still able to induce long term toxicity after consumption, specific to each genetic transformation. The possible combined effects between all these impacts cannot be excluded, inducing chronic pathologies after regular consumption. Only long term testing (more than 3 months in mammals) could answer these possibilities. Thus, regulatory agencies must adapt their methods for health risk assessments of agricultural GMOs, taking into account associated pesticides and their formulations. They should also approach combined effects at different periods of life and on several generations, to be complete, overall when a new food/feed concerns billions of people without traditional knowledge of its consumption.
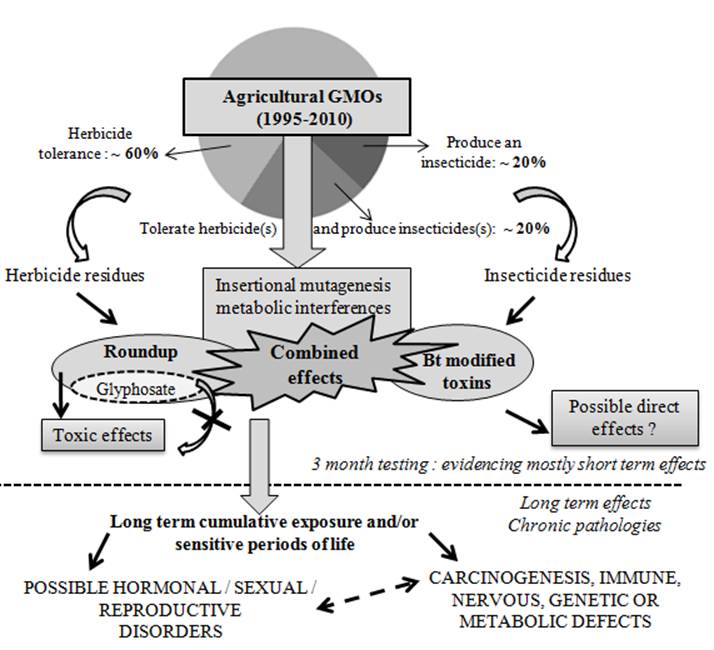
Debate on the shortcomings of the experimental design
All regulatory 90-day rat feeding studies with GMOs have been constructed on the same scheme. The shortcomings of the experimental design are underlined below and summarized (Table 1):
a) Too small number of animals were studied: 10 individuals were measured for biochemical parameters out of 20 per group. This might be enough for long-term experiments, but not over such a short period of time, as naturally a small number of effects or only effects of low amplitude were induced, to be compared to a slowly developing chronic pathology. This kind of protocol could result in low power statistical tests and therefore too many false negative results (for example, it could be misleading, and induce by mistake the research worker to reject a possible effect of the consumption of GMOs).
b) Too many control rats: the number of controls is four times higher in the regulatory tests that have been used all over the world to authorize the main GMOs. Such an imbalance between control and treated rats may conceal the visible effects. Out of 400 rats, there were only 80 eating GMOs (and only 40 biochemically analyzed), thus 4 groups of 10 animals, with 2 dosages (11 and 33% GMO in equilibrated diet), and 2 blood analyses per group (after 5 and 14 weeks). Both sexes were equally represented. But overall we judge the 320 “reference animals” too numerous in comparison to the treated ones. As only half of the rats were studied on all the biochemical and blood parameters, this means that the decisions were made only on 40 rats eating GMOs and assessed from a group of 400 animals, over 90 days.
c) Too many control treatments: the 320 non-GM fed animals were treated in fact with 7 different diets which were supposed to represent a variability of the possible regimen. Six constituted the so-called “reference” groups with feed not demonstrated as substantially equivalent. Moreover, only two dosages in the control groups were chemically equivalent to the GM diets that were made with the isogenic maize or a corn close to the GM variety. But two doses are insufficient to study any dose-related effect.
d) The rat was the only mammal fed with GMOs for 3 months.
e) The regulatory test was only performed once for each GMO, which was then supposed to be eaten all over the world.
f) The duration of 90 days is the longest test on file and only on young adult mammals; it was not long enough to observe chronic effects.
g) The lack of developmental, reproductive as well as chronic or multi-generational tests is the subject of a heated debate for the GMOs currently available on the market.
