Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2012; 8(10):1345-1362. doi:10.7150/ijbs.4699 This issue Cite
Research Paper
Protective Effect of Grape Seed Proanthocyanidins against Liver Ischemic Reperfusion Injury: Particularly in Diet-Induced Obese Mice
Key Laboratory of Medical Cell Biology, China Medical University, Shen Yang City, LiaoNing Province, China, 110001.
Received 2012-6-5; Accepted 2012-10-18; Published 2012-10-27
Abstract
Background: Hepatic ischemia and reperfusion injury (IRI) is a major complication in liver surgery, and hepatic steatosis is a primary factor aggravating cellular injury during IRI. Both pro-inflammatory cytokines and reactive oxygen species (ROS) are key mediators of hepatic IRI. Ischemic preconditioning (IpreC), remote ischemia preconditioning (RIPC) and ischemic postconditioning (IpostC) have offered protections on hepatic IRI, but all these methods have their own shortcomings. Grape seed proanthocyanidins (GSP) has a broad spectrum of pharmacological properties against oxidative stress. Thus, GSP has potential protective effects against hepatic IRI.
Methods: C57BL/6 mice suffering 30mins hepatic ischemia process were sacrificed after 1h reperfusion to build murine warm hepatic IRI model. The mice were injected GSP intraperitoneally 10, 20, 40mg/kg/day for 3 weeks as pharmacological preconditioning. Obese mice fed with high-fat diet for 24 weeks before used. Three pathways related to IRI, including ROS elimination, pro-inflammatory cytokines release and hypoxia responses were examined.
Results: Our data show that GSP could significantly reduce hepatic IRI by protecting hepatocyte function and increasing the activity of ROS scavengers, as well as decreasing cytokines levels. At the same time, GSP also enhance the hypoxia tolerance response. Combined GSP and postconditioning can provided synergistic protection. In the obese mice suffering hepatic IRI group, GSP was more effective than postconditioning on protecting liver against IRI, and the combined strategy was obviously superior to the solo treatment.
Conclusion: GSP could protect liver against IRI: particularly in high-fat diet induced obese mice. GSP used as pharmacological preconditioning and combined with other protocols have huge potential to be used in clinical.
Keywords: Grape seed proanthocyanidins, postconditioning, preconditioning, ischemia, reperfusion injury.
INTRODUCTION
Hepatic Ischemia and Reperfusion Injury (IRI) occurs in a number of clinical hepatic surgeries, particularly in liver transplantation and hepatic resection [1, 2], and hepatic steatosis is a primary factor increasing the extent of cellular injury incurred during IRI. The increasing use of steatotic livers for transplantation induces higher graft nonfunction rates, increased retransplantation rates and increased recipient mortality [3].
Both pro-inflammatory cytokines and reactive oxygen species (ROS) play important part in liver IRI [4, 5]. ROS generated by Kupffer cells and hepatocytes can lead to direct damage on endothelial cells (ECs) and hepatocytes. The use of antioxidants to reduce oxidant injury during ischemia and reperfusion possess is postulated to improve hepatic recovery from dysfunction. The pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNFα) and interleukin-1 beta (IL-1β), released by activated Kupffer cells shortly after reperfusion, have dual role: over range expression of TNFα and IL-1β can induce more production of cytokines and granulocyte colony-stimulating factor, which enhance Kupffer cells activation and promote neutrophil infiltration in microcirculation of liver [6, 7], then aggravate hepatic sterile inflammation after ischemia and reperfusion. One the other hand, TNFα and IL-1 are indispensable for liver regeneration [8].
At the same time, cells can spontaneously respond to injury by activating defensive mechanisms of themselves. Among them, hypoxia inducible factor-1 alpha (HIF-1α) is a key nuclear factor act as the oxygen sensor. It plays an important role in the pathogenesis and development of various hypoxic/ischemic diseases [9, 10]. HIF-1α can also regulate the expression of genes such as heme oxygenase-1 (HO-1) and vascular endothelial growth factor (VEGF) [11-14], which can effect on endothelial cells (ECs) proliferation, migration, and cell organization during recovery phases after hepatic microvascular dysfunction by promoting the secretion of growth and survival factors. The degradation of HIF-1α is mediated by proline hydroxylases (PHDs), which can catalyse the hydroxylation of specific proline residues in the HIF-1α subunits. The activation of PHDs is oxygen-dependent.
The molecular mechanisms underlying IRI are multifactorial, and many investigations have their focus on the intervention of hepatic IRI [15-17]. Ischemic preconditioning (IpreC), defined as brief periods of ischemia and reperfusion before sustained ischemia, and remote ischemia preconditioning (RIPC), which showed several brief ischemia of one organ confers protection on distant organs without direct stress or trauma to blood vessels of the organ, have been reported for reducing myocardial and renal injury [18- 20]. Recent studies have proved that several brief cycles of IR at the onset of sustained reperfusion after ischemia, termed ischemic postconditioning (IpostC), provided effective cardioprotection on IRI. But most of these studies were focused on hearts and little was known about whether IpostC could offer protections on hepatic IRI and the mechanism [21, 22].
Proanthocyanidins, mainly presenting in the seeds of grapes, has been reported to possess a broad spectrum of biological, pharmacological and therapeutic activities against free radicals and oxidative stress [23, 24]. Vitro studies reported that proanthocyanidins were potent scavengers of peroxyl and hydroxyl radicals that were generated in the reperfusion myocardium after ischemia [25]. In addition to the ability to scavenge ROS, GSP has the ability to stimulate NO production in a dose dependent manner, which is a relatively stable free radical and acts as a signaling molecule in diverse physiological and pathological pathways. In our recent study [26], we found that GSP could further improve the oxidation resistance in combined pre- and postconditioning groups, and its implementation was more convenient particularly in combined remote preconditioning and postconditioning group. Therefore, this study was designed to further investigate the effects of GSP on steatotic livers against IRI. The protective effects of GSP as pharmacological preconditioning, compared and combined with postconditioning strategies, were evaluated on the warm hepatic IRI model of standard chow diet and high-fat diet mice. The results show that all these strategies were effective on protecting liver against IRI, and providing the preservation of hepatic function. Combined GSP and postconditioning could offer additional protection over the individual treatment. In the high diet-induced hepatic steatosis with IRI group, which hepatocellular injury more severe than that in standard chow diet mice with IRI group, GSP was more effective than postconditioning on alleviating oxidative damage and cytokines release, as well as promoting hypoxia tolerance. In addition, the combined strategy was obviously superior to the solo treatment on protecting obese mice against hepatic IRI.
All these results provided experimental evidences to evaluate the protective effects of GSP/ postconditioning strategies against hepatic IRI in details and might guide future research on anti-IRI. GSP used as pharmacological ischemic preconditioning and combine with other treatment protocols might have huge potential to be used in clinical surgery.
