10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2012; 8(5):650-662. doi:10.7150/ijbs.3897 This issue Cite
Research Paper
Selection of Peptide Inhibitor to Matrix Metalloproteinase-2 Using Phage Display and Its Effects on Pancreatic Cancer Cell lines PANC-1 and CFPAC-1
Key Laboratory of Human Functional Genomics of Jiangsu Province, Clinical Diabetes Centre of Jiangsu Province, the Department of Biochemistry and Molecular Biology, Nanjing Medical University, Nanjing 210029, Jiangsu, China.
* The two authors contributed equally to this work.
Received 2011-12-1; Accepted 2012-4-22; Published 2012-5-5
Abstract
Despite tremendous advances in cancer treatment and survival rates, pancreatic cancer remains one of the most deadly afflictions and the fourth leading cause of cancer deaths in the world. Matrix Metalloproteinases (MMPs) are thought to be involved in cancer progression. Matrix metalloproteinase (MMP)-2 is known to play a pivotal role in tumor invasion, metastasis and angiogenesis, and validated to be the anticancer target. Inhibition of MMP-2 activity is able to reduce the cancer cell invasion and suppress tumor growth in vivo. Two novel peptides, M204C4 and M205C4, which could specially inhibit MMP-2 activity, were identified by a phage display library screening. We showed that M204C4 and M205C4 inhibited the activity of MMP-2 in a dose dependent manner in vitro. Two peptides reduced MMP-2 mediated invasion of the pancreatic cancer cell lines PANC-1 and CFPAC-1, but not affected the expression and release of MMP-2. Furthermore, these two peptides could suppress tumor growth in vivo. Our results indicated that two peptides selected by phase display technology may be used as anticancer drugs in the future.
Keywords: Pancreatic cancer, MMP-2, Phage display, Peptide, M204C4, M205C4.
INTRODUCTION
For most tumors, intravasation can be facilitated by molecular changes that promote the ability of carcinoma cells to cross the pericyte and endothelial cell barriers that form the walls of microvessels. Matrix metalloproteinases (MMPs), as a family of endopeptidases, play a major role in such processes, in which the extracellular matrix degradation related to cancer cell invasion, metastasis and angiogenesis is induced. According to the specificity and structure of MMPs substrates, all members of the MMP gene family can be classified into subgroups of collagenases, stromelysins, gelatinases, membrane-type MMPs, and other MMPs (1). The two MMPs most closely correlated with metastatic potential are the 72kDa MMP-2 (gelatinase A) and the 92kDa MMP-9 (gelatinase B). They are secreted as zymogen and then cleaved into their active form (2). Experiments with mice lacking MMP-2 suggest that MMP-2 plays an important role in human cancers. First, the number of metastasis colonies in the lung of MMP-2 knockout mice in experimental metastasis decreased significantly after i.v. injection of Lewis lung carcinoma or melanoma cells (3). Furthermore, the angiogenesis and growth of tumors were reduced in MMP-2-deficient mice compared with wildtype mice (4). Deficiency in MMP-2 resulted in suppression of tumor-induced angiogenesis in a dorsal air sac assay and reduction of support of glioma angioarchitecture (5). The importance of stromal MMP-2 in neovascularization was also confirmed in different angiogenic models, including aortic ring assay, retinal angiogenesis, and choroidal neovascularization (4, 6-7). Additional works had shown that MMP-2 expression was correlated with tumor metastasis in various cancers, especially pancreatic cancer and other aggressive malignant tumors (8-10). And the increased capacity of MMP-2 to synergistically promote tumor cell intravasation was tied to their ability to stimulate neoangiogenesis and the formation of leaky blood vessels. Hence, it suggested that MMP-2 played an important role in human cancers and was a prominent predictor of poor prognosis.
In recent years, numerous MMP inhibitors have been tested in different clinical trials, especially MMP-2 inhibitors. In view of MMP-2 involvement in various diseases, inhibition of specific MMPs up-regulation may improve clinical symptoms of patients (11-12). However, these molecules displayed serious side effects and only a few were allowed to attend phase III clinical trials. In addition, Matrix metalloproteinases (MMPs) mediated the homeostasis of the extracellular environment (12). It was unrecommendable to suppress the expression of MMPs blindly. These reasons might partially account for the failure of MMP inhibitors in clinical trials. Until now, specific inhibitors of gelatinases have not been available in clinic. Recently, synthetic peptide inhibitors based on MMPs structure have been thought as a hot spot of study on specific inhibitors.The MMPs are initially synthesized as inactive zymogens with pro-peptide which must be removed from pro-peptide domain before the enzyme is active (13). Therefore, peptide drugs can inhibit extracellular MMPs activation directly, but not affected MMPs intracellular expression. And peptide research on drug design and discovery is one of the most promising fields in the development of new drugs. Compared to small molecule compounds, peptide drugs offer various advantages, such as high specificity and low toxicity.
In the present study, we use phage display technology for the high-throughput screening of protein-peptide to select specific inhibitors of MMP-2, and then obtain two novel peptides that inhibit MMP-2 activity and suppress MMP-2 mediated pancreatic cancer cell invasion in vitro, and attenuated the growth of solid tumor in nude mice. These data indicate that peptides selected from phage display library have potential effects as anticancer agents.
EXPERIMENTAL PROCEDURES
Materials
Recombinant human MMP-2 enzyme was purchased from R&D company, USA. Ph.D.-12TM phage display peptide library kit containing E. coli host strain ER2738 was purchased from New England BioLabs, UK. MMP-2 fluorimetric drug discovery kit was purchased from Biomol, USA. The antibody against MMP-2 and anti-CD31/PECAM-1 were purchased from Cell Signal Technology, USA. QCMTM 24-well cell invasion assay kit was purchased from Chemicon, USA. Dulbecco's modified Eagle media (DMEM) and fetal bovine serum (FBS) were purchased from Gibco, USA. PGE2 was purchased from Sigma-Aldrich Chemical, USA.