This experimental protocol from Monsanto was accepted by several official committees, first confidentially. This procedure is a point of controversy not only with Monsanto, but also with the agencies that have published opinions on our work [1, 2, 5]. We will refer to their opinions collectively as Monsanto et al. in the following, unless otherwise specified, to simplify the reading of this paper.
Insufficiencies of currently used tests, criteria and interpretations; proposed improvements for GMOs health risks assessment. We reviewed here the current protocols used by industry and regulatory committees in commercialized agricultural GMOs. The feeding trials described in column 1 were performed in order to obtain GMOs commercialization, via regulatory agencies. The improvements proposed (column 2) will adapt these tests to modern knowledge in toxicology, in order to avoid the main consequences of overlooked risks (column 3).
| Critical parameters and interpretations | Present regulatory assessment | Improvements proposed | Main consequences if improvements not applied |
|---|---|---|---|
| Number of animals / group | 10 measured on 20 /group | At least 20 rats for 3 months, 10 or more for 24 months / group | Low statistical power |
| Number of controls versus treatments | Too many reference or control groups (320)/ 80 GMO-treated only | Avoid to multiply completely different control groups | Risk of concealing statistical effects |
| Species | Rat only (in mammals with blood analyses) | Rat and other(s) species such as Mice / Rabbit | Results too much species-specific |
| Replication of toxicological test | Only once | At least two | Reproducibility, Reliability not proven |
| Length | Subchronic (3 months) | Chronic (24 months) + developmental + transgenerational | Missing long term, fetal or transgenerational effects |
| Doses | 2 doses | 3 doses | Missing dose response relationship |
| Type of treatment | GMO | GMOs with/without associated pesticides | Confusion between mutagenesis / pesticides effects |
| Food composition | Substantial equivalence | More detailed composition with specific pesticides residues and metabolites, adjuvants | Missing potential contaminants and combined effects |
| Norms followed | OECD 408 strictly or less | OECD 408-453 with other details | Lack of hormonal sex specific data for instance |
| Number of blood analyses | 2 measures only after 5 and 14 weeks | At least 3 the first trimester | Missing punctual phenomena |
| Biological interpretations Dose-effects | “Dose-related”: proportional effects only taken into account with two doses ! | Non linear effects to be studied (U or J curves) | Risk to avoid endocrine, carcinogenic, immune long-term effects… |
| Biological interpretations Sex specificity | Effects studied only if occurring in both sexes | Sex specific effects to be studied | Risk to avoid endocrine-specific effects |
| Biochemical modifications linked to histopathology | Necessary | Not always possible in 3 months | Risk of false negative results |
| Amplitude of effects studied | Effects inside of undefined historical norm of the species not studied | Any statistical difference with controls to be studied | Risk of false negative results |
| Final biological conclusion for an effect | Should be plausible for the regulatory committee | Necessity of more objective criteria: ex. lengthening of the test | Major risk of subjective interpretation |
Debate on statistical tests
If false positive effects are the concern of Monsanto et al. as well as of Séralini et al. [1, 5], we have underlined that false negative effects may be also amplified by the poor experimental design that curbs enough statistical power. Moreover, the fact that EFSA and HCB asked for a revision of statistical methods [13-14], implies that the accepted regulatory tests are insufficient for the time being, at least at this level. Therefore, as such, the experimental protocols admitted by Monsanto et al., even if advocated by the OECD, do not appear capable of offering statistical proofs of health risks nor of their absence except for highly noticeable health risks. Monsanto's tests should have been rejected by the international committees, if this argument fits. Only early warnings of toxicity can be suggested now, as already indicated [2].
Moreover, it has to be noticed that Séralini et al. [1] and Spiroux de Vendômois et al. [2] did not try to test mathematically, as a whole, whether there had been a “GMO effect” on all the parameters: dosage, duration and sex. It was rather established by sex, duration and dosage, a list of all the parameters differentially expressed between control groups, and groups fed with GMOs. Note that Monsanto (raw data of MON 810, MON 863 and NK 603) do not calculate more, and even less, since the 11% dosage is not considered, if the highest dose of 33% is not different. Contrary to what Monsanto claims, the number and the nature of the signs we emphasize are not really different from the ones used in their reports. However, our revealed signs are classified by organs and take into account the differential effect related to dosage and sex. The difference between Monsanto's conclusions and ours is, again, about the biological interpretation rather than mere statistical points.