MATERIALS AND METHODS
Mice
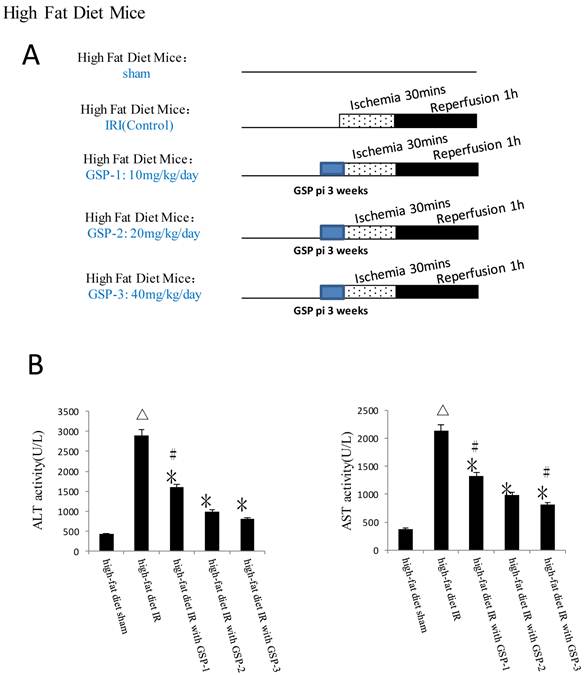
Male inbred wild-type (WT) C57BL/6 mice weighing 20-25gm were used. The animals were housed in China medical university animal facility under specific pathogen-free conditions and received humane care according to National Institutes of Health guidelines. The mice received GSP preconditioning were injected GSP intraperitoneally 10, 20, 40mg/kg/day for 3 weeks before using, while the control group were injected the same volume normal saline. Obese murine model induced by high-fat diet for 24 weeks before used. Obesity was induced by allowing access to a high-fat diet containing (42% kcal from fat, Dyet #112734; Research Diet, New Brunswick, NJ) and the standard chow diet containing 13.5% kcal from fat.
Mouse Hepatic IRI Model
An established mouse warm hepatic IRI model [22, 27] was used with modification. Briefly, mice were injected with heparin (100U/kg), and an atraumatic clip was used to interrupt the arterial and portal venous blood supply to the cephalad lobes of the liver. After 30 minutes of ischemia, the clip was removed, initiating hepatic reperfusion. Mice were sacrificed after 1h to analyze the acute phase of liver IRI [28]. GSP (purity>95%) provided by Tianjin Jianfeng Natural Product R&D, Co. Ltd and was diluted by distilled water before using.
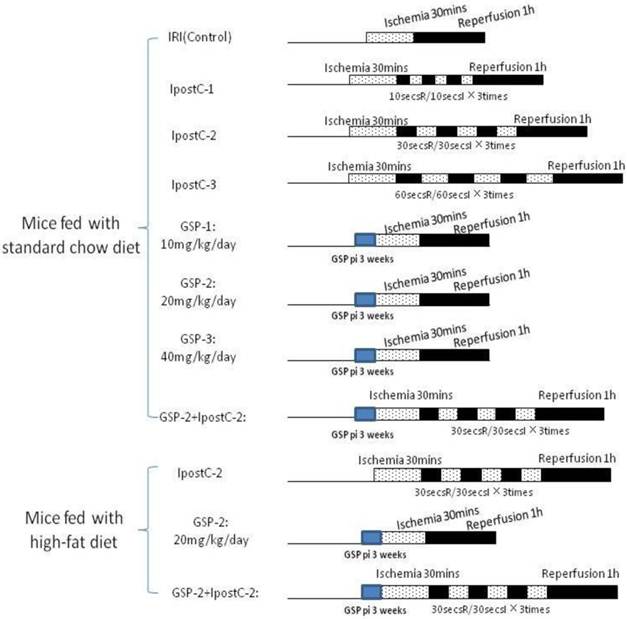
Groupings were list as below (the details were described in Figure 1):
Sham group: mice (standard chow diet and high-fat diet) subjected to the surgical procedures except for liver I/R, and maintained under anesthesia for an equivalent duration.
Sham GSP preconditioning group: mice (standard chow diet and high-fat diet) injected GSP intraperitoneally 10, 20, 40mg/kg/day for 3 weeks as pharmacological preconditioning and subjected to the surgical procedures except for liver I/R consistent with sham group.
Whole experimental grouping. We illustrated the whole experimental processes briefly in this figure: in part one, we detected the protective effect of postconditioning and GSP on IRI livers in the mice fed with standard chow diet; in the second part, we assessed the role of GSP combining with postconditioning in protecting IRI livers of the mice fed with high-fat diet.
Hepatocellular Injury Assay
Aspartase aminotransferase (AST) and alanine aminotransferase (ALT) in serum were used as indicators of hepatocyte function. Blood samples were centrifuged immediately at 8000 × g for 10 minutes. Serum enzyme levels were measured with a Bayer 1650 automatic biochemical analyzer.
Detection of the total antioxidative capacity and the activities of antioxidative enzymes in hepatocellular
Tissue homogenate was made in PBS buffer and centrifuged at 1800× g 10min at 40C to precipitate the insoluble material. The supernatant were used for the followed assay. The total antioxidative capacity (T-AOC), malondialdehyde (MDA) and the activity of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-PX), Nitric Oxide Synthase (NOS) were measured by a commercial testing kit (NanJing JianCheng Bioengineering Institute) according to the manual.
RNA extraction and Quantitative Real-Time PCR
Total RNA was isolated from mouse liver by RNeasy kit (Qiagen) according to the manufacturer's instructions. cDNA was synthesis as suggested by TaKaRa RNA PCR Kit (AMV) Ver3.0. Real-Time PCR was performed using ABI7300 system with the Fermentas SYBR Green PCR pre mix. Primers are listed below:
TNFα: 5'GTGGGTGAGGAGCACGTAGT3'; and 5'CCCCAAAGGGATGAGAAGTT3'
IL-1: 5'CCTACCTTTGTTCCGCACAT3'; and 5'AAGTGTGTCATCGTGGTGGA3'
HIF-1α:5'CTGCCACTGCCACCACAACT3'; and 5'CAGGAAAGAGAGTCATAGAA3'
PHD: 5'CCAGCACCTC GGGGTCTGCC3' ; and 5'GACTCGTCCC TGTCCCACCT3'
VEGF: 5'GTCGGGTGGGAGTTATGG3' ; and 5'GACGAGGTTTGAGGAAGG3'
ELISA and Immunoblotting
Livers were removed from the mice, washed with PBS, and weighed. Livers were homogenized in cold PBS buffer and centrifuged at 1800× g 10mins at 40C. The supernatant were used in the ELISA assay. TNFα and IL-1 levels were determined by the EBIOSCIENCE Mouse ELISA Ready-SET-Go kit. For immunoblotting assay, mouse liver lysate was made in RIPA buffer and separated on SDS-page gel. The HIF-1 α, PHD, VEGF and GAPDH antibodies were bought from Abcam Ltd (USA).
Statistical Analysis
Statistical analyses were performed by SPSS programs. All data are expressed as means ± standard deviation for ten mice. Differences between experimental groups were analyzed by One-Way analysis of variance (ANOVA). Post hoc testing was performed for inter-group comparisons by the Least Significance Difference (L.S.D.) test. All differences were considered statistically significant at a P value <0.05.
RESULTS
The prevalence of hepatic steatosis is coincident with increases seen in obesity, which increase the sensibility of liver to IRI. GSP, a class of polyphenols, is a potent antioxidant that can inhibit fatty acid synthase. In this research, we investigated the effect of GSP on the hepatic fatty content and the marker of hepatocyte injury, then we compared the role of GSP and postconditiong on the sensitivity of liver to IRI between standard chow diet and high-fat diet mice groups.
