10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(12):4642-4647. doi:10.7150/ijbs.72424 This issue Cite
Review
Recipients of COVID-19 vaccines face challenges of SARS-CoV-2 variants
1. Faculty of Health Sciences, University of Macau, Macao SAR, China
2. Ministry of Education-Frontiers Science Center for Precision Oncology, University of Macau, Taipa, Macao SAR, China
Received 2022-2-26; Accepted 2022-5-2; Published 2022-7-11
Abstract
The coronavirus disease 19 (COVID-19) has been rampant since 2019, severely affecting global public health, and causing 5.75 million deaths worldwide. So far, many vaccines have been developed to prevent the infection of SARS-CoV-2 virus. However, the emergence of new variants may threat vaccine recipients as they might evade immunological surveillance that depends on the using of anti-SARS-CoV-2 antibody to neutralize the viral particles. Recent studies have found that recipients who received two doses of vaccination plus an additional booster shoot were able to quickly elevate neutralization response and immune response against wild-type SARS-CoV-2 virus and some initially appeared viral variants. In this review, we assessed the real-world effectiveness of different COVID-19 vaccines by population studies and neutralization assays and compared neutralization responses of booster vaccines in vitro. Finally, as the efficacy of COVID-19 vaccine is expected to decline over time, continued vaccination should be considered to achieve a long-term immune protection against coronavirus.
Keywords: COVID-19, SARS-CoV-2 virus, Vaccines, Viral variants, booster vaccination
Introduction
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has ramparted the whole world since 2019, severely affecting global public health, causing social disruption and significant economic losses. As of February 2022, there were more than 396 million confirmed cases and 5.75 million deaths worldwide [1]. With global spread of SARS-CoV-2, mutations are accumulating during replication, leading to viral variants that increase transmissibility, virulence or reduce effectiveness of vaccines and treatments [2].
SARS-CoV-2 contains four major structural proteins (spike protein, membrane protein, envelope protein, and nucleocapsid protein), which are encoded by individual open reading frames (ORFs) [3, 4]. Spike protein is a glycoprotein located on the surface of SARS-CoV-2 viral particle. This protein can be cleaved into two domains: S1 and S2. S1 domain includes the N-terminal domain (NTD) and receptor-binding domain (RBD), and RBD can help coronavirus bind angiotensin-converting enzyme 2 (ACE2) located at the surface of the host cells such as endothelial cells [5, 6]. Studies showed that the binding free energy (BFE) between RBD and ACE2 was positively correlated with viral infectivity [7, 8]. The function of S2 domain can help the viral particle to enter the host cells via membrane fusion [9]. Among the three major structure proteins of SARS-CoV-2: spike, membrane and nucleocapsid, the gene encodes spike protein has the highest frequency of mutation especially in the RBD region that is used to design the currently used anti-COVID-19 vaccine [10]. Many antibody therapeutics and vaccines have been designed to target the spike protein based on the initially discovered viral strain that caused COVID-19 in early 2020. The accumulation of these spike gene mutations in the new coronavirus is more likely to affect the effectiveness of developed vaccines against these new viral variants [11].
However, during the global spread of SARS-CoV-2, many new variant strains of this coronavirus have been isolated which may be formed due to the accumulation of numerous mutations in the past two years [12]. Many mutations in spike protein, which strengthen the binding between the spike protein and ACE2, have been shown to affect the effectiveness of the existing anti-COVID-19 vaccines and monoclonal antibodies [13]. The mutagenesis has three mechanisms: molecular scale, organism scale, and population level. The first two mechanisms provide numerous candidate mutations in the SARS-CoV-2 genome. The population-level mechanism determines what mutations are predominant by natural selection that have two pathways of infectivity and vaccine resistance [8]. In the summer of 2020, Michigan State University established a model of infection based on natural selection and predicted that SARS-CoV-2 was more infectious on the mutations of residues 452, 489, 500, 501, and 505 in the RBD region, after computing the BFE changes of possible mutations by integrating genotyping, deep learning, biophysics, and mathematics [14]. The result was confirmed by pandemic SARS-CoV-2, in the last two years. Recently, vaccine-resistant mutations that are emerged after vaccinations, have been confirmed. Vaccine-resistant mutations with negative BFE changes, that disrupted the binding between the spike protein and antibodies, have been observed frequently, such as Y449S and Y449H, after many people in many countries were vaccinated in high rates [8]. The trend of increasing frequency of vaccine-resistant mutations correlated strongly with the proportion of fully vaccinated in Europe and America. Mutations in vaccine-resistant pathways also reduce the effectiveness of vaccines and antibody therapies, suggesting that COVID-19 will be a prolonged pandemic.