However, statistics can be discussed. The use of the ANOVA by Monsanto should not exempt them from doing an assessment of the power of the tests. At no time in the company studies this aspect was highlighted, although it is essential. As soon as a statistical test is used (a Student t test as well as an ANOVA), the result interpretation can only be based on the one on the p-value that allows an estimation of the risk α of a Type I error when the null hypothesis is rejected, and on the other hand on the statistical power 1-β that allows an estimation of the risk β of a Type II error, when the null hypothesis is accepted. This power allows the estimation of the effect size. It is not because a hypothesis is not rejected that it is inevitably true.
We know that this test power depends on the sample size, on the Type I risk α, and on the effect size we want to pick up. In Spiroux de Vendômois et al. [2], the only example of the power calculus in a Student test is just an illustration and not a demonstration of the fact that Monsanto's power tests are weak, even if they are true. The statistical power is never calculated for any of their ANOVA.
As a matter of fact, in their regulatory reports Monsanto et al. use an ANOVA without statistical power for each parameter, both for each sex and duration (week 5 and 14). Even if there are ANOVA calculations, they lead to the implementation of a large number of statistical tests: 4 times the number of parameters (4 = 2 sexes x 2 durations). Also in this case of multiple comparisons, the study of the false positives is not specific to the Student t test, and is not treated in these Monsanto's studies.
Our goal while reviewing Monsanto's data [1, 2] was to make a list of all the differentially expressed parameters. Thus it was essential to suggest a study of the false positives. The FDR method, accepted with Benjamini-Yekutieli's correction, makes it possible to take into account the potential dependency between the parameters. Also, we disagree the argument which claims that what is picked up by this method (but still statistically different) is inevitably obtained “by chance” (developed by some experts or agencies, Le Monde, 10/02/2010). The outcome still needs to be interpreted biologically.
The normal use conditions of the Student t tests are not always satisfied: small samples (equal or fewer than 10) in subchronic tests, normality's rejection by the Shapiro test and non homogeneity of variances. Thus we did apply nonparametric methods. But, even if Monsanto do make it clear (in all the “materials and methods” section of their reports of the raw data) that they do the same, there is not any application of it in their statistical test data: for each parameter, each duration, each sex, only the ANOVA is indicated, even when the data normality or the variance homogeneity is not satisfied. And yet, the physiological interpretation is supposed to be based on these results.
Consensus on statistical effects
Not only does Monsanto admit major statistical differences for some parameters, but most of our results demonstrate huge discrepancies [2]. Significant and non-significant effects correspond to Monsanto data (94-96% of the total, depending on the GMO, deduced from Table E p 723 [2]). In the comment of our study, EFSA [15] admits the presence of visible statistical effects in the results « The significant differences highlighted by Spiroux de Vendômois et al. have all been considered previously by the GMO panel... ». Ergo, the major scientific disagreements are only about the biological interpretations of the statistical effects.
There remains a discussion about the weight curves for MON 863 treated rats which we have published [1], but that were not in the original report of Monsanto. The French committee CGB criticized the failure to take into account the individual variability for each rat in our first paper [1]. However, even when taking that into account, they admitted a significant effect on the female weights' variations of the GMO-fed group in their report on our work, which was used by EFSA, but still disregarded in EFSA's opinion. The authorities should then have reacted to such a serious sign. We certainly consider this as a shortcoming.
Divergent biological interpretations
Therefore, the biological interpretations become crucial after a global statistical consensus. Two possible issues here: either a demonstration of innocuousness (Monsanto et al.'s opinion), or disturbing disruptions that should be followed by longer tests before approvals (in our opinion).
There are at least two arguments used by EFSA [15] and Monsanto et al. in general, to reject our study [2]. First, they said that our data were only presented in percentages and not in absolute values. On the contrary, we indeed published absolute values to give an idea of the crude effects for MON 810, NK 603 [[2], pp 724-5] and MON 863 [[1], p 600]. However, it does not change the results in any way. Secondly, the parameter values in our studies are compared to the controls and references (boxes and double boxes in the tables), contrary to what was claimed in EFSA's official opinion.
