Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(12):4669-4676. doi:10.7150/ijbs.74676 This issue Cite
Review
Natural tannins as anti-SARS-CoV-2 compounds
1. Graduate Institute of Biomedical Sciences, College of Medicine, China Medical University, Taichung, Taiwan, 40402
2. Center for Molecular Medicine, China Medical University Hospital, Taichung, Taiwan, 40447
3. Research Center for Cancer Biology, China Medical University, Taichung, Taiwan, 40402
4. Department of Biotechnology, Asia University, Taichung, Taiwan, 41354
*These two authors contributed equally.
Received 2022-5-3; Accepted 2022-5-21; Published 2022-7-11
Abstract
Tannins are polyphenols enriched in wood, bark, roots, leaves, seeds and fruits of a variety of plants. Over the last two decades, there has been an increasing interest in understanding the biological functions of tannins and their applications as antioxidants, anticancer drugs, and food additives. Since the outbreak of the COVID-19 pandemic, much effort has been devoted to finding an expedient cure. Tannins have been put forward as having possible anti-COVID-19 properties; however, owing to the profuse nature of the structurally diverse derivatives of tannins, the tannin species in the family associated with an indication of anti-COVID-19 have been poorly defined, compounded by frequent terminology misnomers. This article reviews the tannin family in fruits and the current knowledge about the activities of the compounds with regard to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It will aid molecular and cellular biologists in developing natural anti-viral chemicals as means of overcoming the current and future pandemics.
Introduction
Beginning in early 2020, at time of writing the COVID-19 coronavirus pandemic has lasted for over two years, with about 507 million cases of infection and more than 6.2 million deaths (April 2022; Global Change Data Lab). Several anti-COVID vaccines have been rushed into the clinic globally, debuting mRNA technology in vaccines. However, although vaccination has significantly reduced the extent and duration of symptoms, the efficacy of anti-viral immunity declines rapidly and breakthrough infections frequently occur even in the fully vaccinated population [1-3]. Thus, on the edge of surging infections worldwide, coexisting with the virus is becoming a more realistic solution [4, 5]. Thus, in the post-pandemic world, regimens including anti-SARS-CoV-2 small molecules are highly desirable. Currently, three SARS-CoV-2 inhibitors, remdesivir/VEKLURY (Gilead) [6], ritonavir/PAXLOVID (Pfizer) [7], and molnupiravir/LAGEVRIO (Merck) [8] have been authorized by the US FDA to treat patients with symptomatic SARS-CoV-2. However, they are mostly used for treatment of patients with mild-to-moderate symptoms [1, 9]. Furthermore, concerns about the effectiveness of these drugs in blocking the emerging variants and the potential mutagenic activity of molnupiravir in causing viral variants have been raised [10, 11]. Alternatively, repurposing the existing FAD-approved drugs may also be a promising strategy for timely development of anti-COVID molecules. For example, multiple groups have reported that imatinib/GLEEVEC, a clinically approved targeted tyrosine kinase inhibitor in cancer therapy, is effective in suppressing COVID-19 infection through different mechanisms [12-14].
Considering the fact that living with infectious pathogens such as coronaviruses is likely to be a norm in the future world [15-17], safe and potent anti-viral compounds with the potential to become acute treatments as well as chronic prevention methods are becoming an attractive approach [18]. Intuitively, natural products are the promising sources of such chemicals [19, 20]. A long track record of past and recent studies has demonstrated the activities of the abundant tannins derived from numerous common fruits in suppressing viral infection (see below). This review summarizes the current understanding of the tannin compounds which are relatively abundant in fruits, for their biological activities, with particular focus on COVID-19 inhibition. Reviews of the chemistry and biochemistry of the naturally occurring tannins compounds can be found elsewhere by others [21-23].
Classification of tannins
Characterization based on chemical structures classifies tannins into different categories: hydrolysable tannins, condensed tannins, complex tannins, and phlorotannins. Phlorotannins, are of low abundance and present mainly in brown macroalgae; however, tannins of the other categories are abundant in terrestrial plants [24]. Hydrolysable tannins can be further subdivided into gallotannins, commonly referred to as tannic acid (TA) and ellagitannins such as punicalagin. Gallotannins are relatively rare in nature [25]. The complex tannins are structurally characterized by a gallotannin or ellagitannin unit followed by a catechin unit. Condensed tannins include proanthocyanidins, catechins, and procyanidins, and are the most abundant of all tannins followed by the hydrolysable tannins [26]. The hydrolysable tannins are prone to oxidation [27] and characterized by their short half-lives [28]. In an oxidative environment, tannic acid can be rapidly degraded into gallic acid [29]. Due to the unstable nature of tannic acid, there have been only few studies investigating its exact content in natural products or wines. In some other studies, the nomenclatures are overlap in tannic acid and other tannins.