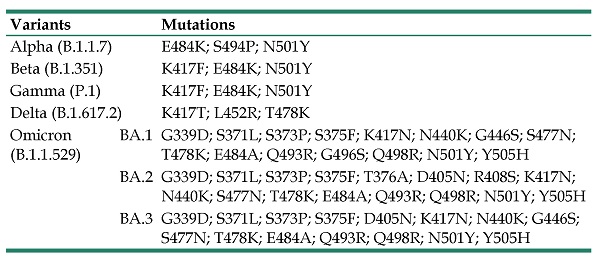
As of February 2022, World Health Organization (WHO) listed five designated variants of concern (VOCs) for coronavirus: Alpha (B.1.1.7, earliest documented in the UK in September 2020), Beta (B.1.351, earliest documented in South Africa in May 2020), Gamma (P.1, earliest documented in Brazil in November 2020), Delta (B.1.617.2, earliest documented in India in October 2020), and Omicron (B.1.1.529, earliest documented in multiple countries in November 2021) (Table 1). WHO has also listed two designated variants of interest (VOIs): Lambda (C.37, earliest documented in Peru in December 2020) and Mu (B.1.621, earliest documented in Colombia in January 2021) [15].
Vaccines are constantly been developed to achieve immune responses to SARS-CoV-2 virus [16]. However, vaccine development is a long-lasting and challenging process, taking years to complete. Currently, there are 195 vaccines in preclinical development and 142 vaccines in clinical trials [17]. As of 12 January 2022, 10 vaccines are listed under WHO Emergency Use Listing Procedure (EUL), which include four types: mRNA vaccines, viral vector vaccines, recombinant spike vaccines and inactivated vaccines. These vaccines are being widely used around the world to achieve immune protection against SARS-CoV-2. By February 2022, more than 3.7 billion people were vaccinated with at least 1 dose worldwide, about 1/4 of the total population. There are 2.2 billion people that were fully vaccinated worldwide [18]. Many countries are working together to roll out a COVID-19 vaccine globally.
Variants of SARS-CoV-2 and mutations in RBD.
| Variants | Mutations | |
|---|---|---|
| Alpha (B.1.1.7) | E484K; S494P; N501Y | |
| Beta (B.1.351) | K417F; E484K; N501Y | |
| Gamma (P.1) | K417F; E484K; N501Y | |
| Delta (B.1.617.2) | K417T; L452R; T478K | |
| Omicron (B.1.1.529) | BA.1 | G339D; S371L; S373P; S375F; K417N; N440K; G446S; S477N; T478K; E484A; Q493R; G496S; Q498R; N501Y; Y505H |
| BA.2 | G339D; S371L; S373P; S375F; T376A; D405N; R408S; K417N; N440K; S477N; T478K; E484A; Q493R; Q498R; N501Y; Y505H | |
| BA.3 | G339D; S371L; S373P; S375F; D405N; K417N; N440K; G446S; S477N; T478K; E484A; Q493R; Q498R; N501Y; Y505H | |
But the emergence of these new, potentially more infectious SARS-CoV-2 variants challenges vaccine protection [19]. Based on the changes of BFE, it was found that some mutations made SRAS-COV-2 more infectious. Three key RBD mutations sites of N501, L452 and Y505H were proved in VOCs that promote transmission and break through the protection of vaccines (Table 1). The Delta and Alpha variants documented in 2020 were found to be more infectious, leading to a global pandemic. The Omicron variant is currently the most dominant pandemic strain, which accounted for 85% of new cases in January of 2022 [20]. The Omicron variant, designated by WHO as VOC within two days, has three lineages, BA.1, BA.2, and BA.3 (Table 1) [21]. BA.1 contains up to 32 mutations in the spike gene and 15 of these mutations clustered in the region encoding RBD strengthen infectivity and disrupt monoclonal antibodies [22-27]. Spike proteins are critical for viral entry and the main target of neutralizing antibodies. The Omicron variant has thus raised concerns that this viral stain may escape from protection conferred by vaccines. Recently, Omicron BA.2, which was more infectious than BA.1 based on changes of BFE and could lead to breakthroughs in vaccines, is replacing the BA.1 in China [28]. The lack of clinical data on the effectiveness of vaccines against new variants creates an uncertainty that could prolong the duration of the pandemic. We need to understand the effectiveness of COVID-19 vaccines, especially against VOCs.