In addition, biological interpretations strongly diverge between us and Monsanto et al., on several key-points. We have previously developed this debate, at least in part, in two reviews [5, 10], and suggested improvements in regulatory tests: relative to transparency, length, with a duration corresponding to the lifespan of animals (2 years for the rat in laboratory for instance, as is done for some pesticides and drugs). It has become essential to organize counter-evaluation.
EFSA and other national official committees have accepted to recommend the commercial release and consumption of these GMOs, based on Monsanto's own tests and interpretation. The main differences between their biological conclusions and ours, following statistical differences in biochemical and organ parameters, are listed below:
a) For the record, we would like to state that besides the controversy on the shortcomings of the protocol design outlined above, any early sign of difference should be collected in a table to get a global picture of the animal physiology after GMO consumption. It is really impossible within 90 days, with a single experiment worldwide and such a small number of rats, to get a consistent toxicological picture, as requested by Monsanto et al., and to consider the disturbing signs they indicate. This is a major point, because we are concerned by possible chronic pathologies.
Some effects may not be of major amplitude as yet; however, some are. For instance, the increase of the hearts' weight by 11% in males for NK603, or 40% increase in plasmatic triglycerides in females eating MON 863 (together with a pre-diabetic profile), could be considered as enough to trigger a moratorium. As a matter of fact, Monsanto did not repeat their studies or made them take place over a longer period of time. They even routinely prevent independent reproducibility by refusing to supply the material needed [12] and by blocking access to confidential data, as they did by bringing the case before the Court of Appeal in Germany [1] (however, they lost the case).
b) The statistical differences are often considered by Monsanto et al. between the GM-treated groups and the so-called “historical standards of the species” which are undefined, as the also undefined “normal range”. This approach makes it possible for them to consider larger variations as normal, for subjective reasons. The differences have to be considered first with the closest control group. It is only afterwards that it might be possible to compare it with experimental reference groups (Monsanto et al. did that first) receiving a non-equivalent regimen (for instance where salts or sugars are concerned). For the record, we wish to underline that the reference groups are still too numerous in comparison to the treated rats.
c) The significant effects are taken into account by Monsanto et al. only when they are similar in both sexes. This is not acceptable, since by the current knowledge [5] chronic pathologies, as well as the endocrine disturbances or some cancers, are usually sex-related and not proportional to the carcinogen dose taken over a short period of time. The data specificity of the parameters changing and depending on sex has just been admitted in Monsanto's answer to our study ([16], p. 12).
d) For Monsanto et al., the absence of dose-dependent effects is a reason to overlook the significant differences. This is also unacceptable, simply because, for instance, the potential endocrine disrupting antagonistic actions need to be taken into account [17]. Moreover, it has to be underlined that dose-dependency cannot be studied only with the two-dose study presented to the authorities by Monsanto (11 and 33% of GMO in the diet).
e) Since anatomo-pathological lesions or plasmatic biochemical disruptions could arise long after the beginning of a treatment, it is not necessary to establish correlations between these statistical differences and histopathological findings (overall within three months) to conclude on a disturbing sign, despite what Monsanto et al are claiming. In addition, histological slides and embedded organs are the property of the company, and were not double-checked by official committees or independent authors. We ask for an official counter-analysis, in particular of the male kidneys in these studies, that concentrate more than 43% of all disrupted parameters in a meta-analysis of all published data on commercialized GMOs [10].
We already know that during the MON 863 study, Monsanto highlighted anatomic signs of “chronic progressive nephropathy” on GM-fed male rats' kidneys. However, Monsanto did not see these signs as being noteworthy due to the fact that, according to them, they were well known to occur in old Sprague-Dawley rats. This explanation was then publicly repeated by the president of the CGB, the French evaluation committee for the GMO in question. But these rats were only 5 months old, and still quite young at the end of the experiment. Oddly enough, these anatomo-pathological signs on kidneys were not noticed during the studies on MON 810 and NK603 maize. Yet the rats were the same age and from the same strain.
f) The chemical composition of food/feed is an important indication. However all insecticide toxins/herbicide residues/unintended or unknown metabolites (due for instance to insertional mutagenesis or new metabolites) are not assessed; thus the substantial equivalence with non GM products is not a proof of innocuousness.