Screening phage display library against MMP-2
MMP-2 was immobilized on the NUNC wells. The MMP-2-coated well was blocked with 0.5% BSA/NaHCO3 for 2 hours at room temperature. For selection, phages (1.5×1015 pfu/ml) from the linear 12-amino acid random peptide phage display library diluted in 0.1%BSA/ NaHCO3 were added into MMP-2-coated wells and incubated overnight at 4ºC. After extensive washing with TBST (TBS+0.1%(v/v)Tween-20), the bound phages were eluted with a low pH buffer (0.2M glycine-HCl, pH 2.2). Recovered phages were amplified using competent Escherichia coli cells and then subjected to two subsequent rounds of selection on MMP-2-coated wells. Phage binding was quantified by counting the phage titer in eluted aliquots as protocol described. Phages from selected clones were sequenced at Invitrogen Life Technologies (Shanghai, China). Peptide sequences were determined according to the inserted sequence in the phage genome. Peptides used in our assays were synthesized in the Huachen Limited company (Xian province, China).
Fluorescent MMP-2 activity assay
Protease activity of MMP-2 was measured by using a self-quenching fluorogenic peptide substrate (Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2) as previously referenced (14). Mca fluorescence is quenched by the Dpa group until cleavage by MMPs at the Gly-Leu bond separates the two moieties. The assays are performed in a convenient 96-well microplate format. The kit is useful to screen inhibitors of MMP-2, a potential therapeutic target. The compound NNGH(i) is also included as a prototypic control inhibitor. To analyze inhibition, MMP-2 was pre-incubated with selected phage clones or a range of concentrations of synthesized peptide for 45 minutes at 37ºC in reaction buffer to allow inhibitor/enzyme interaction, and then the substrate was added. Finally, enzymatic activity was determined by continuous monitoring with a fluorescence spectrophotometer at excitation and emission wavelengths of 328 nm and 393 nm, respectively. Drug concentrations inhibiting MMP-2 activity by 50% (IC50) was calculated from relative reactive curve using the median-effect principle.
Cell culture
Human pancreatic cancer cell lines, PANC-1 and CFPAC-1 were obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in DMEM containing 100 μg/ml penicillin, 100 μg/ml streptomycin in the presence of 10% fetal bovine serum (FBS) at 37ºC in a humidified CO2 incubator and subcultured every 2-3 days. For the experiments described below, cells were trypsinized and harvested on reaching 80% to 90% confluency.
Zymography
Zymography was performed as described previously (15). Cells (1×105) were seeded onto wells of 24-well plates and allowed to adhere in the presence of serum. Media subsequently were replaced by 0.5 ml of serum-free medium per well containing PGE2 or not. After 24 hours of incubation, the conditioned media were collected. After collection, the media were spun at 800 × g for 3 min at 4ºC to remove cell debris. The supernatant was frozen at -20ºC for later zymography. Zymography was carried out as described previously. In brief, 25μL of the conditioned medium for each sample was subjected to 10% SDS/PAGE 1mg/mL gelatin incorporated into the gel mixture. Following the electrophoresis, the gels were soaked in 2.5% triton X100 for 40 minutes, to remove SDS, and transferred to a bath containing 50mmol/L Tris (pH8.0), 5 mmol/L CaCl2, and 2μmol/L ZnCl2 at 37ºC for 20 hours. The gel then was stained with 0.1% coomassie blue in 45% methanol and 10% acetic acid. Experiments were repeated three times.
Cell invasion assay
Invasion of pancreatic cancer cells in vitro was measured by the invasion of cells through Matrigel-coated trans-well inserts as protocol described (16). Briefly, thaw gel overnight at 2-8 °C before use, which was diluted up to two-fold with DMEM containing different concentrations of peptides onto the Matrigel-coated inserts, and in the lower wells DMEM with 5% FBS was added as the chemoattractant. After 48h incubation with PGE2, the noninvasive cells on the upper surface of the membrane were removed with a cotton swab. Cells that solubilized the Matrigel, passed through the 8 μm pores of the transwell, and attached to the lower surface of membrane were fixed with 10% formaldehyde, stained with 0.1% crystal violet solution, and photographed. For quantification, the transwell membranes were detached and solubilized in 10% acetic acid solution (90% deionized water and 10% glacial acetic acid), and the intensity of the colored solution was quantified by spectrophotometrical analysis at 590 nm. The intensity of the untreated control transwell was set to 100%. Each experiment performed with two replicate transwells was repeated three times independently.
Animal Models
Cell suspension of PANC-1 or CFPAC-1 human pancreatic cancer cells was prepared (108 cells/ml). A total of 0.1 ml of the cell suspension was injected (s.c.) into the right upper flank of 6~8-week-old female nude BALB/c mice (20~22g, n=6). When tumors mass was established (~50 mm in diameter), each 100µl of PBS containing synthetic peptides (100µM, 100ul each injection) was injected (s.c.) into tumor-surrounding tissues of a nude mouse once a week for 4 weeks. Tumor measurements and animal behavior observations were recorded daily. The short (r) and long (l) diameters of the tumors were measured and the tumor volume of each was calculated as (r2× 1)/2. 30 days after the treatment, all mice were sacrificed and then the size and weight of tumors were recorded. Inhibitive rate of tumor was calculated by using the formula: inhibitive rate of tumor (%) = (1- average tumor weight in treated group/average tumor weight in control) × 100%. Tumor volume and weight were presented as the mean ±SD. Animal studies were carried out according to the Guidelines for Animal Experiments drawn up by the Committee for Animal Experiments of the National Cancer Center.