Therefore, the purpose of this article is to review the recent studies on the effectiveness of vaccines against different SARS-CoV-2 variants. We hope this review will provide an overview on the effectiveness of various types of vaccines, which will contribute to the development of new treatments and new vaccines.
Effectiveness of clinical vaccines against VOCs
VOCs were able to break through the protection of vaccines, evade immunological surveillance and escape neutralizing antibodies after vaccination [23, 29-33]. So far, the Alpha variant has been found to have limited effect on the efficacy of the vaccine. Numerous data and research have demonstrated that neutralization response and vaccine-induced immune protection declined against the Omicron variant compared to wild-type virus and other VOCs (Table 2). The emerging evidence supports that the neutralizing antibodies against SARS-CoV-2, which can block the interaction between the spike protein with ACE2, play an important role in protective immunity after vaccination or infection. B and T lymphocytes also contribute to the protective effect against symptomatic infection. Several monoclonal antibody-based therapeutics have been approved to treat patients with SARS-CoV-2 [34]. However, the anti-Omicron activity of many monoclonal antibodies was diminished [23, 25, 35, 36]. Next, this review will discuss the efficacy of several commonly used vaccines against the coronavirus variants from published preclinical and clinical data in population studies and laboratory tests.
Efficacy and neutralization efficiency of different vaccines
| Outcome | Wild type | Alpha | Delta | Beta/Gamma | Omicron |
|---|---|---|---|---|---|
| Efficacy against infection % | |||||
| mRNA-1273 (Moderna) | 94 | 73 | 70 | 61 | 44 |
| BNT162b2 (Pfizer-BioNTech) | 95 | 94 | 88 | 72 | 70 |
| AZD1222 (AstraZeneca) | 74 | 70 | 60 | 64 | N/A |
mRNA vaccine
The mRNA-1273 (Moderna) vaccine was co-developed by researchers at the National Institute of Allergy and Infectious Disease in USA. In population-based studies, effectiveness of mRNA-1273 vaccine after full doses was approximately 73% against infection of the Alpha variant, but 61% against infection of the Beta variant [37, 38] (Table 2). Only 55% of serum samples, randomly selected from participants in the Coronavirus Efficacy (COVE) phase 2 and phase 3 trials, were detected to have neutralization response against the Omicron variant by geometric mean neutralizing titers (GMTs). Together, neutralization titers were 34.8-fold lower against the Omicron variant than the wild-type virus [33]. The effectiveness against omicron infection was approximately 44% at two weeks after vaccination, but 5.9% after 9 months [39].