g) A bias for biological interpretations could also be seen in the fact that the regulatory toxicological tests were presented and commented for the authorities only by the companies developing industrial products, and this has been the case, for at least the last fifty years. Few studies have been conducted by independent groups such as Malatesta et al. [17-21] who found ultrastructural alterations of hepatic cells of mice that had eaten Roundup- tolerant GMOs. Finamore's study, which focused on an insecticide-producing variety, suggested gut and peripheral immune response to GM crop ingestion [22]. No industry-funded studies suggest potential side effects of GMO consumption. It is a well-known problem; for instance in the bisphenol A controversy, the meta-analysis of all studies performed showed that none of the industry-funded studies showed adverse effects of bisphenol A, whereas 90% of government funded ones showed hazards at various levels and various doses [23].
A proposition for studies conducted independently from companies to tackle this issue has been made to the Council of European Ministers by some of us [24].
Conclusions and perspectives
Controversy on biological interpretations is a usual way of advancement in science. It would however have been beneficial for the acceptance of biotechnologies by the public at large, to close this scientific debate by longer, more detailed, and transparent toxicological tests on GMOs, and in particular twenty years ago when the most widely grown GMOs were still experimental.
We wish to reassert that our work does not claim to demonstrate the chronic toxicity of the GMOs in question, especially since it is based on the data originating from insufficient tests that were accepted by regulatory authorities and Monsanto et al., a fact for which we are not in any way responsible. For the regulatory authorities, as well as Monsanto et al, these tests prove chronic innocuousness for mammalian and human public health. And they claim it is not essential to demonstrate the GMOs innocuousness. This again raises the same issues and consequences. We have revealed the inefficiency both of these tests and of their statistical analysis and biological interpretations, for the various reasons detailed above. However, some of the in vivo 90-day tests are not performed any longer today to get worldwide commercial authorizations, especially for GMO with “stacked events” (i.e., producing one or several insecticides and tolerating one or two herbicides), and this is even more seriously inadequate since the so-called “cocktail effects” are not taken into consideration.
The same controversy took place (February 2010) in India, in relation to the authorization process for a transgenic eggplant that produces a new Bt insecticide. This authorization was based on three-month tests on three mammals and other animals for shorter times, which presented significant biological effects after this GM consumption [10, 25]. The same arguments were used in the debate in India. But in this case, the government decided to take the time to study chronic health effects, following our expertise, and therefore to implement a moratorium [26].
In the present case, we wish to underline that the commercial GMOs in question contain pesticide residues, some of which have been demonstrated as human cellular endocrine disruptors at levels around 1000 times below their presence in some GM feed [27]. Such Roundup residues are present in more than 80% of edible cultivated GMOs. This does not exclude other possible effects.
As a conclusion, we call for the promotion of transparent, independent and reproducible health studies for new commercial products, the dissemination of which implies consequences on a large scale. Lifetime studies for laboratory animals consuming GMOs must be performed, by contrast to what is done today, like the two-year long tests on rats for some pesticides or some drugs. Such tests could be associated to transgenerational, reproductive or endocrine research studies. And moreover, shortcomings in experimental designs may raise major questions on other chemical authorizations.
Acknowledgements
We are grateful to the administrative and scientific councils of CRIIGEN for fruitful discussions and to Herrade Hemmerdinger for the English revision of the manuscript.