Levels of tannins in wine and fruits
Some of the winemaking factors determining the flavor of wine products are the phenolic substances extracted from the seeds, skin, and stems of grapes as well as the wooden casks during the fermentation process. Due to technical limitations and short half-lives, the exact contents of tannic acid in different wines or fruits have been poorly characterized. Most studies measured the tannin content rather than tannic acid. It was indicated in some studies that hydrolysable tannins do not exist naturally in grape-derived wines [30]. Others identified tannic acid in grape-derived wines sourced from the wooden barrels and the various parts of grapes during the temperature-dependent brewing process [31, 32]. Depending on the winery, tannic acid can be added to the wines to enhance the astringent taste [33]. Tannic acid in the solution can interact with the major wine protein VVTL1 (Vitis vinifera Thaumatin-Like-1), resulting in conformational change of the protein VVTL1 as demonstrated by circular dichroism spectra in the near-UV region [30]. It is estimated that the tannic acid content (dry weight) is 4.1g/100g in grape seeds [34]. For tannins in general, their contents are relatively high in fruits such as persimmon, pomegranate, grape, cashew apple, guava, chokeberries, blackberries, raspberries, apple, banana, etc [25, 26, 35, 36]. The sources and analytical methods of studies measuring tannins are summarized in Table 1. Most grape-derived tannins are condensed tannins [37]. In grapes and berries, it has been estimated that 80% and 20% of tannins are from seed and skin, respectively [38]. In wines, 60% of total tannins are generated from grape skin [38]. The average tannin concentration of over 1300 commercial red wines from different regions (California, Oregon, Washington, Australia, and France) was 544 ± 293 mg/L catechin equivalents (CE). Among them, the top five high-tannin red wines are Cabernet Sauvignon (672 mg/L CE), Zinfandel (652 mg/L CE), Merlot (559 mg/L CE), Syrah (455 mg/L CE) and Pinot noir (348 mg/L CE) [39]. Thus, tannin concentrations in wines vary dramatically.
The biological effects of fruit-derived tannins
The health benefit of red wine extracts as nutritional supplements is a popular topic on social media. However, little research provides controlled experimental evidence about the effects of red wine extracts on human subjects. The biological effects of tannins in natural products and wines have been investigated by different groups (Table 2). Tsuchiya et al., for example, showed that administration of a test beverage including 200 mg/day oligomeric procyanidins (condensed tannins) isolated from red wines improved skin condition of moisturizing and whitening in a group of 30-60 year-old healthy women in 12 weeks [40]. Kitada et al. reported that daily supplementation of resveratrol (19.2 mg) and polyphenols (136 mg) extracted from red wine for 8 weeks improved the metabolic profile including enhancing insulin sensitivity and decreasing serum low-density lipoprotein-cholesterol as well as triglyceride in non-diabetic individuals [41]. Moreover, Ueda et al. demonstrated the anti-viral effects of persimmon-derived tannins against viruses including influenza virus, herpes simplex virus-1 and vesicular stomatitis virus by inhibition of viral infection [42]. Mechanistically, it has been shown that the catechol units of tannic acid function as scavengers of reactive oxygen species (ROS), contributing to the anti-oxidative, anti-inflammatory and anti-carcinogenic activities of tannic acid [43, 44].
Dietary supplementation with tannic acid helped improve cognition and behavioral dysfunctions, in part by inhibiting the processing of amyloidogenic precursor proteins, reducing the production and cerebral vascular deposits of β-amyloid, and reducing neuroinflammation manifested by β-amyloid plaque-associated microgliosis in aged Alzheimer-like transgenic mice [45]. Tannic acid protected rat kidneys after ischemia-reperfusion from oxidative stress through activation of nuclear factor erythroid-2-Related factor 2 (NRF2) [46]. Subcutaneously administered tannic acid decreased myocardial fibrosis and reduced the levels of inflammatory cytokines and apoptosis-associated mediators such as toll-like receptor 4 (TLR4), Nuclear factor kappa B (NF-κB), B-cell lymphoma-2 (Bcl-2), Bcl-2-associated protein (Bax) and the stress-responding kinase p38 in a myocardial fibrosis mouse model induced by isoproterenol [47]. Additionally, tannic acid reduced kidney damage modulated by NF-κB and NRF2 pathways, resulting in the reduction of oxidative stress, apoptosis and inflammatory cytokines such as IL-6, IL-8 and TNF-α in an arsenic trioxide-induced nephrotoxicity rat model [48].