In population-based studies, researchers in the UK found that vaccination with BNT162b2 (Pfizer-BioNTech) vaccine or AZD1222 (AstraZeneca) vaccine decreased the risk of infection with the Alpha variant, but the risk of infection with the Delta variant increased relative to the Alpha variant [40]. The effectiveness of BNT162b2 vaccine against infection was approximately 94% against the Alpha variant, 88% against the Delta variant [41], 72% against the Beta variant [42] and 70% against the Omicron variant [43] (Table 2). The neutralization titers of serum samples also verified these results against various viral strains (wild-type virus ˃ Delta variant ˃ Beta variant ˃ Omicron variant) and the neutralization efficiency with two doses of BNT162b2 vaccine against Omicron variant was lower (by a factor of about 20) than wild-type virus [43, 44].
In summary, these reports showed that after administration of aforementioned vaccines, the antibody titers against Omicron variant were lower than those against the wild-type virus and some stains of VOCs, especially the Omicron variant, may escape mRNA vaccines-induced immune protection.
Viral vector vaccines
AZD1222 (also known as ChAdOx1 nCoV-19) consists of a replication-deficient chimpanzee adenoviral vector containing the sequence of SARS-CoV-2 spike gene. The efficacy after two-doses of AZD1222 vaccination was approximately 70% against symptomatic infection of the Alpha variant [45] (Table 2). Likewise, a study by University College London Hospitals and the Francis Crick Institute in London showed that 37% serum samples with two-doses of AZD1222 vaccination were detected with quantifiable neutralizing antibody titer against the Omicron variant, which was lower than the detected rate against the Alpha and the Delta variants [46]. The neutralization titers of serum samples against different viral strains ranged in the following order: Alpha ˃ Delta ˃ Omicron. The research by College of Life Science and Technology, Beijing University of Chemical Technology also found that serum samples from some recipients of two-dose AZD1222 did not exhibit any detectable titer of neutralizing antibody against the Omicron variant and those data suggested that the Omicron variant is antigenically more different from the wild-type virus than the Beta and Delta variants [47]. So, those results showed that recipients who received viral vector vaccines (i.e. AZD1222) displayed a decreased neutralization response and immune response relative to wild-type virus than to some of VOCs, especially the Omicron variant.
Recombinant spike vaccines
ZF2001, a recombinant dimeric RBD protein vaccine, induces RBD-directed neutralizing response, and is undergoing phase 3 clinical trials [48-50]. All the serum samples of ZF2001 recipients displayed positive neutralization response against wild-type virus and ZF2001 also preserved the neutralizing activity against the Delta variant [51]. However, it was almost impossible for ZF2001 to neutralize the Omicron variant [52].
Inactivated vaccines
SARS-CoV-2 inactivated vaccines elicit immune response directed against the entire viral particle instead of only targeting the spike protein or RBD of the virus [53]. After 4 months, neutralizing antibodies against Gamma and Delta variants were limitedly detected with two-dose CoronaVac (Sinovac) [54]. Among the vaccine recipients, all serum samples were negative for both Omicron and Delta variants [55]. The third dose of CoronaVac was found to stimulate cross-protective B and T cell responses against variants [30, 56].
BBIBP-CorV (Sinopharm, Beijing CNBG), authorized by the Hungarian National Drug and Food Evaluation Authority, contains SARS-CoV-2 inactivated in Vero cells. Ninety percent of participants were below the age of 50 and they were detected to have RBD-binding antibody after two doses of BBIBP-CorV vaccination [57]. Neutralization titer was decreased against the Beta variant, a greater degree was found with the Delta variant. RBD binding antibodies of the serum samples were also decreased against the VOCs [58]. It was also reported that the neutralizing activity against wild-type virus was 80% after two doses of inactivated BBIBP-CorV and only 10% serum samples showed successful neutralization against the Omicron variant. Neutralization titer against Omicron variant was also significantly reduced relative to wild-type virus in serum samples recovered from Delta variant infection [35].
However, the BBIBP-CorV vaccine was effective in generating cross-protective B and T cell responses to prevent infection of other variants, which were mutated in spike protein [59, 60]. Those cross-protective responses have also been found after receiving vaccination of mRNA-1273 and BNT162b2 mRNA [61, 62]. It thus suggests that the inactivated vaccine can generate cell-mediated immune responses against other variants.