Immunohistochemistry
Subcutaneous tumors in all groups were harvested and processed for immunostaining as previously described (17). Tumors that had been frozen in OCT were sectioned in 8-μm slices, mounted on positively charged slides, and air-dried for 30 min. Tissue sections were then fixed in cold acetone followed by 1:1 acetone/chloroform and acetone and then washed with PBS. Specimens were then incubated with 3% H2O2in methanol at room temperature to block endogenous peroxidase. After 12min, these sections were washed three times with PBS (pH 7.5), and incubated for 20 min at room temperature in a protein-blocking solution consisting of PBS supplemented with 1% serum. Anti-CD31/PECAM-1 rat monoclonal antibody, which recognizes platelet-endothelial cell adhesion molecule-1 (PECAM-1) on endothelial cells, and anti-MMP-2 rabbit monoclonal antibody were diluted 1:800 in protein-blocking solution and applied to the sections, which were incubated overnight at 4°C. Negative controls were stained with nonspecific immunoglobulin G (IgG) and the appropriate horseradish peroxidase-conjugated secondary antibody was used. All sections were counterstained with Gill's hematoxylin. The immunostained tumor sections were examined by bright field microscopy.
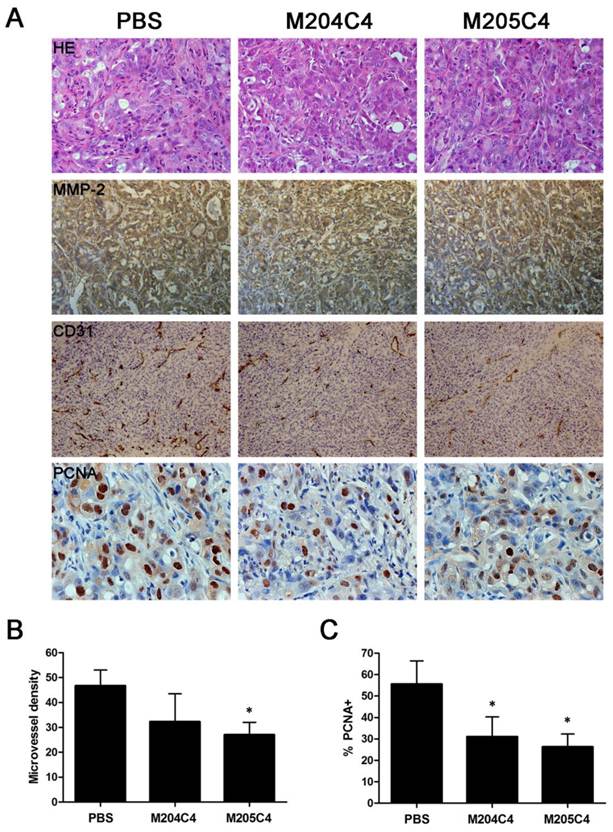
Microvascular density (MVD) was assessed by immunohistochemical analysis with antibodies to the endothelial marker CD31 and determined according to the method (18). Briefly, the immunostained sections were initially screened at low magnifications (40× and 100×) to identify hot spots, which are the areas of highest neovascularization. Any brown stained endothelial cell or endothelial cell cluster that was clearly separated from adjacent microvessels, tumor cells, and other connective tissue elements was considered as a single, countable microvessel. Within the hot spot area, the stained microvessels were counted in a single high-power (200×) field, and the average vessel count in 3 hot spots was considered as the value of MVD. All counts were performed by three investigators in a blinded manner. Microvessel counts were compared between the observers and discrepant results were reassessed. The consensus was used as the final score for analysis. Cell proliferation was determined by using anti-PCNA immunoassaying. The nuclei resulting in brown staining as PCNA-positive indicated proliferative nuclei. The number of cells was counted in three random fields from three different slides at 200× magnification. An average for the percentage of PCNA-positive cells was taken over these fields. The sections were observed by two pathologists in a blinded manner.
Pharmacokinetic
Before animal experiments, the 8~10-week-old female ICR mice (24~26g) were fasted overnight. Two peptides in PBS were administrated through tail vein injection (20mg/kg, n=6). Animals were sacrificed at different time points (from 0.2 min to 30min). Blood was centrifuged (3000rpm, 5min, 4°C) and plasma was collected. The amount of peptides in plasma was quantified by UPLC-DAD-MS as references described (19-21).
Chromatographic Conditions and Instrumentation
Ultra-performance liquid chromatography-tandem mass spectrometry. Analysis was performed on the ACQUITY UPLC H-Class System (Waters, Milford, MA), which consists of a quaternary pump solvent management system, an online degasser, an autosampler, and an Acquity photodiode array detector. An Acquity UPLC BEH (50mm ×2.1mm, 1.7 μm) column was applied for all analyses. The raw data were acquired and processed with MassLynx 4.1 software. The mobile phase was composed of A ((0.1% formic acid in Acetonitrile, v/v) and B (0.1% formic acid in H2O, v/v) with a gradient elution: 0-0.2min, 5%A; 0.2-1min, 5-100% A; 1-4 min, 100% A; 4-5 min, 100-5% A; 5-8 min, 5% A. The flow rate of the mobile phase was 0.3ml/min, and the column temperature was maintained at 30°C. Detection wavelength was set at 218nm for peptides. MS analysis was performed on a TQD mass spectrometer connected to the Acquity UPLC instrument via an electrospray ionization interface (ESI). High-purity nitrogen was used as the nebulizer and auxiliary gas; argon was utilized as the collision gas. The TQD mass spectrometer was operated in positive ion mode with a capillary voltage of 3.9kV, a sampling cone voltage of 81V, a extractor voltage of 3V, a RFLens voltage of 0.1V, a desolvation temperature of 250 °C, a source temperature of 150°C, a collision energy of 6 V, and the mass range was set from m/z 400 to 2000.