The sources and analytical methods of tannins
| Research title | Source | Tannin type | Method | Ref. |
|---|---|---|---|---|
| Comparison of analytical methods for the determination of condensed tannins in grape skin | 38 grape skin samples | Condensed tannin | protein and methylcellulose precipitation and by HPLC-phloroglucinolysis | [31] |
| Inhibitory effect of tannic acid extracted from grape seeds and pomegranate peels on some microorganisms | Seeds of local grape fruit and peels of pomegranate fruit | Hydrolysable tannin (tannic acid) | Chromatographic separation and automated analysis | [34] |
| Tannin contents of selected plants used in Jordan | Anise, broad beans, chamomile chard, chickpea, clary, cocoa, coriander, dates, eggplant, fennel, fenugreek, figs, garden rocket, gundelia, hawthorn, Jew's mallow, lentils, liquorice locust, mint, oak, okra, parsley, plums, pomegranate, red and green grapes, rosemary, sage, sumac, tea, thyme, tomatoes, turmeric, verbena, vine leaves, wheat | Total tannins | HPLC | [76] |
| Antioxidant properties of different fruit seeds and peels | Gooseberry, watermelon, apple, plum, melon, red grapes, white grapes, lemon, red grapefruit, white grapefruit, kiwi fruit, orange | Total tannins | Folin-Ciocalteu method | [36] |
| Variability of tannin concentration in red wines | 1325 commercial red wines | Total tannins | Protein precipitation | [39] |
| Tannins in fruit juices and their removal [REVIEW] | Fruit juice (apple, banana, cashew apple, guava, grape, jamun, persimmon, pomegranate, myrobalan, sugarcane) | Total tannins; condensed tannins, hydrolysable tannins (punicalagin, ellagic acid) | protein precipitation method, Folin-Denis method | [35] |
| Technological application of tannin-based extracts [REVIEW] | Nut, black currant, strawberry, European blackberry, black raspberry, Pomegranate, Guava, mango, almond. | Condensed tannins, hydrolysable tannins | HPLC, vanillin-HAL, gas chromatography, spectrophotometric techniques, radial diffusion, acid butanol (spectrophotometric) assays | [26] |
| Proanthocyanidins and hydrolysable tannins: occurrence, dietary intake and pharmacological effects [REVIEW] | Fruits (cranberries, chokeberries, plums, black diamond, blueberries, black currants, red currants, blackberries, crowberries, lingonberries, red grapes, grape seeds, strawberries, peaches, apricot, raspberries, pears, apple, pomegranate, guava, mango), juice (cranberry, apple, grape), nuts (almonds, hazelnuts, pecans, pistachio nuts, walnuts), legumes (beans, carob fiber, cowpeas, lentils, peanuts), cereal grains (barley, buckwheat, sorghum, rice), beverages (wine, tea), cacao beans, chocolate | Condensed tannin (proanthocyanidins), hydrolysable tannins (ellagitannins and gallotannins) | HPLC | [25] |
| Health effects, sources, utilization and safety of tannins: a critical review [REVIEW] | Fruits (majuphal, babul, amla, ripened banana, red supari, munakka, dates, raisins, badillayachi, persimmon, mango, samgiri), leafy vegetables (canola, drumstick, bathua, gotu kola, joseph's coat, edible amaranth, fenugreek, desert horse purslane, plumed cockscomb, snakeroots, punarnava, Mexican mint, false amaranth, benghal dayflower, shona cabbage, buttercup, white gulmohur, musk thistle), cereals and millets (rice, wheat, sorghum, bajra, ragi, finger millets, pearl millet), seeds/nuts (cumin seeds, mango seeds, fenugreek, coffee, castor seeds, faba beans, tamarind seeds, almond, brazil nut, cashew nut, virginia peanut, walnut, pistachio, pecan, pine nut, hazelnut, macadamia nut), legumes (pigeon pea, chickpea, green gram, red gram, soya bean, kidney bean, cowpea) | Total tannins | Spectrophotometric method, HPLC, Folin-Denis method | [77] |
Administration and effects of tannins in humans.