Effectiveness of vaccine booster against VOCs
Although the efficiency of vaccination could be decreased over time, some studies have shown that both inactivated vaccines and mRNA vaccine booster significantly increased the neutralization titers to wild-type virus and other variants, restored efficiency of vaccine and reduced virus transmission [30, 36, 63-65].
It has been observed that the neutralization efficiency against the wild-type virus was decreased by 7.6 times after 6 months of vaccination with a second dose of the mRNA-1273 vaccine [33]. The results obtained from 239 COVID-19 vaccinees showed that booster mRNA vaccines (mRNA-1273 and BNT162b) increased neutralizing antibody response compared to two-dose mRNA vaccines, especially against the Omicron variant. After the third dose of mRNA-1273 vaccine, neutralization against the Omicron variant was detected in all serum samples, but not in some serum samples after two-dose mRNA-1273 vaccine [30, 33]. Data from 49 states of U.S. in December 2021 showed that receipts of BNT162b2 vaccine booster was protective against the Omicron and Delta variants, while there was less protection for the Omicron variant than for the Delta variant [63]. It was also reported that the third dose of BNT162b2 vaccine was 100 times more effective in neutralizing the Omicron variant than the two-dose vaccination [44].
The neutralization titers against the Omicron variant and wild-type virus were elevated after the third inactivated vaccine [35]. The neutralization titer of BBIBP-CorV/ZF2001 heterologous booster group was higher relative to homologous booster group [66]. Both homologous and heterologous inactivated vaccine booster increased neutralizing antibodies and improved the protection against the Omicron variant [35]. Results demonstrated that the ZF2001 booster was more efficient against SARS-CoV-2 variants that have mutations in NTD of the spike, such as the Delta variant [54]. The study from Fudan University found that after BBIBP-CorV booster, the levels of antibody in the blood samples were increased, and humoral immune responses of spike-specific memory B and T cells were quickly elevated [67]. Therefore, it is very necessary to carry out COVID-19 vaccine booster to restore the efficiency of vaccine and limit virus transmission.
Conclusion
During the current pandemic, SARS-CoV-2 variants are continuously selected, which can evade immunological surveillance and promote transmission. Vaccines can loss immunity to SARS-CoV-2 variants due to the mutations in the spike proteins. The emergence of new variants threat vaccine recipients and researchers are finding new solutions to attenuate immune evasion.
Interestingly, recipients who received an additional booster vaccine after two doses quickly elevated neutralization response and immune responses of spike-specific memory B and T cells and similar results were also observed in rhesus macaques [68]. However, the efficiency of vaccination decreased over time. For example, even after the second dose of the mRNA-1273 vaccination, the neutralization efficiency against the wild-type virus was decreased by 7.6 times after 6 months [33]. Similarly, after a third booster dose of mRNA-1273 vaccine, the neutralization efficacy against the Omicron variant was decreased by 6.3 times after five months. Therefore, continued vaccination even after the vaccine booster should be considered to achieve long-term immune protection against new emerging variants of SARS-CoV-2.
Competing Interests
The authors have declared that no competing interest exists.