Statistical analysis
Differences between groups were analyzed using two-sided t test and ANOVA with P < 0.05 considered statistically significant.
RESULTS
Identification of Peptide Sequences That Bind to Recombinant Human MMP-2 Enzyme
In this study, the phage display technique was used to identify peptide sequences that bound to MMP-2. A random 12-mer peptide phage display library composed of ~2.7×109 independent peptide sequences was screened for binding to immobilized recombinant human MMP-2 enzyme. In the experiment, the MMP-2-bound phages were recovered by elution with acidic buffer (0.2M glycine-HCl, pH2.2). A marked relative enrichment in the MMP-2-binding phage was detected, and bovine serum albumin (BSA) was used as a control target protein without enrichment after three round biopannings (data not shown).
Selected Phages on MMP-2 Enzyme Activity in vitro
At the end of the biopanning, a total of 22 clones were isolated. DNA from these phages was sequenced, and the predicted 12-mer peptides were aligned (Fig.1). To further determine the effects of selected phage clones on the MMP-2 activity, the selected phage clones were amplified, and the titer was detected via blue/white plaque assay. The 20μl of selected phages at the concentration of 5.0×1012 pfu/ml, were added to the reaction solution as procedure described above, respectively. All clones could inhibit the activity of MMP-2 enzyme with different effects (Fig.1). To further determine peptide potency, M201 to M219 were identified for the following screening.
Synthetic Peptides inhibit MMP-2 Enzyme Activity in vitro
19 linear peptides binding to MMP-2 were synthesized. The inhibiting effects of these peptides were analyzed in fluorescent MMP-2 activity assay. It was disappointed that the expected effect was not observed from all identified peptides. In order to conserve the original right conformation displayed on the phage surface according to the protocol suggestion, all polypeptides containing GGGS at the C-terminal end were synthesized respectively. Finally, M204C4 and M205C4 efficiently inhibited MMP-2-mediated self-quenching fluorogenic peptide substrate degradation in a dose-dependent manner (Fig.2) with a median effective inhibiting concentration (IC50) of 78.0 and 38.8 nM. Therefore, M204C4 and M205C4 (shown in Table 1) were chosen as drug candidates for our studies.
Characterization of peptide candidates, M204C4 and M205C4.
| Sequence | Mol Wt | Purity | |
|---|---|---|---|
| M204C4 | HWWQWPSSLQLRGGGS | 1881.06 | >95% |
| M205C4 | HNWTRWLLHPDRGGGS | 1889.68 | >95% |
Effects of different phage clones on the MMP-2 activity. The MMP-2 Fluorescent Assay was used to measure protease activity of MMP-2 with a quenched fluorescent peptide. Measure fluorescence at Ex/Em = 328/393 nm in a micro-plate reader.C: control; I: MMP-2 inhibitor NNGH; M201-M219: phage clones which have different peptide sequences. Black: different phage clones which have the same sequence. Values are the means±SD (n=3) of three individual experiments.
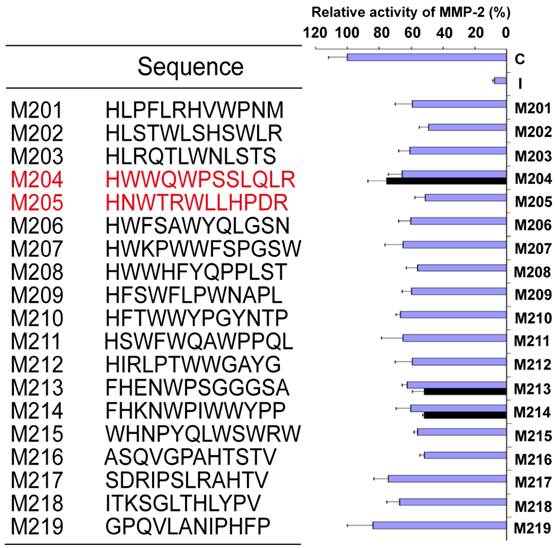
M204C4 and M205C4 inhibit MMP-2 Enzyme Activity in vitro. To evaluate the effect of M204C4 and M205C4 on MMP-2 Enzyme Activity, the MMP-2 Fluorescent Assay was used. After recombinant human MMP-2 was incubated with the indicated concentrations of peptides for 45 minutes at 37°C, and then the substrate was added. The plates were read continuously in a fluorescence microplate reader (Molecular Devices) over 30 min, at Ex/Em = 328/393. Values are the means±SD (n=3) of three individual experiments.
Synthetic peptides reduce pancreatic cancer cellular invasion
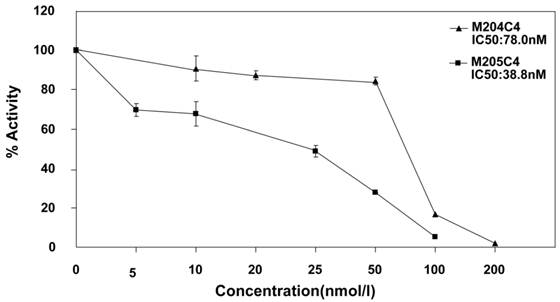
It is well known that MMP-2 is involved in cancer cellular invasion. In order to confirm the inhibiting effects of synthetic peptide M204C4 and M205C4 on cancer cellular invasion, the pancreatic cancer cell lines PANC-1and CFPAC-1 were applied for this assay. To observe the invasion, the pancreatic cancer cells were treated with PGE2 to promote the expression and activation of MMP-2 (22-23). As documented, PGE2 increased MMP-2 extracellular expression but not MMP-9 (Fig.3A) and pancreatic cancer cells invasion significantly compared with control subjects (Fig.3B). To examine the effect of peptides on the invasion of pancreatic cancer cell lines, matrigel invasion assays were conducted in the presence of M204C4 or M205C4. As shown in Fig.4B, the PGE2-stimulated pancreatic cancer cells invaded the bottom of the wells as detected by 0.1% crystal violet solution staining. And PGE2-mediated invasion through Matrigel was inhibited by synthetic peptides in a dose-dependent manner, respectively (Fig.3B and 3C). These results suggest that M204C4 and M205C4 have the inhibitory effects on pancreatic cancer cellular invasion through Matrigel.