| Tannin source | Type of tannin | Route | Disease (the number of subjects) | Biological effects | Outcome-based potential benefits for COVID-19 prevention and cure | Ref. |
|---|---|---|---|---|---|---|
| Grape seed extract | Condensed tannins (proanthocyanidins) | Oral | Healthy (8) | Lipid hydroperoxide↓; oxidants↓; antioxidant capacity↑; resistance to oxidative modification of LDL↑; prevent plasma postprandial oxidative stress in humans. | Antioxidant capacity↑ | [78] |
| Grape seed extract | Total tannins | Oral | Metabolic syndrome (27) | Systolic and diastolic blood pressures↓; no significant changes in serum lipids or blood glucose values. | --- | [79] |
| Lyophilized grape powder | Total tannins (grape polyphenols) | Oral | Women (24 premenopausal and 20 postmenopausal) | Triglyceride↓; LDL↓; apolipoproteins B and E↓; cholesterol ester transfer protein activity↓; whole-body oxidative stress↓; plasma TNF-α↓; exert a cardioprotective effect by lowering plasma lipids and reducing oxidative stress. | Plasma TNF-α↓ | [80] |
| Flavonoids and phenolic acids from cranberry juice | Condensed tannins (proanthocyanidins) | Oral | Healthy postmenopausal women (10) | Plasma total antioxidant capacity↑; resistance of LDL against oxidation↑ | Plasma total antioxidant capacity↑ | [81] |
| Bath additive containing tannic acid | Hydrolysable tannin (tannic acid) | Bath | Atopic dermatitis (21) | Improvement of pruritus↑ | --- | [82] |
| A tannic acid-based medical food, Cesinex® | Hydrolysable tannin (tannic acid) | Oral | Diarrhea (10) | Diarrhea↓; transepithelial resistance↑; CFTR-dependent or the calcium-activated Cl- secretion↓; improve the impaired epithelial barrier function induced by TNFα; Cesinex® has high antioxidant capacity. | Improve the impaired epithelial barrier function induced by TNFα; high antioxidant capacity | [83] |
| Grape seed proanthocya-nidin | Condensed tannins (oligomeric proanthocya-nidin) | Oral | Asymptomatic carotid plaques or abnormal plaque free carotid intima-media thickness (287) | progression of carotid atherosclerotic plaques↓; carotid plaque size↓; the number of plaques↓; incidence rate for transitory ischemic attack↓; arterial revascularization procedure↓; clinical vascular events↓ | --- | [84] |
| Cherry juice | Condensed tannins (procyanidin B2) | Oral | Insomnia (11) | sleep time↑; sleep efficiency↑; serum kynurenine to tryptophan ratio↓; serum PGE2↓; inflammation↓; inhibition of inhibited indoleamine 2, 3-dioxygenase with a reduction in the degradation of tryptophan | Serum kynurenine to tryptophan ratio↓; serum PGE2↓; inflammation↓; inhibition of inhibited indoleamine 2, 3-dioxygenase | [85] |
| Grape seed extract | Total tannins | Oral | Postmenopausal women (46) | Grape seed extract did not decrease plasma estrogens (estrone, estradiol, estrone sulfate) and did not increase precursors of androgens (testosterone and androstenedione). | --- | [86] |
| Flavonoid-rich blueberry beverage | Condensed tannins (antho- and pro-cyanidins) | Oral | Healthy (18) | Improved cognitive function. | --- | [87] |
| Flavonoid-rich apple with skin | Total tannins | Oral | Healthy (30) | Improves endothelial function assessed using flow-mediated dilation of the brachial artery in individuals at risk for cardiovascular disease. | --- | [88] |
| Red grape seed extract | Total tannins | Oral | Mildly hyperlipidemia (52) | Total cholesterol↓; LDL cholesterol↓; Ox-LDL↓; the risk of atherosclerosis and cardiovascular disorders↓ | --- | [89] |
| Cranberry beverage | Total tannins | Oral | Healthy (54) | Human γδ-T cell proliferation (percentage of the CD3+ population)↑; the number of symptoms associated with colds and influenza↓; the ability of PBMC to secrete IFN-γ ↑ | Human γδ-T cell proliferation (percentage of the CD3+ population)↑; the ability of PBMC to secrete IFN-γ ↑ | [90] |
| Grape seed extract | Condensed tannin (proanthocyanidins) | Oral | Heathy (11) | Condensed tannin does not affect iron status or bioavailability. | --- | [91] |
| Actitan F | Total tannins | Oral | Acute gastroenteritis (60) | The number of stools↓ | --- | [92] |
| Persimmon fruit tannin-rich fiber | Condensed tannins | Oral | Healthy (40) | Plasma total cholesterol levels↓; plasma low-density lipoprotein cholesterol levels↓ | --- | [93] |
| Oligomeric proanthocya-nidins of red wine | Condensed tannins | Oral | Healthy (100) | skin whitening and moisturizing↑ | --- | [40] |