References
1. WHO. WHO Coronavirus (COVID-19) Dashboard. 2022; Available from: https://covid19.who.int/.
2. He X, Hong W, Pan X, Lu G, Wei X. SARS-CoV-2 Omicron variant: Characteristics and prevention. MedComm. 2021;2(4):838-845
3. Wu A P, Peng Y S, Huang B Y, Ding X, Wang X Y, Niu P H. et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe. 2020;27(3):325-328
4. Arya R, Kumari S, Pandey B, Mistry H, Bihani S C, Das A. et al. Structural insights into SARS-CoV-2 proteins. J Mol Biol. 2021;433(2):166725
5. Wang Q H, Zhang Y F, Wu L L, Niu S, Song C L, Zhang Z Y. et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell. 2020;181(4):894-904.e9
6. Ou X Y, Liu Y, Lei X B, Li P, Mi D, Ren L L. et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nature Communications. 2020;11(1):1620
7. Walls A C, Park Y J, Tortorici M A, Wall A, McGuire A T, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181(2):281-292.e6
8. Wang R, Chen J, Wei G W. Mechanisms of SARS-CoV-2 Evolution Revealing Vaccine-Resistant Mutations in Europe and America. J Phys Chem Lett. 2021 12(4)9: 11850-11857
9. Pinto D, Park Y J, Beltramello M, Walls A C, Tortorici M A, Bianchi S. et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815):290-295
10. Wu R F, Luo K Q. Developing effective siRNAs to reduce the expression of key viral genes of COVID-19. Int J Biol Sci. 2021;17(6):1521-1529
11. Plante J A, Liu Y, Liu J, Xia H, Johnson B A, Lokugamage K G. et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2021;592(7852):116-121
12. Zhou W, Wang W. Fast-spreading SARS-CoV-2 variants: challenges to and new design strategies of COVID-19 vaccines. Signal Transduct Target Ther. 2021;6(1):226
13. Malik J A, Mulla A H, Farooqi T, Pottoo F H, Anwar S, Rengasamy K R R. Targets and strategies for vaccine development against SARS-CoV-2. Biomedicine & Pharmacotherapy. 2021;137:111254
14. Chen J H, Wang R, Wang M L, Wei G W. Mutations Strengthened SARS-CoV-2 Infectivity. J Mol Biol. 2020;432(19):5212-5226
15. WHO. Tracking SARS-CoV-2 variants. 2022; Available from: https://www.who.int/activities/tracking-SARS-CoV-2-variants/tracking-SARS-CoV-2-variants.
16. Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252-260
17. WHO. COVID-19 Vaccine Tracker and Landscape. 2022; Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
18. WHO. Vaccine uptake. 2022; Available from: https://app.powerbi.com/view?r=eyJrIjoiMWNjNzZkNjctZTNiNy00YmMzLTkxZjQtNmJiZDM2MTYxNzEwIiwidCI6ImY2MTBjMGI3LWJkMjQtNGIzOS04MTBiLTNkYzI4MGFmYjU5MCIsImMiOjh9.
19. Forni G, Mantovani A, on behalf of the COVID-19 Commission of Accademia Nazionale dei Lincei, Rome. COVID-19 vaccines: where we stand and challenges ahead. Cell Death and Differentiation. 2021;28(2):626-639
20. Nextstrain. Genomic epidemiology of novel coronavirus - Global subsampling. 2022; Available from: https://nextstrain.org/ncov/gisaid/global.
21. Chen J and Wei G W. Omicron BA.2 (B.1.1.529.2): high potential to becoming the next dominating variant. J Phys Chem Lett. 2022: 5; 13(17): 3840-3849
22. Chen J, Wang R, Gilby N B, Wei G W. Omicron Variant (B.1.1.529): Infectivity, Vaccine Breakthrough, and Antibody Resistance. J Chem Inf Model. 2022;62(2):412-422
23. Chen R E, Zhang X, Case J B, Winkler E S, Liu Y, VanBlargan L A. et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med. 2021;27(4):717-726
24. Guo S X, Liu K F, Zheng J. The Genetic Variant of SARS-CoV-2: Would it matter for Controlling the Devastating Pandemic? Int J Biol Sci. 2021;17(6):1476-1485
25. VanBlargan L A, Errico J M, Halfmann P J, Zost S J, Crowe J E Jr, Purcell L A. et al. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med. 2022;28(3):490-495
26. Ghosh N, Nandi S, Saha I. A review on evolution of emerging SARS-CoV-2 variants based on spike glycoprotein. Int Immunopharmacol. 2022;105:108565
27. Lippi G, Mattiuzzi C, Henry B M. Updated picture of SARS-CoV-2 variants and mutations. Diagnosis (Berl). 2021;9(1):11-17
28. Rahimi F, Talebi Bezmin Abadi A. The Omicron subvariant BA.2: Birth of a new challenge during the COVID-19 pandemic. Int J Surg. 2022;99:106261
29. Taylor L. Covid-19: Omicron drives weekly record high in global infections. BMJ. 2022;376:o66
30. Garcia-Beltran W F, St Denis K J, Hoelzemer A, Lam E C, Nitido A D, Sheehan M L. et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185(3):457-466.e4
31. Ren S Y, Wang W B, Gao R D, Zhou A M. Omicron variant (B.1.1.529) of SARS-CoV-2: Mutation, infectivity, transmission, and vaccine resistance. World J Clin Cases. 2022;1:1-11
32. Song Y, Masaki F. Preparation for the challenge of heavily mutated Omicron variant. Clin Transl Med. 2021;11(12):e679
33. Pajon R, Doria-Rose N A, Shen X, Schmidt S D, O'Dell S, McDanal C. et al. SARS-CoV-2 Omicron Variant Neutralization after mRNA-1273 Booster Vaccination. N Engl J Med. 2022: 386(11): 1088-1091.