References
1. Séralini GE, Cellier D, Spiroux de Vendômois J. New analysis of a rat feeding study with a genetically modified maize reveals signs of hepatorenal toxicity. Arch Environ Contam Toxicol. 2007;52:596-602
2. Spiroux de Vendômois J, Roullier F, Cellier D. et al. A comparison of the effects of three GM corn varieties on mammalian health. Int J Biol Sci. 2009;5:706-26
3. Domingo JL. Health risks of GM foods: many opinions but few data. Science. 2000;288:1748-9
4. Domingo JL. Toxicity studies of genetically modified plants: a review of the published literature. Crit Rev Food Sci Nutr. 2007;47:721-33
5. Séralini GE, Spiroux de Vendomois J, Cellier D. et al. How subchronic and chronic health effects can be neglected for GMOs, pesticides or chemicals. Int J Biol Sci. 2009;5:438-43
6. Clive J. Global Status of Commercialized Biotech/GM Crops: 2009. ISAAA Brief. 2009;41:1-44
7. Rosati A, Bogani P, Santarlasci A. et al. Characterisation of 3' transgene insertion site and derived mrnas in mon810 yieldgard maize. Plant Mol Biol. 2008;67:271-81
8. Manetti C, Bianchetti C, Casciani L. et al. A metabonomic study of transgenic maize (zea mays) seeds revealed variations in osmolytes and branched amino acids. J Exp Bot. 2006;57:2613-25
9. Natarajan SS, Xu C, Cregan P. et al. Utility of proteomics techniques for assessing protein expression. Regul Toxicol Pharmacol. 2009;54:32-6
10. Séralini GE, Mesnage R, Clair E. et al. Genetically modified crops consumption at large scale: possible negative health impacts due to holes in assessment. Environ Sci Pollut Res. submitted
11. Support to Gilles-Eric Séralini and his co-authors in respect of scientific controversy and counter evaluation. CRIIGEN. http://www.criigen.org/SiteEn/index.php?option=com_content&task=blogcategory&id=80&Itemid=119
12. Waltz E. GM crops: Battlefield. Nature. 2009;461:27-32
13. EFSA. Statistical considerations for the safety evaluation of GMOs. EFSA Journal. 2010;8:1250-59
14. HCB. Avis sur le dossier EFSA/GMO/NL/2005/22. France: HCB, French Council of Biotechnologies. 2009
15. GMO Panel deliberations on the paper by de Vendômois et al, 2009. EFSA. http://www.efsa.europa.eu/en/events/event/gmo100127.htm
16. Monsanto. Response: de Vendômois (Séralini) et al. US: Monsanto. 2009
17. Moslemi S, Seralini GE. Estrogens and Breast Cancer: Aromatase and Activity Disruption. Trends in Breast Cancer Research. 2005:101-127
18. Malatesta M, Caporaloni C, Gavaudon S. et al. Ultrastructural morphometrical and immunocytochemical analyses of hepatocyte nuclei from mice fed on genetically modified soybean. Cell Struct Function. 2002;27:173-180
19. Malatesta M, Biggiogera M, Manuali E. et al. Fine structural analyses of pancreatic acinar cell nuclei from mice fed on genetically modified soybean. Eur J Histochem. 2003;47:385-388
20. Malatesta M, Baldelli B, Battistelli S. et al. Reversibility of hepatocyte nuclear modifications in mice fed on genetically modified soybean. Eur J Histochem. 2005;49:237-42
21. Vecchio L, Cisterna B, Malatesta M. et al. Ultrastructural analysis of testes from mice fed on genetically modified soybean. Eur J Histochem. 2004;48:449-454
22. Finamore A, Roselli M, Britti S. et al. Intestinal and peripheral immune response to MON810 maize ingestion in weaning and old mice. J Agric Food Chem. 2008;56:11533-9
23. Vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol a shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926-33
24. 2008. Lepage Reports: CRIIGEN. http://www.criigen.org/SiteEn/index.php?option=com_content&task=blogcategory&id=77&Itemid=116
25. Jayaraman KS. Transgenic aubergine put on ice. Nature. 2009;461:1041
26. 2009. Effets on health and environment of transgenic (or GM) Bt Brinjal: CRIIGEN. http://www.criigen.org/SiteFr//images/stories/Dossiers/Divers/btbrinjal-ges_%200109.pdf
27. Gasnier C, Dumont C, Benachour N. et al. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology. 2009;262:184-91
Author contact
![]() Corresponding author: Prof. Gilles-Eric Séralini, Institute of Biology, EA 2608, University of Caen, Esplanade de la Paix, 14032 Caen Cedex, France. Phone +33 2 31 56 56 84; Fax +33 2 56 53 20; Email: criigenfr.
Corresponding author: Prof. Gilles-Eric Séralini, Institute of Biology, EA 2608, University of Caen, Esplanade de la Paix, 14032 Caen Cedex, France. Phone +33 2 31 56 56 84; Fax +33 2 56 53 20; Email: criigenfr.

 Global reach, higher impact
Global reach, higher impact