Body and liver weights
The average body weights and liver weights (gm) of the mice in different groups were shown in Table 1.
The comparation of body and liver weight in mice under different diet (mean±SD, n=10).
| standard chow diet | standard chow diet add | high-fat diet | high-fat diet add | |
|---|---|---|---|---|
| GSP preconditioning | GSP preconditioning | |||
| Body weight | 29.76±1.35 | 23.59±0.92 * | 45.8±1.87▲ | 39.6±1.44△ |
| Liver weight | 1.02±0.07 | 0.97±0.04 * | 1.86±0.11▲ | 1.02±0.09△ |
*: compared with mice fed with standard chow diet, P<0.05.
▲: compared with mice fed with standard chow diet, P<0.01.
△: compared with mice fed with high-fat diet, P<0.05.
The comparation of liver triglyceride in mice under different diet (mean±SD, n=10).
| Group | n | liver triglyceride (mg/100mg liver tissue) |
|---|---|---|
| Standard chow diet | 10 | 8.65±0.83 |
| standard chow diet add GSP preconditioning | 10 | 6.79±0.54 * |
| high-fat diet | 10 | 28.45±2.06 ▲ |
| high-fat diet add GSP preconditioning | 10 | 11.36±1.12 △ |
*: compared with mice fed with standard chow diet, P<0.05.
▲: compared with mice fed with standard chow diet, P<0.01.
△: compared with mice fed with high-fat diet, P<0.01.
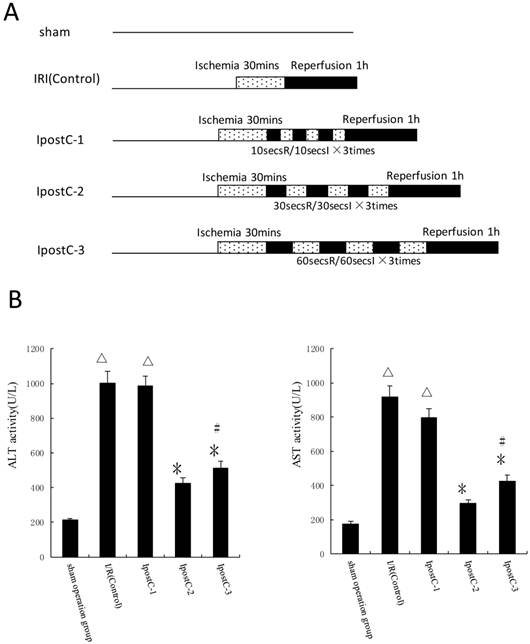
Ischemic postconditioning reduce hepatic ischemic reperfusion injury
In the first set of experiments, ALT and AST activities were used as markers to evaluate the effects of various ischemic postconditioning strategies against hepatic ischemic reperfusion injury. Compared to IRI control group, the plasma activities of ALT and AST significantly decreased in IpostC-2 and IpostC-3 groups (P<0.01), decreased more obviously in group IpostC-2, as shown in Figure 2B. Due to the significant protection function of IpostC-2, IpostC-2 was used in the combined GSP procedure in the following experiments.
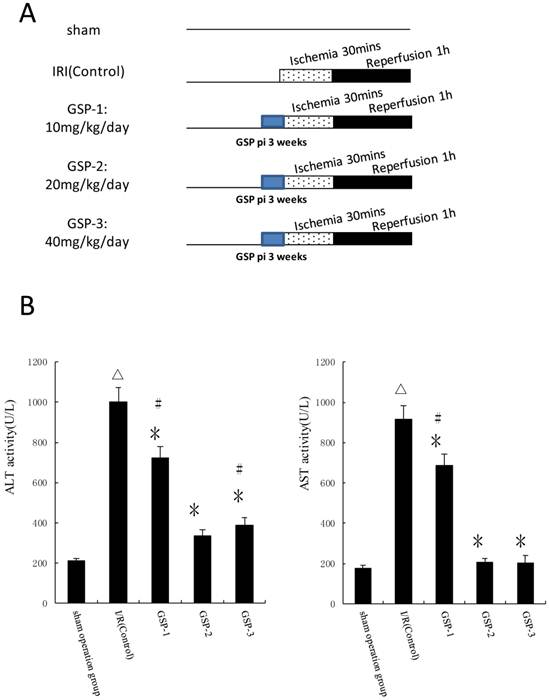
GSP preconditioning reduce hepatic ischemic reperfusion injury
We chose different doses of GSP as pharmacological preconditioning and evaluated its protection against hepatic ischemic reperfusion injury by examining ALT and AST activities in plasma. Compared to IRI control group, the plasma activities of ALT and AST significantly decreased in GSP-2 and GSP-3 groups (P<0.01) as shown in Figure 3B. From ecomomic and reducing damage angles, intraperitoneal injection GSP 20mg/kg/day preconditioning protocol was used to combine with IpostC-2.
Effects of ischemic postconditioning on the activities of ALT and AST in serum. (A) Experimental protocols of grouping and ischemic postconditioning groups. Sham: mice fed standard chow diet subjected to the surgical procedures except for liver I/R, and maintained under anesthesia for an equivalent duration. IRI control: 30 minutes of ischemia followed with 1 hour reperfusion. Dotted areas represent the periods of ischemia; Black areas represent the periods of perfusion. IpostC procedure: 10, 30, 60 seconds of reperfusion followed by 10, 30, 60 seconds of reocclusion for three times at the onset of reperfusion. (B) ALT and AST activities in mice serum were measured at 1 hour after perfusion. (mean±SD, n=10/group). Activities of these two enzymes are significantly lower in serum in IpostC-2 and IpostC-3 groups. (△indicate P<0.01 when compared with sham group; *indicate P<0.01 when compared with IRI control group; #indicate P<0.05 when compared with IpostC-2 group).
Effects of GSP on the activities of ALT and AST in serum. (A) Experimental protocols of grouping and different doses of GSP injection. Sham: mice fed standard chow diet subjected to the surgical procedures except for liver I/R, and maintained under anesthesia for an equivalent duration. IRI control: 30 minutes of ischemia followed with 1 hour reperfusion. Dotted areas represent the periods of ischemia; Black areas represent the periods of perfusion. GSP procedures: mice injected GSP intraperitoneally 10, 20, 40mg/kg/day for 3 weeks as pharmacological preconditioning. (B) ALT and AST activities in mice serum were measured at 1 hour after perfusion. (mean±SD, n=10/group). Activities of these two enzymes are significantly lower in serum in GSP-2 and GSP-3 groups. (△indicate P<0.01 when compared with sham group; *indicate P<0.01 when compared with IRI control group; #indicate P<0.05 when compared with GSP-2 group).