34. Ning L, Abagna H B, Jiang Q, Liu S, Huang J. Development and application of therapeutic antibodies against COVID-19. Int J Biol Sci. 2021;17(6):1486-1496
35. Wang X, Zhao X, Song J, Wu J, Zhu Y, Li M. et al. Homologous or heterologous booster of inactivated vaccine reduces SARS-CoV-2 Omicron variant escape from neutralizing antibodies. Emerg Microbes Infect. 2022;11(1):477-481
36. Mattiuzzi C, Lippi G. Primary COVID-19 vaccine cycle and booster doses efficacy: analysis of Italian nationwide vaccination campaign. Eur J Public Health. 2022;32(2):328-330
37. Chemaitelly H, Yassine H M, Benslimane F M, Al Khatib H A, Tang P, Hasan M R. et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nature Medicine. 2021;27(9):1614-1621
38. Park J W, Lagniton P N P, Liu Y, Xu R H. mRNA vaccines for COVID-19: what, why and how. Int J Biol Sci. 2021;17(6):1446-1460
39. Tseng H F, Ackerson B K, Luo Y, Sy L S, Talarico C A, Tian Y. et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. 2022;28(5):1063-1071
40. Eyre D W, Taylor D, Purver M, Chapman D, Fowler T, Pouwels K B. et al. Effect of Covid-19 Vaccination on Transmission of Alpha and Delta Variants. N Engl J Med. 2022;386(8):744-756
41. Bernal J L, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S. et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. New Engl J Med. 2021;385(7):585-594
42. Abu-Raddad L J, Chemaitelly H, Butt A A, Vaccinati N S G C-. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. New Engl J Med. 2021;385(2):187-189
43. Collie S, Champion J, Moultrie H, Bekker L G, Gray G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N Engl J Med. 2022;386(5):494-496
44. Nemet I, Kliker L, Lustig Y, Zuckerman N, Erster O, Cohen C. et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N Engl J Med. 2022;386(5):492-494
45. Emary K R W, Golubchik T, Aley P K, Ariani C V, Angus B, Bibi S. et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397(10282):1351-1362
46. Wu M, Wall E C, Carr E J, Harvey R, Townsley H, Mears H V. et al. Three-dose vaccination elicits neutralising antibodies against omicron. Lancet. 2022;399(10326):715-717
47. Li M C, Lou F X, Fan H H. SARS-CoV-2 variant Omicron: currently the most complete "escapee" from neutralization by antibodies and vaccines. Signal Transduct Tar. 2022;7(1):28
48. Dai L P, Zheng T Y, Xu K, Han Y X, Xu L L, Huang E Q. et al. A Universal Design of Betacoronavirus Vaccines against COVID-19, MERS, and SARS. Cell. 2020;182(3):722-733 e11
49. Yang S L, Li Y, Dai L P, Wang J F, He P, Li C G. et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;21(8):1107-1119
50. Emary K R W, Golubchik T, Aley P K, Ariani C V, Angus B, Bibi S. et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397(10282):1351-1362
51. Zhao X, Zheng A Q, Li D D, Zhang R, Sun H, Wang Q H. et al. Neutralisation of ZF2001-elicited antisera to SARS-CoV-2 variants. Lancet Microbe. 2021;2(10):e494
52. Hu J, Peng P, Cao X X, Wu K, Chen J, Wang K. et al. Increased immune escape of the new SARS-CoV-2 variant of concern Omicron. Cell Mol Immunol. 2022;19(2):293-295