Synthetic peptides attenuate the rate of tumor growth in vivo
Human xenograft tumor model in nude mice was utilized for efficacy studies (24). Treatments were initiated when the size of the tumor was approximately 0.05 cm3. During the treatment with peptides, the growth of tumors originating from human pancreatic cancer cell lines PANC-1 and CFPAC-1 was inhibited (Fig.4). As shown in Fig.4B, tumors (weight) inhibitive rate of M205C4 rose to more than 50%, compared to controls. Therefore, the therapeutic effect of M205C4 was stronger than that of M204C4 at the same dose, and the failure of solid tumor formation from PANC-1 cells in nude mice treated with M205C4 was also observed (Fig.4A). Recent studies have shown that gelatinases also play a role in the angiogenesis and affect the formation of the new blood vessels nurturing the tumor (25-26). To evaluate whether the suppression of tumor growth is associated with angiogenesis, we examined the expression of endothelial marker CD31, MMP-2 and Proliferating Cell Nuclear Antigen (PCNA) in PANC-1 tumors by immunostaining (Fig.5A). Microvascular density (MVD) was determined by counting the number of the microvessels per high-power field (hpf) in section with an antibody reactive to CD31 (Fig.5B). The mean MVD that had a significant positive correlation with tumor size was reduced in tumors by peptides. A marked decrease of PCNA-positive cells was observed in the groups treated with peptides (Fig.5C). It indicated that two synthetic peptides could suppress the proliferation of tumor cells.In addition; it was notable that no significant difference in MMP-2 expression was found in three groups (Fig.5A). And after incubation with peptides in vitro for 48h in PANC-1 and CFPAC-1 cell lines, M204C4 and M205C4 did not reduce MMP-2 mRNA and protein levels (data not shown). The results suggested that two peptides possibly reduced tumors proliferation by inhibiting MMP-2 activation to prevent the angiogenesis of tumors.
Effects of M204C4 and M205C4 on cellular invasion in the pancreatic cancer cell lines. After incubation with PGE2 for 24h without peptides, MMP-2 activity in media was examined by gelatin zymography (A). In transwell assays, cells were treated with indicated concentrations of peptides and PGE2 (5μM) for 48h. Representative pictures in all groups (B). Spectrophotometric quantification of transwell assays (C), stained cells were dissolved in 10% acetic acid, and the absorbance was read on a spectrophotometer set at 590 nm. The values are expressed as percentage relative to the untreated control (PEG2).Values are the means±SD (n=3) of three individual experiments. *p<0.05, **p<0.01 vs control (PANC-1 group with PEG2); #p<0.05, ##p<0.01 vs control (CFPAC-1 group with PEG2).
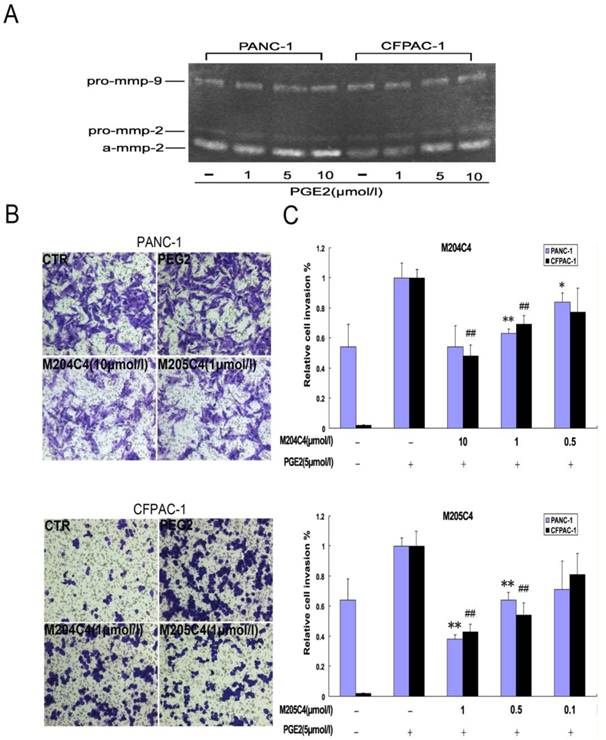
The effect of M204C4 and M205C4 on the growth of human xenograft tumor model in mice. All mice (n=6/group) with tumor were injected s.c. with peptides (100µM) or PBS weekly. Macroscopic appearance of solid tumors after ending the treatment (A). Tumor weight and inhibition rate (B), W: average tumor weight in each group; R: inhibitive rate of tumor. Tumor growth curve(C). Tumor volume = (width2 × length)/2; Inhibitive rate of tumor (%) = (1- average tumor weight in treated group/average tumor weight in control) × 100%. Data are mean ± SD.