| Red wine extract | Total tannins | Oral | Healthy (12) | Insulin sensitivity↑; peripheral blood mononuclear Sirt1 and p-AMPK↑; LDL-C↓; triglyceride↓; IL-6↓ | Peripheral blood mononuclear Sirt1 and p-AMPK↑; IL-6↓ | [41] |
The anti-inflammatory and anti-oxidant activities of tannic acid were exploited by a nanogel-based delivery system into the murine macrophage cell line RAW264.7. Treatment with tannic acid significantly lowered the levels of intracellular ROS, TNF-α and IL-6 induced by phorbol 12-myristate 13-acetate (PMA) [49] as an inflammation activator. Similar benefits have also been observed in a mouse model of zymosan-induced peritonitis [50]. In a chronic alcohol-induced mice model, treatment with tannic acid-encapsulated Poly D, L-lactide-co-glycolic acid (PLGA) nanoparticles mitigated the levels of triglyceride, total cholesterol, and the inflammation cytokines such as TGF-β, IL-6, TNF-α and IL-1β, resulting in lowering liver oxidative stresses, hepatic apoptosis, and promoting functional recovery [51]. It was proposed that the hepato-protection of tannic acid is through its binding with the receptor tyrosine kinase receptor EGFR, which in turn activates the EGFR-AKT-STAT3 pathway [51].
The potential anti-tumor activities of tannins have been explored in numerous studies. The addition of tannic acid in cholangiocarcinoma chemotherapeutic regimens such as mitomycin C and 5-fluorouracil can achieve a synergistic effect which is believed to result from modulating the drug efflux pathways by tannic acid, thus increasing chemotherapy-induced apoptosis by the treatment [52].
It seems that the function of tannic acid in mediating oxidoreductive stresses is cell context dependent. In contrast to its anti-ROS anti-inflammatory cell-protective activities as observed in non-transformed cells, tannic acid induced apoptosis in prostate cancer cells through increasing the level of ROS, inducing ER stress, inhibiting tumor lipogenesis, and disrupting the plasma membrane as well as the nuclear structure [53]. It is also reported that tannic acid possesses a dual pathway to induce human embryonic carcinoma apoptosis [54]. Tannic acid repressed the Wnt/β-catenin signaling pathway and induced TNF-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis by increasing ROS levels in the mitochondria [54]. Fruits and vegetable juices enriched with biologically active phytochemicals have been suggested to contribute to proliferation inhibition and apoptosis in breast cancer cell lines such as MCF-7 and MDA-MB-231, as well as suppression of breast cancer xenograft tumor growth in rodent models [55]. Indeed, fruit and juice consumption is associated with reduced risk of breast cancer in a dose-dependent manner in a cohort study [56]. It is speculated that the polyphenols of the fruits and vegetables are the main source of the anti-inflammatory and anti-oxidant components which in turn inhibit cancer development [55].
The anti-COVID-19 activities of tannins
The two major frontiers in battling the COVID-19 are infection and propagation of the SARS-CoV-2 coronaviruses, and the possible extreme reaction of the body's immune system in response to pathogenic intrusion [57]. While most research efforts have been devoted to preventing viral infection or eradicating the virus from the body, relatively less attention has been focused on developing methods to cope with the inflammatory responses, the “cytokine storm”, over the course of COVID-19. Elevations of IL-6, IL-1β, TNF-α, IFN-γ and IL-10, as well as reductions in CD4+ and CD8+ T lymphocytes have been observed in COVID-19 patients, especially in severe cases [58]. Currently, several polyphenol-based [59] and tannin-based clinical trials (NCT04911777 [60], NCT04403646 [61], and IRCT20200418047122N1 [62]) have been conducted with COVID-19 patients. Oral tannins extracted from quebracho and chestnut in combination with B12 vitamin and standard treatment reduced macrophage inflammatory protein-1α (MIP-1α) and TNF-α levels in hospitalized COVID-19 patients [63]. In a Syrian hamster model of COVID-19, persimmon-derived tannins pre-administered by oral gavage were able to suppress SARS-CoV-2 titers, reduce the severity of pneumonia and decrease inflammation-related gene expression such as IL-6, TNF-α, and IFN-γ [64].