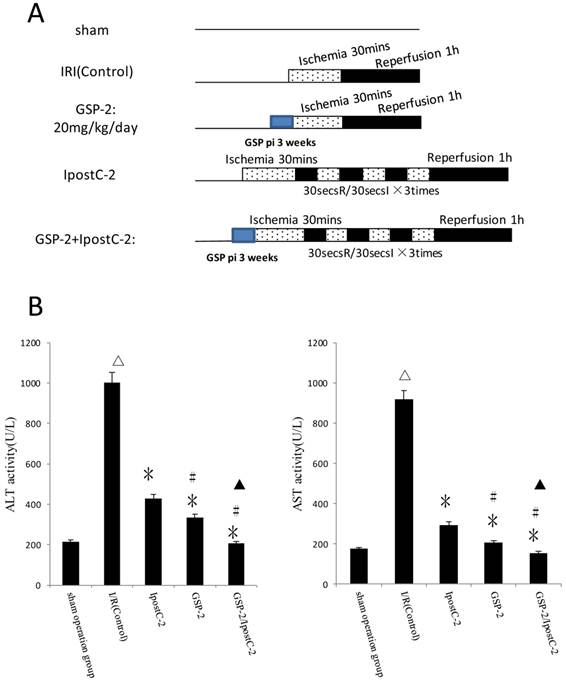
Combination of GSP and postditioning procedure provide synergistic protection against the ischemia and reperfusion injury
Our hypothesis was that combination of GSP and postconditoning procedure would show more effective protection effects against hepatic ischemic reperfusion injury than the individual treatments. We designed protocols measured and compared the plasma activities of ALT and AST in serum from different experimental groups as shown in Figure 4B. The activities ALT and AST were significantly decreased in GSP-2/ IpostC-2 group (P<0.05) compared to solo treatment groups.
TNF-α and IL-1β levels reduced in GSP preconditioning and postconditioning groups
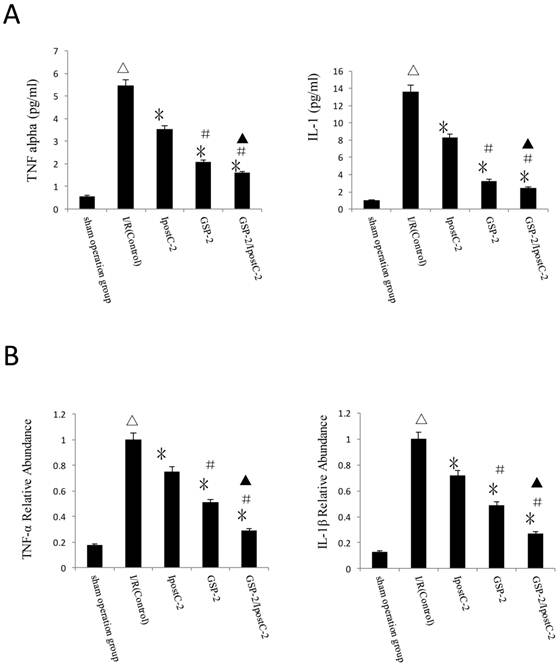
Compared to IR control samples, the concentration of TNF-α and IL-1β in serum were dramatically decreased in IpostC-2, GSP-2 and GSP-2/ IpostC-2 groups (P<0.01) (Figure 5A). And the mRNA expression reduction of pro-inflammatory cytokines was shown in Figure 5B. The combination of GSP-2 and IpostC-2 can further reduce TNFα and IL-1β level in serum than individual procedures (P<0.05).
Effects of combining GSP-2 with IpostC-2 against liver IRI. ALT and AST activities in mice serum were measured at 1 hour after perfusion. (mean±SD, n=10/group). Activities of these two enzymes are decreased in IpostC-2 and GSP-2 group. (△indicate P<0.01 when compared with sham group; *indicate P<0.01 when compared with IRI control group; #indicate P<0.05 when compared with IpostC-2 group). Combined GSP and postconditioning can provided synergistic protection. (▲indicate P<0.05 when compared with GSP-2 group).
Cytokines level in mouse serum after IpostC and GSP treatment. (A) TNF-α and IL-1β level were measured in mice serum. (mean±SD, n=10/group). (△indicate P<0.01 when compared with sham group; *indicate P<0.01 when compared with control group; #indicate P<0.01 when compared with IpostC-2 group; ▲indicate P<0.05 when compared with GSP-2 group). (B) TNF-α and IL-1β mRNA abundance in serum were quantified by RT-PCR. (mean±SD, n=10/group). Results obtained with postconditioning, GSP groups and GSP/Ipost were normalized to results obtained with control group, which were given a value of 1. (△indicate P<0.01 when compared with sham group; *indicate P<0.01 when compared with control group; #indicate P<0.01 when compared with IpostC-2 group; ▲indicate P<0.05 when compared with GSP-2 group).
Response of antioxidative pathways in GSP preconditioning and ischemic postconditioning during hepatic IRI process
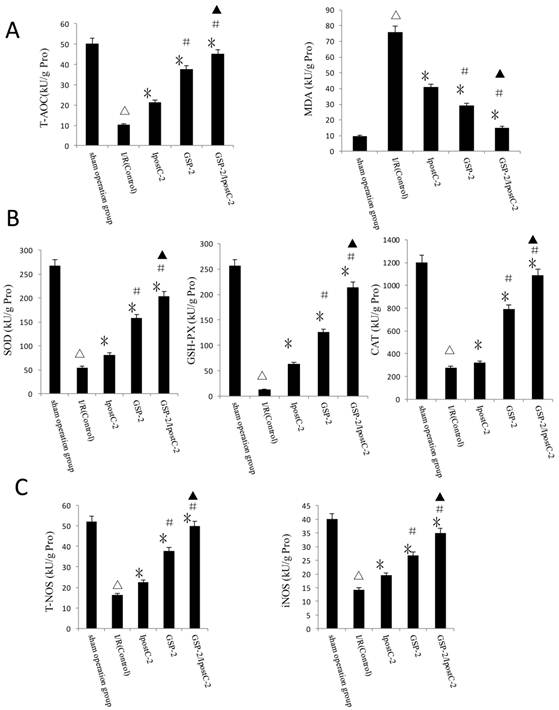
Another important factor causing ischemia reperfusion injury is ROS mediated cell apoptosis. To investigate the role of ROS in IR injury, we first measured the total antioxidative capacity (T-AOC) and malondialdehyde (MDA) level that indicate the overall level of the tissue oxidative stress. As shown in Figure 6A, either individual or combination protocols could decrease oxidative stress by reducing MDA content and elevating T-AOC content. To quantify the contribution of different ROS scavengers, the activity of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-PX), total nitric oxide synthase (T-NOS) and iNOS were measured in the mouse serum 1 hour after perfusion. The activities of all these enzymes were elevated in IpostC-2, GSP-2 and GSP-2/ IpostC-2 groups compared to IR control (P<0.01) (Figure 6B and C). The combined procedure could increase the activity of all these ROS scavengers and antioxidants more obviously (P<0.05).