Pharmacokinetic Disposition
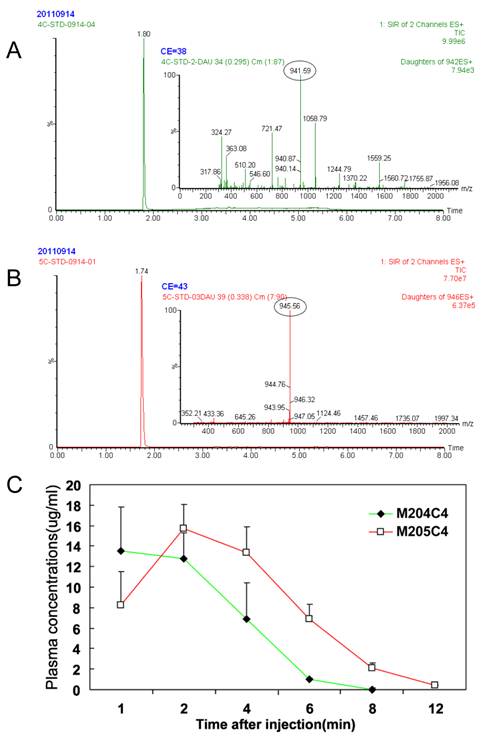
A major drawback of the use of peptides as anticancer drugs is their rapid metabolism by peptidase in vivo (27-29). To understand their metablism in vivo, the pharmacokinetics of two peptides were priorly evaluated in mice. The results are summarized in Fig.6. After i.v. administration, peptides in plasma were not detected nearly about ten minutes. And no significant difference in the parameters was observed between two peptides. For this reason, we suggested that M204C4 and M205C4 should be used locally but not systematically in the drug administration.
Immunohistochemical analysis in cancer tissues. PANC-1 tumors were analyzed by hematoxylin-eosin staining and immunohistochemical staining for the expression of MMP-2, CD31 and PCNA (A). Microvessel density of the tumor tissues was assessed by CD31 immunohistochemical analysis(B). Anti-PCNA staining in tumor sections from each group, the percentage of PCNA-positive cells per 200× field was accounted for as described in the methods(C). Data are mean ± SD. *p<0.05, vs control (PBS).
Metabolic stabilities in vivo: Plasma pharmacokinetics of peptides (20mg/kg) i.v. in ICR mice (n=6). Reversed-phase UPLC separation of peptides with total ion current (TIC) chromatogram of samples extracted from the supernatant of protein precipitated mice plasma, M204C4(A) and M205C4(B). The concentration-time profile for the UPLC-MS analysis of two peptides in vivo(C). Data are mean ± SD.
DISCUSSION
Pancreatic cancer is the fourth most common cause of cancer death in the world. For all stages combined, the 1- and 5-year relative survival rates are 25% and 6%, respectively. Clinical drugs did not show strong therapeutic efficacy combined with a lack of side effects.And according to the American Cancer Society, there are no established guidelines to prevent pancreatic cancer.
MMP-2 (gelatinase A), which is able to degrade type IV collagen, is considered to be especially important in the degradation of the extracellular matrix that is associated with the malignant behavior of tumor cells (30-31). There are no exceptions in pancreatic cancer, MMP-2 shows high levels of expression and positively associates with tumor progression in clinical and experimental models (32). Therefore, MMP-2 has been considered as a potential therapeutic target for pancreatic cancer and other aggressive malignant tumors. Since small molecules with non-specific inhibition present inevitable side effects in patients, researchers have focused on biochemical drugs. Bevacizumab (Avastin) working as a humanized monoclonal antibody, which prevented the formation of new blood vessels by targeting and inhibiting the function of VEGF, was approved by the U.S. Food and Drug Administration (FDA) for cancers in 2004 (33). And Avastin, as a positive control of angiogenesis, was applied for our experiments in vivo (See Supplementary Material). Although MMP-2 inhibitors are not used for clinical application, many studies about biological inhibitors of MMPs have been reported (34-36).
Phage display is a powerful method to screen in vitro random libraries of peptides and to select short sequences with high affinity and specificity for biological targets. Using this technique Koivunen et al (37) obtained several cyclic peptides containing the amino acids sequence histidine-tryptophane-glycine-phenylalanine (HWGF) as selective inhibitors of MMP-2 and MMP-9. In our study, two consecutive biopanning of a 12-mer peptide phage display library against MMP-2 immobilized on Nunc ELISA wells resulted in identification of 22 different phage clones capable of binding to immobilized MMP-2. All these clones can inhibit the MMP-2 enzyme activities at the same titer with different potency (Fig.1). Chemically synthetic peptides M201 to M219 were tested for their ability to inhibit MMP-2 activity. Unfortunately, the inhibiting effects were very mild (data not shown). In order to conserve their construction as the original shape on the surface of phage, the amino acid sequence GGGS was added to the C-terminal end of 19 peptides according to the protocol suggestions (38), and M204C4 and M205C4 showed higher binding potencies to recombinant human MMP-2 enzyme and markedly inhibited the activity of recombinant human MMP-2 enzyme in vitro compared with other peptides. To measure cell invasion assay, Prostaglandin E2 (PGE2) promoted cellular invasive potential by inducing matrix metalloproteinase-2 expression and activity, and PGE2 -mediated invasion was reversed to different extents by M204C4 and M205C4 (Fig.3C). Moreover, M209C4 and M214C4 that were randomly chosen for invasion assay had no effect on inhibition of cellular invasion (data not shown). For these reasons, it suggested that selected peptides might be used as MMP-2 specific inhibitors for the following studies.
As reported, rapid degradation of peptides limits its application in the clinic, and these two peptides had such problems (Fig.6) and were not used systematically. In order to obtain high local concentration, we injected two peptides directly into tumor basement, which was prone to deliver peptides directly into tumors to be available against the growth of tumors in vivo. And peptides were put into diluted Matrigel without mixing with culture medium for invasion assay. These processes were effective to avoid rapid degradation of peptides.The expression of matrix metalloproteinase-2 (MMP-2) has been linked to tumor invasion, angiogenesis, and metastasis. (39-40). Angiogenesis is required for tumor growth and metastasis and constitutes an important point in the control of cancer progression (41-42). The relationship of MMP-2 expression and angiogenesis has been previously reported and its biological and therapeutic implications remained the focus of investigations (43-44). MMP-2 might facilitate endothelial cell invasion by removing matrix barriers or initiating signaling pathways that promote or cooperate the angiogenic phenotype (45), so that tumor cells could get enough oxygen and other essential nutrients.
