10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2020; 16(7):1166-1179. doi:10.7150/ijbs.41293 This issue Cite
Research Paper
Potential Crosstalk between Liver and Extra-liver Organs in Mouse Models of Acute Liver Injury
1. Department of Physiology and Pathophysiology, State Key Laboratory of Medical Neurobiology, School of Basic Medical Sciences, Fudan University, Shanghai 200032, China
2. Department of Pathology, School of Basic Medical Sciences, Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China
3. State Key Laboratory of Genetic Engineering, School of Life Sciences, Metabolomics and Systems Biology Laboratory, Human Phenome Institute, Fudan University, Shanghai 200433, China
4. Department of Cardiology, Huashan Hospital, Fudan University, Shanghai 200032, China
5. Department of Internal Medicine, Huashan Hospital West Campus, Fudan University, Shanghai 200032, China
6. Shanghai Key Laboratory of Clinical Geriatric Medicine, Fudan University, Shanghai 200032, China
*These authors contributed equally to this work.
Abstract
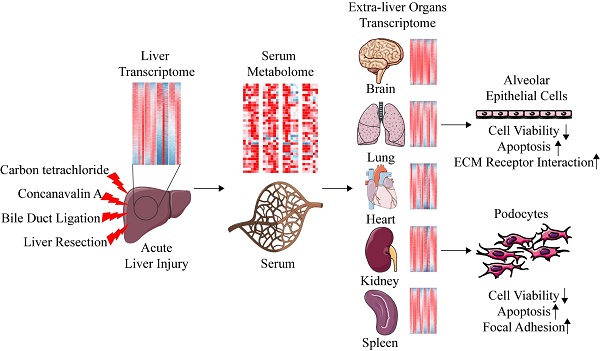
Carbon tetrachloride (CCl4), Concanavalin A (ConA), bile duct ligation (BDL), and liver resection (LR) are four types of commonly used mouse models of acute liver injury. However, these four models belong to different types of liver cell damage while their application situations are often confounded. In addition, the systematic changes of multiple extra-liver organs after acute liver injury and the crosstalk between liver and extra-liver organs remain unclear. Here, we aim to map the morphological, metabolomic and transcriptomic changes systematically after acute liver injury and search for the potential crosstalk between the liver and the extra-liver organs. Significant changes of transcriptome were observed in multiple extra-liver organs after different types of acute liver injury despite dramatic morphological damage only occurred in lung tissues of the ConA/BDL models and spleen tissues in the ConA model. Liver transcriptomic changes initiated the serum metabolomic alterations which correlated to transcriptomic variation in lung, kidney, and brain tissues of BDL and LR models. The potential crosstalk might lead to pulmonary damage and development of hepatorenal syndrome (HRS) and hepatic encephalopathy (HE) during liver injury. Serum derived from acute liver injury mice damaged alveolar epithelial cells and human podocytes in vitro. Our data indicated that different types of acute liver injury led to different transcriptomic changes within extra-liver organs. Integration of serum metabolomics and transcriptomics from multiple tissues can improve our understanding of acute liver injury and its effect on the other organs.
Keywords: acute liver injury, systematic change, metabolomics, transcriptomics, crosstalk

 Global reach, higher impact
Global reach, higher impact