Effect of GSP preconditioning and ischemic postconditioning on hypoxia response
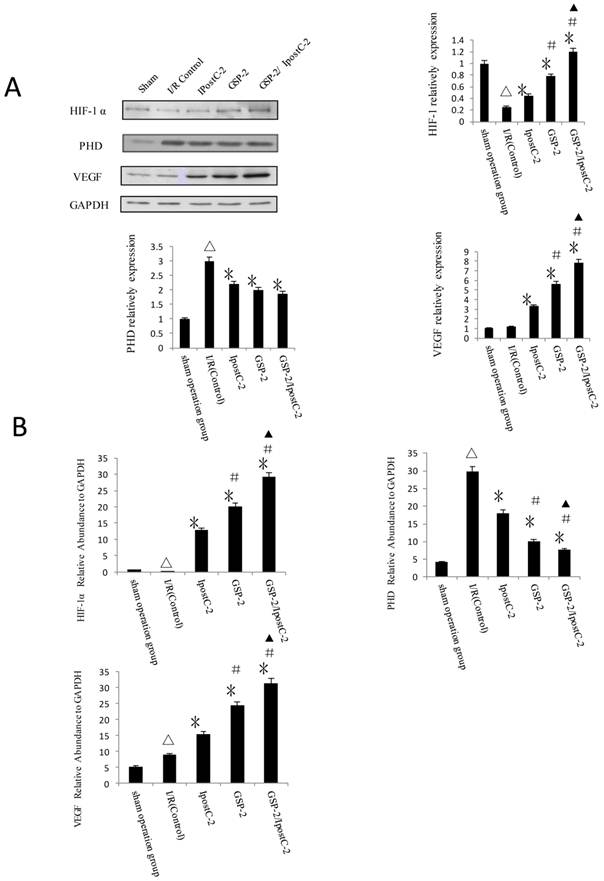
HIF-1α and its signaling pathway component PHD level were analyzed by immunoblotting (Figure 7A) and Real-Time PCR (Figure 7B). The expression of HIF-1α and VEGF were clearly increased in GSP-2, IpostC-2 and GSP-2/ IpostC-2 groups compared to control. We showed bar graph of the gray-scale value of the protein bands besides the Western blot image. The expression of PHD decreased in GSP-2, IpostC-2 and GSP-2/ IpostC-2 groups compared to IR control. Compared to solo treatments, GSP-2/ IpostC-2 could further increase (P<0.05) the expression of HIF-1α and VEGF.
Effects of ischemic postcondioning protocols and GSP on hepatic oxidative stress. (A)Total antioxidative capacity (T-AOC) and the malondialdehyde (MDA) contents in mouse liver. (mean±SD, n=10/group). (△indicate P<0.01 when compared with sham group; * indicate P<0.01 when compared with IRI control group; # indicate P<0.05 when compared with IpostC-2 group; ▲indicate P<0.05 when compared with GSP-2 group). (B)SOD, CAT and GSH-PX enzymatic activities and (C) T-NOS and iNOS activities in mouse liver. (mean±SD, n=10/group). (△indicate P<0.01 when compared with sham group; * indicate P<0.01 when compared with IRI control group; # indicate P<0.01 when compared with IpostC-2 group; ▲indicate P<0.05 when compared with GSP-2 group).
Effect of postconditioning and GSP on the expression level of HIF-1 alpha, PHD and VEGF. (A) Whole cell extracts made from mouse liver were separated on SDS- page gel. (mean±SD, n=10/group) HIF-1 α, PHD, VEGF and GAPDH protein level were determined by immunblotting with the specific antibodies against the proteins listed on the right. GAPDH was used as a loading control. (△indicate P<0.01 when compared with sham group; *indicate P<0.01 when compared with control group; #indicate P<0.01 when compared with IpostC-2 group; ▲indicate P<0.05 when compared with GSP-2 group). (B) HIF-1 α, PHD and VEGF mRNA expression relative to GAPDH were determined by Real-Time PCR. (mean±SD, n=10/group). (△indicate P<0.01 when compared with sham group; *indicate P<0.01 when compared with control group; #indicate P<0.01 when compared with IpostC-2 group; ▲indicate P<0.05 when compared with GSP-2 group, )
GSP preconditioning decreased the marker of hepatocyte injury and the sensitivity of steatotic liver to IRI
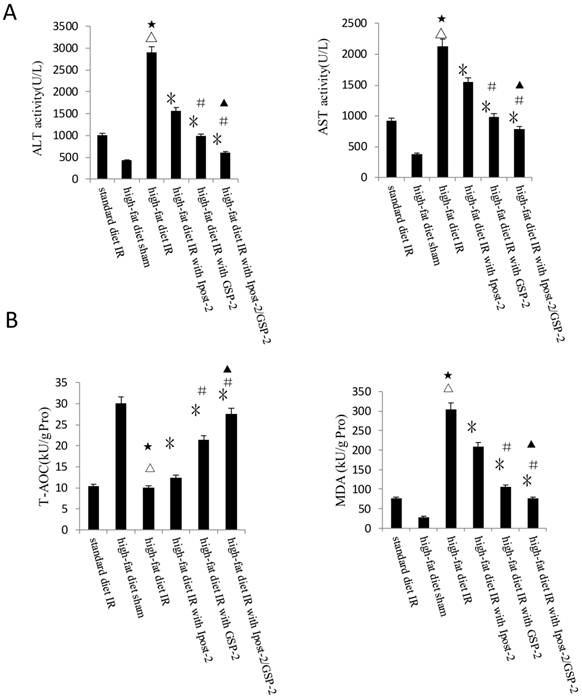
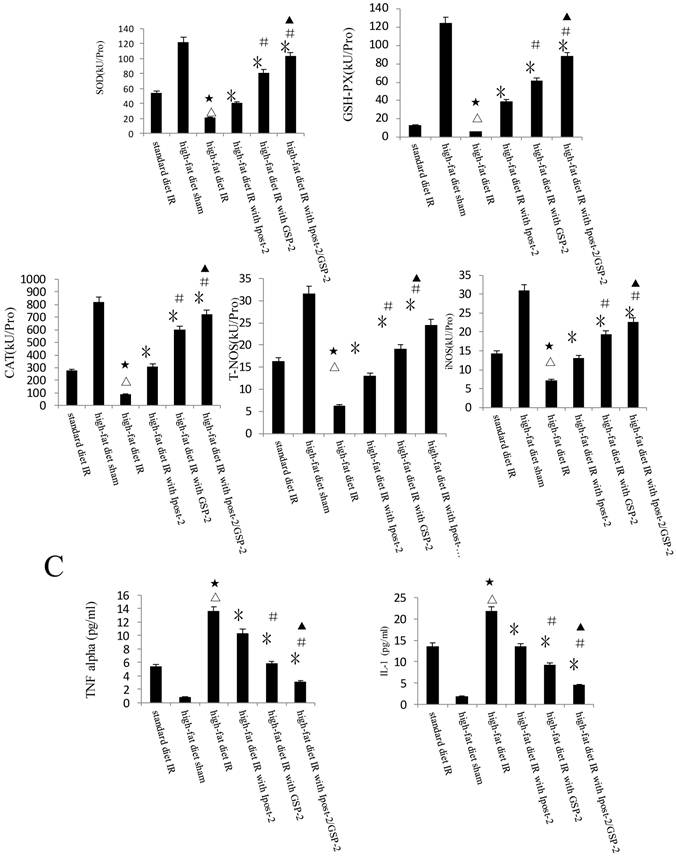
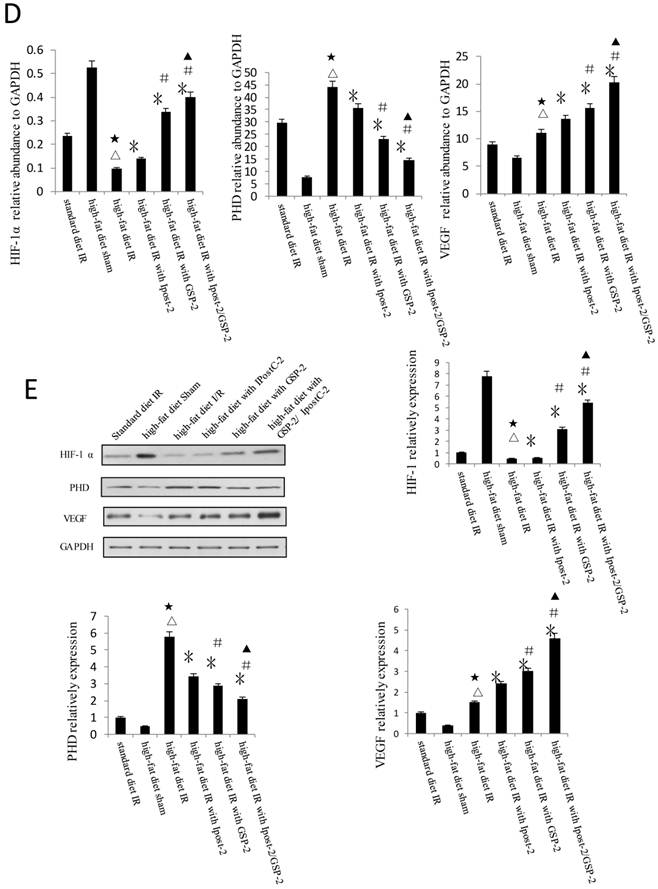
GSP preconditioning decreased the marker of hepatocyte injury: as showed in Figure 8B, the plasma activities of ALT and AST significantly decreased in GSP-2 and GSP-3 groups (P<0.01) compared to IRI control group. Effect of GSP on alleviating oxidative stress (Figure 9B) and pro-inflammation cytokines release (Figure 9C), as well as promoting hypoxia tolerance (Figure 9D, E) in obese mice group, in which the high-fat diet increased the sensitivity of liver to IRI. Although the IR injury was more severe in high-fat diet group than standard chow diet group, GSP and IpostC could still protect the steatotic livers from IRI compared with sham group (P<0.01). GSP and GSP combined with IpostC strategies were more effective than IpostC treatment (P<0.05).
Effects of GSP on the activities of ALT and AST in obese mice under hepatic IRI. (mean±SD, n=10/group) (△indicate P<0.01 when compared with high-fat diet sham group; *indicate P<0.01 when compared with high-fat diet IR group; #indicate P<0.01 when compared with high-fat diet with GSP-2 group).
Protective effect of GSP on steatotic livers against IRI. (A) Effects of GSP on the activities of ALT and AST in mice fed with standard chow diet and high-fat diet. (mean±SD, n=10/group). (△indicate P<0.01 when compared with high-fat diet sham group; ★indicate P<0.01 when compared with standard chow diet IR control group; *indicate P<0.01 when compared with high-fat diet IR control group; #indicate P<0.05 when compared with high-fat diet IR under IpostC-2 group; ▲indicate P<0.05 when compared with high-fat diet IR under GSP-2 group). (B) Effects of GSP on the expression of TNF-α and IL-1β mRNA abundance in serum were quantified by RT-PCR. (mean±SD, n=10/group). (△indicate P<0.01 when compared with high-fat diet sham group; ★indicate P<0.01 when compared with standard chow diet IR control group; *indicate P<0.01 when compared with high-fat diet IR control