In our studies, two peptides inhibited the growth of tumors from human pancreatic cancer cell lines effectively. MVD of the tumor exposed to the peptides was reduced in a dose dependent manner, especially in the high dose group. Meanwhile, PCNA-positive cells were decreased in treated groups, and such change resulted from deficient supplies for tumor growth. And no metastic colonies were found at tissues sections in all groups. It suggested that M204C4 and M205C4 might reduce tumor growth by inhibition of tumor angiogenesis. And similar to the results in vitro, M205C4 had a stronger inhibitory effect on the tumor than M204C4. In fact, we have synthesized C-to-N terminally cyclized peptides (c-M204C4 and c-M205C4), Backbone cyclization can help to stabilize peptides against degradation, but the ability of inhibition to MMP-2 was declined obviously in MMP-2 Fluorescent Assay, with IC50 of 1495.3 nM and 2309.7nM. And cyclized peptides had on significant effect on the growth of tumors from PANC-1 cell line (See Supplementary Material). Therefore, the effect of peptides on tumor growth was potentially associated with inhibition of MMPs-mediated tumorigenesis and metastasis. The hypotheses were supported by the results that peptides did not induce the apoptosis and cycle arrest of pancreatic cancer cells in vitro (data not shown).
A key consideration in the design of antimetastatic therapeutic agents is the fact that the decreased significant numbers of disseminated tumor cells should been observed in patient's blood, bone marrow, and distant organ sites upon initial presentation in the oncology clinic. However, it was hard to exactly estimate the unitary effect of peptides on tumor metastasis with some drawbacks, such as rapid degradation of peptides and animal model of tumor metastasis. In order to investigate the anti-metastatic efficacy of new therapeutic agents, orthotopic models of pancreatic cancer had been constructed in nude mice (39, 46). After improving the pharmacokinetic profile by constitutional modification to raise retention time in mice, it will be able to evaluate the therapeutic effects of peptides in vivo for further studies.
In conclusion, this study describes the discovery of new peptides, M204C4 and M205C4, which are effective inhibitors of MMP-2. These molecules may have future utility in clinical applications for treating cancer and other diseases associated with MMP-2 up-regulation.
Supplementary Material
Figure S1: Cell suspension of PANC-1 human pancreatic cancer cells was prepared (108 cells/ml).
Acknowledgements
This work was supported by grants from the National Science and Technology Major Project (2009ZX09103-608) and the Natural Science Foundation of Jiangsu Province (BK2003003).
Competing Interests
All authors declare that there are no conflicts of interest and agree with the contents of themanuscript for publication and support open access publishing to allow unlimited access and high publicity of published paper.
References
1. Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43(Suppl):S42-51
2. Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161-174
3. Itoh T, Tanioka M, Yoshida H. et al. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58(5):1048-1051
4. Berglin L, Sarman S, van der Ploeg I. et al. Reduced choroidal neovascular membrane formation in matrix metalloproteinase-2-deficient mice. Invest Ophthalmol Vis Sci. 2003;44(1):403-408
5. Takahashi M, Fukami S, Iwata N. et al. In vivo glioma growth requires host-derived matrix metalloproteinase 2 for maintenance of angioarchitecture. Pharmacol Res. 2002;46(2):155-163
6. Kato T, Kure T, Chang JH. et al. Diminished corneal angiogenesis in gelatinase A-deficient mice. FEBS Lett. 2001;508(2):187-190
7. Ohno-Matsui K, Uetama T, Yoshida T. et al. Reduced retinal angiogenesis in MMP-2-deficient mice. Invest Ophthalmol Vis Sci. 2003;44(12):5370-5375
8. Pellikainen JM, Ropponen KM, Kataja VV. et al. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res. 2004;10(22):7621-7628
9. Atkinson JM, Pennington CJ, Martin SW. et al. Membrane type matrix metalloproteinases (MMPs) show differential expression in non-small cell lung cancer (NSCLC) compared to normal lung: correlation of MMP-14 mRNA expression and proteolytic activity. Eur J Cancer. 2007;43(11):1764-1771
10. Ellenrieder V, Alber B, Lacher U. et al. Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int J Cancer. 2000;85(1):14-20
11. Turpeenniemi-Hujanen T. Gelatinases (MMP-2 and -9) and their natural inhibitors as prognostic indicators in solid cancers. Biochimie. 2005;87(3-4):287-297
12. Overall CM, Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6(3):227-239
13. Pei D, Kang TQi H. Cysteine array matrix metalloproteinase (CA-MMP)/MMP-23 is a type II transmembrane matrix metalloproteinase regulated by a single cleavage for both secretion and activation. J Biol Chem. 2000;275(43):33988-33997
14. Wallon UM, Overall CM. The hemopexin-like domain (C domain) of human gelatinase A (matrix metalloproteinase-2) requires Ca2+ for fibronectin and heparin binding. Binding properties of recombinant gelatinase A C domain to extracellular matrix and basement membrane components. J Biol Chem. 1997;272(11):7473-7481
15. Mazzoni A, Mannello F, Tay FR. et al. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. J Dent Res. 2007;86(5):436-440
16. M D, Brooks SA. In Vitro Invasion Assay Using Matrigel(R). Methods Mol Med. 2001;58:61-70
17. Saad RS, Kordunsky L, Liu YL. et al. Lymphatic microvessel density as prognostic marker in colorectal cancer. Mod Pathol. 2006;19(10):1317-1323