53. Kwok H F. Review of Covid-19 vaccine clinical trials ? A puzzle with missing pieces. Int J Biol Sci. 2021;17(6):1461-1468
54. Cao Y L, Hao X H, Wang X, Wu Q H, Song R, Zhao D. et al. Humoral immunogenicity and reactogenicity of CoronaVac or ZF2001 booster after two doses of inactivated vaccine. Cell Research. 2022;32(1):107-109
55. Lu L, Mok B W, Chen L L, Chan J M, Tsang O T, Lam B H. et al. Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clin Infect Dis. 2021 ciab1041
56. Zhao X, Li D D, Ruan W J, Chen Z H, Zhang R, Zheng A Q. et al. Effects of a Prolonged Booster Interval on Neutralization of Omicron Variant. New Engl J Med. 2022;386(9):894-896
57. Ferenci T, Sarkadi B. RBD-specific antibody responses after two doses of BBIBP-CorV (Sinopharm, Beijing CNBG) vaccine. BMC Infect Dis. 2022;22(1):87
58. Jeewandara C, Aberathna I S, Pushpakumara P D, Kamaladasa A, Guruge D, Wijesinghe A. et al. Persistence of immune responses to the Sinopharm/BBIBP-CorV vaccine. Immun Inflamm Dis. 2022;10(6):e621
59. Ai J, Zhang Y, Zhang H, Zhang Q, Fu Z, Lin K. et al. Safety and immunogenicity of a third-dose homologous BBIBP-CorV boosting vaccination: interim results from a prospective open-label study. Emerg Microbes Infect. 2022;11(1):639-647
60. Zhang H, Liu Y, Liu D, Zeng Q, Li L, Zhou Q. et al. Time of day influences immune response to an inactivated vaccine against SARS-CoV-2. Cell Res. 2021;31(11):1215-1217
61. Stamatatos L, Czartoski J, Wan Y H, Homad L J, Rubin V, Glantz H. et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;372(6549):1413-1418
62. Reynolds C J, Pade C, Gibbons J M, Butler D K, Otter A D, Menacho K. et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021;372(6549):1418-1423
63. Accorsi E K, Britton A, Fleming-Dutra K E, Smith Z R, Shang N, Derado G. et al. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. Jama-J Am Med Assoc. 2022;327(7):639-651
64. Gruell H, Vanshylla K, Tober-Lau P, Hillus D, Schommers P, Lehmann C. et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat Med. 2022;28(3):477-480
65. YangYang Gong X, Yang L Li J, Zhang J Wei L. et al. Regular and booster vaccination with inactivated vaccines enhance the neutralizing activity against Omicron variant both in the breakthrough infections and vaccinees. J Infect. 2022;84(4):579-613
66. Ai J W, Zhang H C, Zhang Y, Lin K, Zhang Y L, Wu J. et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infec. 2022;11(1):337-343
67. Liu Y, Zeng Q, Deng C, Li M, Li L, Liu D. et al. Robust induction of B cell and T cell responses by a third dose of inactivated SARS-CoV-2 vaccine. Cell Discov. 2022;8(1):10
68. Corbett K S, Gagne M, Wagner D A, O' Connell S, Narpala S R, Flebbe D R. et al. Protection against SARS-CoV-2 Beta variant in mRNA-1273 vaccine-boosted nonhuman primates. Science. 2021;374(6573):1343-1353
Author contact
![]() Corresponding author: kluoedu.mo
Corresponding author: kluoedu.mo

 Global reach, higher impact
Global reach, higher impact