group; #indicate P<0.05 when compared with high-fat diet IR under IpostC-2 group; ▲indicate P<0.05 when compared with high-fat diet IR under GSP-2 group). (C) Effects of GSP on hepatic steatosis oxidative stress under IRI. (mean±SD, n=10/group). (△indicate P<0.01 when compared with high-fat diet sham group; ★indicate P<0.01 when compared with standard chow diet IR control group; *indicate P<0.01 when compared with high-fat diet IR control group; #indicate P<0.05 when compared with high-fat diet IR under IpostC-2 group; ▲indicate P<0.05 when compared with high-fat diet IR under GSP-2 group). (D) Effect of GSP on HIF-1 α, PHD, VEGF and GAPDH mRNA level in obese mice under hepatic IRI. (mean±SD, n=10/group). (△indicate P<0.01 when compared with high-fat diet sham group; ★indicate P<0.01 when compared with standard chow diet IR control group; *indicate P<0.01 when compared with high-fat diet IR control group; #indicate P<0.05 when compared with high-fat diet IR under IpostC-2 group; ▲indicate P<0.05 when compared with high-fat diet IR under GSP-2 group). (E) Effect of GSP on the expression level of HIF-1 alpha, PHD and VEGF in obese mice under hepatic IRI. (mean±SD, n=10/group). (△indicate P<0.01 when compared with high-fat diet sham group; ★indicate P<0.01 when compared with standard chow diet IR control group; *indicate P<0.01 when compared with high-fat diet IR control group; #indicate P<0.05 when compared with high-fat diet IR under IpostC-2 group; ▲indicate P<0.05 when compared with high-fat diet IR under GSP-2 group).
DISSCUSSION
Grape seed proanthocyanidins, the major polyphenols component of grape seeds is a potent antioxidant against oxidative stress relative diseases. As reactive oxygen species (ROS) play important part in hepatic IRI, we speculated GSP would have potential protective effects against hepatic IRI. In this model, GSP showed its ideally protective effects on reducing hepatic fat content and against liver IRI: particularly in high-fat diet induced obese mice. Meanwhile, GSP was more effective than postconditioning on protecting liver against IRI, and the combined strategy was obviously superior to solo treatment.
The effect of GSP on reducing hepatic fatty content and decreasing the marker of hepatocyte injury
Pharmacologic treatments are under investigation in an effort to increase the success of organ transplants. Liver steatosis is now a primary factor in determining the usability of potential organs because fatty livers are acutely more sensitive to IR injury than lean livers. As a result, nearly one third of all donated livers are discarded because of steatosis. Currently no agents are approved that can be administered to donors or recipients to increase the success of transplantation of steatotic livers. As showed in Table 1, Table 2 and Figure 3, mice received high-fat diet can increase their body weight and liver weight, and the content of triglyceride in livers also enhanced. After GSP preconditioning, obese induced by high-fat diet was alleviated: the liver weight and content of triglyceride were decreased. The markers of hepatocyte injury: the activity of ALT and AST were decreased either. Coupled with its apparent low toxicity, GSP is an ideal candidate for use as a therapeutic agent to increase the success of liver transplantation, especially for those patients with sreatotic liver.
Evaluate the protective effects of GSP and ischemic postconditioning against the IRI
Numerous studies have investigated the underlying mechanisms of ischemia and reperfusion injury with the goal of finding therapeutic approaches to enhance ischemia tolerance [2-10]. Among them, ischemic conditioning (preconditioning, postconditioning and remote conditioning) was widely used and these strategies could provide tissue-protective effects after ischemia and reperfusion injury. But Ischemia preconditioning (IpreC) application time is hard to control (hard to implementation before unpredictable long time of ischemia), while remote organ ischemic preconditioning (RIPC) and ischemic postconditioning were concentrated on the treatment of heart IRI. In our study, hepatic IpostC were established by three sets: 10, 30 and 60 seconds of reperfusion followed by 10, 30 and 60 seconds of ischemia for three times at the onset of reperfusion. The results showed that 30 and 60 seconds IpostC could offer effective protection but the 30 seconds protocol could provide the best protection against hepatic IRI.