18. Su D, Deng H, Zhao X. et al. Targeting CD24 for treatment of ovarian cancer by short hairpin RNA. Cytotherapy. 2009;11(5):642-652
19. Yang JZ, Bastian KC, Moore RD. et al. Quantitative analysis of a model opioid peptide and its cyclic prodrugs in rat plasma using high-performance liquid chromatography with fluorescence and tandem mass spectrometric detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;780(2):269-281
20. Guo S, Duan JA, Tang YP. et al. Characterization of nucleosides and nucleobases in fruits of Ziziphus jujuba by UPLC-DAD-MS. J Agric Food Chem. 2010;58(19):10774-10780
21. Su S, Guo J, Duan JA. et al. Ultra-performance liquid chromatography-tandem mass spectrometry analysis of the bioactive components and their metabolites of Shaofu Zhuyu decoction active extract in rat plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(3-4):355-362
22. Zahner G, Harendza S, Muller E. et al. Prostaglandin E2 stimulates expression of matrix metalloproteinase 2 in cultured rat mesangial cells. Kidney Int. 1997;51(4):1116-1123
23. Ito H, Duxbury M, Benoit E. et al. Prostaglandin E2 enhances pancreatic cancer invasiveness through an Ets-1-dependent induction of matrix metalloproteinase-2. Cancer Res. 2004;64(20):7439-7446
24. Schultz RM, Merriman RL, Toth JE. et al. Evaluation of new anticancer agents against the MIA PaCa-2 and PANC-1 human pancreatic carcinoma xenografts. Oncol Res. 1993;5(6-7):223-228
25. Belotti D, Paganoni P, Manenti L. et al. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63(17):5224-5229
26. Munaut C, Noel A, Hougrand O. et al. Vascular endothelial growth factor expression correlates with matrix metalloproteinases MT1-MMP, MMP-2 and MMP-9 in human glioblastomas. Int J Cancer. 2003;106(6):848-855
27. Wearley LL. Recent progress in protein and peptide delivery by noninvasive routes. Crit Rev Ther Drug Carrier Syst. 1991;8(4):331-394
28. Adessi C, Frossard MJ, Boissard C. et al. Pharmacological profiles of peptide drug candidates for the treatment of Alzheimer's disease. J Biol Chem. 2003;278(16):13905-13911
29. Werle M, Bernkop-Schnurch A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids. 2006;30(4):351-367
30. Somiari SB, Somiari RI, Heckman CM. et al. Circulating MMP2 and MMP9 in breast cancer -- potential role in classification of patients into low risk, high risk, benign disease and breast cancer categories. Int J Cancer. 2006;119(6):1403-1411
31. Stahtea XN, Roussidis AE, Kanakis I. et al. Imatinib inhibits colorectal cancer cell growth and suppresses stromal-induced growth stimulation, MT1-MMP expression and pro-MMP2 activation. Int J Cancer. 2007;121(12):2808-2814
32. Bloomston M, Zervos EERosemurgy AS 2nd. Matrix metalloproteinases and their role in pancreatic cancer: a review of preclinical studies and clinical trials. Ann Surg Oncol. 2002;9(7):668-674
33. Prager GW, Lackner EM, Krauth MT. et al. Targeting of VEGF-dependent transendothelial migration of cancer cells by bevacizumab. Mol Oncol. 2010;4(2):150-160
34. Hesek D, Toth M, Meroueh SO. et al. Design and characterization of a metalloproteinase inhibitor-tethered resin for the detection of active MMPs in biological samples. Chem Biol. 2006;13(4):379-386
35. Xu X, Mikhailova M, Chen Z. et al. Peptide from the C-terminal domain of tissue inhibitor of matrix metalloproteinases-2 (TIMP-2) inhibits membrane activation of matrix metalloproteinase-2 (MMP-2). Matrix Biol. 2011;30(7-8):404-412
36. Hu J, Dubois V, Chaltin P. et al. Inhibition of lethal endotoxin shock with an L-pyridylalanine containing metalloproteinase inhibitor selected by high-throughput screening of a new peptide library. Comb Chem High Throughput Screen. 2006;9(8):599-611
37. Koivunen E, Arap W, Valtanen H. et al. Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol. 1999;17(8):768-774
38. Desjobert C, de Soultrait VR, Faure A. et al. Identification by phage display selection of a short peptide able to inhibit only the strand transfer reaction catalyzed by human immunodeficiency virus type 1 integrase. Biochemistry. 2004;43(41):13097-13105
39. Stetler-Stevenson WG. The role of matrix metalloproteinases in tumor invasion, metastasis, and angiogenesis. Surg Oncol Clin N Am. 2001;10(2):383-392
40. John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res. 2001;7(1):14-23
41. Fang JH, Zhou HC, Zeng C. et al. MicroRNA-29b suppresses tumor angiogenesis, invasion and metastasis by regulating MMP-2 expression. Hepatology. 2011Nov;54(5):1729-40
42. Kargiotis O, Chetty C, Gondi CS. et al. Adenovirus-mediated transfer of siRNA against MMP-2 mRNA results in impaired invasion and tumor-induced angiogenesis, induces apoptosis in vitro and inhibits tumor growth in vivo in glioblastoma. Oncogene. 2008;27(35):4830-4840
43. Masson V. [Roles of serine proteases and matrix metalloproteinases in tumor invasion and angiogenesis]. Bull Mem Acad R Med Belg. 2006;161(5):320-326
44. Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9(2):267-285
45. Stetler-Stevenson WG. Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest. 1999;103(9):1237-1241
46. Katz MH, Takimoto S, Spivack D. et al. An imageable highly metastatic orthotopic red fluorescent protein model of pancreatic cancer. Clin Exp Metastasis. 2004;21(1):7-12
Author contact
![]() Corresponding author: Xiao Han, Telephone: +86 25 86862733, Fax: +86 25 86862731, E-mail: hanxiaoedu.cn.
Corresponding author: Xiao Han, Telephone: +86 25 86862733, Fax: +86 25 86862731, E-mail: hanxiaoedu.cn.

 Global reach, higher impact
Global reach, higher impact