A recent upsurge of interest in herbal remedies has practically resulted in manufacturing of numerous natural products and herbal concoctions once used as medicinal remedies. Among all these natural products with a broad range of biological activities, antioxidant class has gained the most popularity. Natural antioxidants primarily constitute selected enzymes, vitamins, and a spectrum of flavonoids, in addition to the numerous synthetic ones (e.g., dithiothreitol, butylated hydroxytoluene, etc.). These naturally occurring flavonoids/ phenolic antioxidants demonstrate a wide range of biochemical, pharmacological, and toxicological activities in animals and humans such as anti-inflammatory, anti-hyperlipemia, anti-ulcer, anti-proliferative, neuro-, cardio-, and hepatoprotective, skin tumor prevention, and immune-modulatory effects [29-31]. Some botanical extracts such as trans-resveratrol was recently used in hepatic IRI model and showed its benefits on hepatocyte protection [32]. Proanthocyanidins, the main components of grape seed extracts in general, are antioxidants which are nontoxic, and if absorbed, very active in vivo [33]. Grape seed proanthocyanidins are a group of natural antioxidants, which are known to possess a broad spectrum of pharmacological activity and protect cells from a variety of toxic insults. Science fewer reports demonstrated the effects of GSP on hepatic ischemic and reperfusion injury, we are the first to evaluate the protective effect of GSP and postconditioning against hepatic IRI and select the optimum dose of GSP for using. Our data showed that GSP could curb the obesity on mice and different dose of GSP injections were effectively reduce hepatic IRI. The injection dose of GSP 20 mg/kg/day for 3 weeks was more economic and gentle for application. Significantly, GSP was superior to IpostC on protecting liver against IRI. Indeed, the activities ALT and AST were significantly decreased in GSP-2/ IpostC-2 group (P<0.05) compared to solo treatment groups (Figure 2B). Hence, the combinations of the GSP and postconditioning produce better hepatic protection than individual treatments.
The mechanisms by which GSP and postconditioning decreasing hepatocellular injury
The mechanisms involved in GSP and IpostC on hepatic IRI also had been investigated in our study in detail. Our results suggested that the protective effects of GSP and IpostC against hepatic IRI were related with their roles in reducing the tissue oxidative stress level [34, 35]. Both GSP and postconditioning could regulate the activities of SOD, GSH-PX, NOS and CAT. Combined GSP with ischemic postconditioning could offer additive protection by increase the GSH-PX and CAT activities.
GSP and IpostC could decrease the cellular injuries and promote cell survival through suppressing cytokines release. Combined GSP and IpostC could further reduce the cytokines level, but still higher than sham group because TNFα and IL-1 are indispensable for liver regeneration. So, our data confirmed that maintain the concentration of TNFα and IL-1 at certain level might help liver function recovery in liver regeneration process.
Hypoxia was an important environmental alteration in the process of liver IR injury. Adaptation to hypoxia are mediated by HIF-1, which is a master transcriptional factors activated by low oxygen tension. The expression of HIF-1α and VEGF were increased in GSP-2, IpostC-2, and GSP-2/ IpostC-2 groups compared with IR control which would help illustrating the speed of cells regeneration and the recover status of hepatic function. PHDs can degrade HIF-1α by catalysing the hydroxylation of specific proline residues in the HIF-1α subunits. The expression of PHD was decreased in GSP-2, IpostC-2, and GSP-2/ IpostC-2 groups compared with IR control, which helped illustrating GSP could protect HIF-1α from degradation and further improve hypoxia tolerance in the combined strategy.
The protective effects of GSP on steatotic livers against IRI
At present, the increase in the prevalence of obesity is a worldwide problem and hepatic steatosis was the most common manifestation of liver diseases [36]. This situation has resulted in fewer usable donors for liver transplantation. Higher levels of fat in the liver are associated with an increased level of recipient morbidity and mortality because hepatic steatosis increases the extent of cellular injury incurred during ischemia and reperfusion injury [34]. Currently, nearly one third of all donated livers are discarded because of steatosis. The increasing use of fatty livers for transplantation induces higher graft nonfunction rates, increased retransplantation rates, and increased recipient mortality. Liver steatosis is now the primary factor in determining the usability of potential organs. Fast-acting pharmacologic treatments are under investigation in an effort to increase the success of transplants using livers with higher fat contents, but no agents are approved that can be administered to donors or recipients to increase the success of transplantation of steatotic livers [37, 38].
The utilazation of GSP as pharmacological preconditioning was more convenient particularly in combined strategies [26]. More important, this kind of implementation abandoned the main disadvantage of technical preconditioning, which brought trauma to major vessels and stress to the target organ. It was more humane and relatively easier to be operated, especially during the warm liver transplantation. Our published study has first showed that GSP exerted an antioxidant effect on hepatic ischemic and reperfusion injury: GSP could improve the activities of ROS scavengers and oxidation resistance in combined pre- and postconditioning groups. More important, the utilization of GSP was a well-accepted, noninvasive way to be operated as pharmacologic preconditioning during clinical liver transplantation and had minimal negative effects for patients to take without aggravating liver metabolic burden [26]. In this research, GSP preconditioning not only improve the donors' physical conditions which can withstand high-fat diet induced hepatic steatosis then modulated the health status of donors in the whole, but also provide a nondestructive preconditioning for following operation. Coupled with the convenience of postconditioning, this combined strategy will alleviate hepatic IRI and elevate the achievement ratio of liver transplantation operation, as well as enhancing recovery programme of the patients after surgery.
In summary, the comparation between grape seed proanthocyanidins (GSP) and IpostC was associated with their comparable protection on mouse livers against warm IRI. Combined GSP with IpostC could offer additive protection and the protocol of this combined strategy was more convenient for clinical application. Our data showed that the mechanism underlying the protection of GSP against hepatic IRI is associated with its ability of improving the oxidation resistance and inhibiting pro-inflammatory cytokines release, as well as augmenting the hypoxia tolerance responses. In high-fat diet induced obese mice suffering hepatic IRI, GSP also showed its powerful ability on protecting hepatocyte function and decreasing the damage of warm hepatic IRI. Further studies are necessary to determine other mechanisms involved in GSP conditioning and screen out the most appropriate dosage and mode of administration for clinical usage, as well as the development of new novel drugs, which can mimic the function of GSP and postconditioning.
Acknowledgements
The authors would like to thank Rui Ye for critical comments on the manuscript. This study was supported by Natural Science Foundation of China (NO.81130042 and NO.31171323) and University Innovation Team support plan of Liaoning (NO.LT2011011).
CONFLICT OF INTERESTS
The authors have declared that no conflict of interest exists.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284(1):G15-G26
2. Rougemont O, Lehmann K, Clavien PA. Preconditioning, organ preservation, and postconditioning to prevent ischemia-reperfusion injury to the liver. Liver Transpl. 2009;15(10):1172-1182
3. Angulo P. Medical progress-Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221-1231
4. Zhou G, Dada LA, Wu M, Kelly A, Trejo H, Zhou Q. et al. Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. Am J Physiol Lung Cell Mol Physiol. 2009;297(6):1120-1130
5. Penna C, Mancardi D, Rastaldo R, Pagliaro P. Cardioprotection: A radical view Free radicals in pre and postconditioning. Biochim Biophys Acta. 2009;1787(7):781-793
6. Sínay L, Kürthy M, Horváth S, Arató E, Shafiei M, Lantos J. et al. Ischemic postconditioning reduces peroxide formation, cytokine expression and leukocyte activation in reperfusion injury after abdominal aortic surgery in rat model. Clin Hemorheol Microcirc. 2008;40(2):133-142
7. Kin H, Wang NP, Mykytenko J, Reeves J, Deneve J, Jiang R. et al. Inhibition of myocardial apoptosis by postconditioning is associated with attenuation of oxidative stress-mediated nuclear factor-kappa B translocation and TNF alpha release. Shock. 2008;29(6):761-768
8. Jia C. Advances in the regulation of liver regeneration. Expert Rev Gastroenterol Hepatol. 2011;5(1):105-121
9. Koshikawa N, Hayashi J, Nakagawara A, Takenaga K. Reactive oxygen species -generating mitochondrial DNA mutation up-regulates hypoxia-inducible factor -1alpha gene transcription via phosphatidylinositol 3-kinase-Akt/protein kinase C/histone deacetylase pathway. J Biol Chem. 2009;284(48):33185-33194
10. Molitoris KH, Kazi AA, Koos RD. Inhibition of oxygen-induced hypoxia-inducible factor-1alpha degradation unmasks estradiol induction of vascular endothelial growth factor expression in ECC-1 cancer cells in vitro. Endocrinology. 2009;150(12):5405-5414
11. Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD. et al. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104(11):1631-1639
12. Law AY, Wong CK. Stanniocalcin-2 is a HIF-1 target gene that promotes cell proliferation in hypoxia. Exp Cell Res. 2010;316(3):466-476
13. Lemus ML, Flores ME, Cervantes R, Torres BM, Gudiño G, Chaparro V. et al. Expression of HIF-1 alpha, VEGF and EPO in peripheral blood from patients with two cardiac abnormalities associated with hypoxia. Clin Biochem. 2010;43(3):234-239
14. Bockhorn M, Goralski M, Prokofiev D, Dammann P, Grünewald P, Trippler M. et al. VEGF is important for early liver regeneration after partial hepatectomy. J Surg Res. 2007;138(2):291-299
15. Hausenloy Derek J, Yellon Derek M. Preconditioning and postconditioning: Underlying mechanisms and clinical application. Atherosclerosis. 2009;204(2):334-341
16. Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nature Medicine. 2011;17(11):1391-1401
17. De Rougemont O, Lehmann K, Clavien P.A. Preconditioning, Organ Preservation, and Postconditioning to Prevent Ischemia-Reperfusion Injury to the Liver. Liver Transpl. 2009;15(2):1172-1182
18. Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR. Remote postconditioning: A novel protective method from ischemia reperfusion injury - A review. J Surg Res. 2008;150(2):304-330
19. Kanoria S, Jalan R, Seifalian AM, Williams R, Davidson BR. Protocols and mechanisms for remote ischemic preconditioning: a novel method for reducing ischemia reperfusion injury. Transplantation. 2007;84(4):445-458
20. Saxena P, Newman MA, Shehatha JS, Redington AN, Konstantinov IE. Remote ischemic conditioning: evolution of the concept, mechanisms, and clinical application. J Card Surg. 2010;25(1):127-134
21. Granfeldt Asger, Lefer David J, Vinten-Johansen J. Protective ischaemia in patients: preconditioning and postconditioning. Cardiovascular Research. 2009;83(2):234-246
22. Yadav SS, Sindram D, Perry DK, Clavien PA. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology. 1999;30(5):1223-1231
23. Bagchi D, Garg A, Krohn RL, Bagchi M, Bagchi D J, Stohs S. Protective effects of grape seed proanthocyanidins and selected antioxidants against TPA-induced hepatic and brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen. Pharm. 1998;30(5):771-776
24. Terra X, Montagut G, Bustos M, Llopiz N, Ardevol A, Blade C. et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J Nutr Biochem. 2009;20(3):210-218
25. Pataki T, Bak I, Kovacs P, Baqchi D, Das DK, Tosaki A. Grape seed proanthocyanidins improved cardiac recovery during reperfusion after ischemia in isolated rat hearts. Am J Clin Nutr. 2002;75(5):894-899
26. Song X, Zhang N, XU H, Cao L, Zhang H. Combined preconditioning and postconditioning provides synergistic protection against liver ischemic reperfusion injury. Int J Biol Sci. 2012;8(5):707-718
27. Shao ZH, Woicik KR, Dossumbekova A, Hsu C, Mehendale SR, Li CQ. et al. Grape seed proanthocyanidins protect cardiomyocytes from ischemia and reperfusion injury via Akt-NOS signaling. J Cell Biochem. 2009;107(4):697-705
28. Ferrera R, Benhabbouche S, Bopassa JC, Li B, Ovize M. One hour reperfusion is enough to assess function and infarct size with TTC staining in Langendorff rat model. Cardiovasc Drugs Ther. 2009;23(4):327-331
29. Blade C, Arola L, Salvado ML. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol Nutr Food Res. 2010;54(1):31-59
30. Bhat KPL, Kosmeder JW, Pezzuto JM. Biological effects of resveratrol. Antioxid Redox Signal. 2001;3(6):1041-1064
31. Guler A, Sahin MA, Yucel O, Yokusoglu M, Gamsizkan M, Ozal E. et al. Proanthocyanidin prevents myocardial ischemic injury in adult rats. Med Sci Monit. 2011;17(11):326-331
32. Sahar HK, Cottart CH, Wendum D, Vibert F, Clot JP, Savouret JF. et al. Postischemic treatment by trans-resveratrol in rat liver ischemia-reperfusion: A possible strategy in liver surgery. Liver Transpl. 2008;14(4):451-459
33. Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64(5):923-933
34. Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142(4):711-725
35. Seifalian AM, Piasecki C, Agarwal A, Davidson BR. The effect of graded steatosis on flow in the hepatic parenchymal microcirculation. Transplantation. 1999;68:780-784
36. Chavin KD, Yang S, Lin HZ, Chatham J, Chacko VP, Hoek JB. et al. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem. 1999;274:5692-5700
37. Chavin KD, Fiorini RN, Shafizadeh SF, Wan C, Cheng G, Polito C. et al. Fatty acid synthase blockade protects steatotic livers from warm ischemia/reperfusion injury and transplantation. Am J Transplant. 2004;4(9):1440-1447
38. Fiorini RN, Donovan JL, Rodwell D, Evans Z, Cheng G, May HD. et al. Short-term administration of (-)-epigallocatechin gallate reduces hepatic steatosis and protects against warm hepatic ischemia/reperfusion injury in steatotic mice. Liver Transpl. 2005;11(3):298-308
Author contact
![]() Corresponding author: Xiaoyu Song, Email: xysongcmu.edu.cn; and Liu Cao, caoliucmu.edu.cn.
Corresponding author: Xiaoyu Song, Email: xysongcmu.edu.cn; and Liu Cao, caoliucmu.edu.cn.

 Global reach, higher impact
Global reach, higher impact