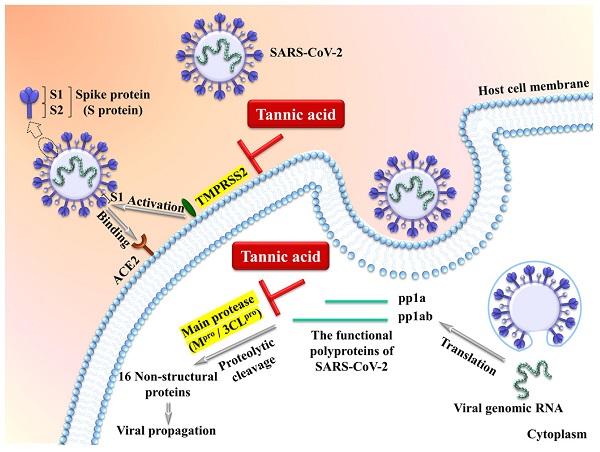
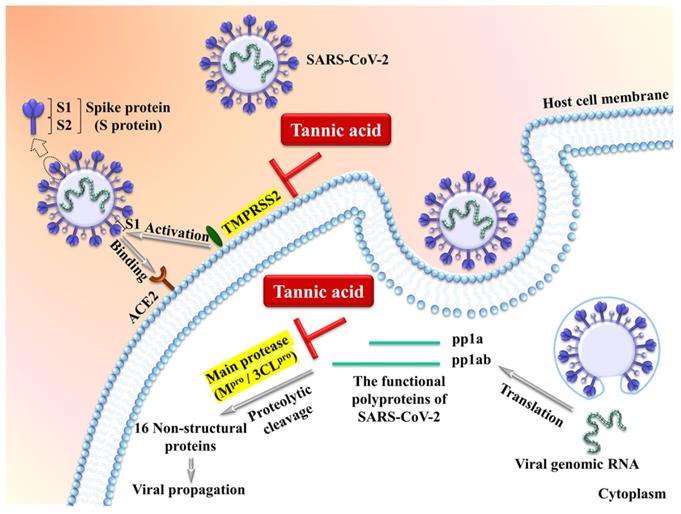
The transmembrane protease serine 2 TMPRSS2 is an essential cell surface protease which processes the viral surface spike protein (S protein) to enhance its binding to the cell surface receptor ACE2 (angiotensin converting enzyme 2) followed by cell entry via membrane fusion. The intracellular viral genomic RNA is then translated into polyproteins which are subject to further proteolytic cleavage by the viral main protease/3-chymotrypsin-like cysteine protease (Mpro/3CLpro) to produce non-structural functional proteins essential for viral propagation (Figure 1) [65-70]. It has been demonstrated by multiple groups that tannic acid is a potent dual inhibitor of TMPRSS2 and Mpro/3CLpro to inhibit the SARS-CoV-2 activity [65-69]. Tannic acid inhibits Mpro/3CLpro with IC50 ranging from 1 μM to 13.4 Μm [65-69], and inhibits TMPRSS2 with IC50 ranging from 2.31 μM to 50 μM [68, 69]. The variable IC50 may result from the different methodologies such as fluorescence resonance energy transfer and ELISA-based enzymatic assays. Importantly, the dual inhibition of TMPRSS2 and Mpro/3CLpro by tannic acid can be translated into suppression of cellular entry of the virus [68]. Thus, tannic acid shows high potential for inhibition of SARS-CoV-2 among plant-derived polyphenols. Similar inhibitory activities of Mpro/3CLpro by the tannins of dimeric proanthocyanidins [71], punicalagin [72] and mixtures of tannic acid with plant-derived polyphenols [67] have also been observed in in vitro studies.
Consistently, it has been reported that green tea-derived tannins inhibited viral replication of SARS-CoV-2 in vitro and stably persist in the pharyngeal mucosa 1 hour after administration by throat spray [73], supporting the potential of green tea tannins in anti-COVID-19 therapies. Moreover, the hydrolysable tannins such as pedunculagin, tercatain, and castalin have been demonstrated to be potential inhibitors of SARS-CoV-2 using the molecular operating environment software (MOE 09) which is an integrated computer-aided molecular design platform [74]. These natural compounds are predicted to be able to bind to the catalytic dyad residues of Mpro/3CLpro and inhibit the enzyme activity [74]. It is worth mentioning that oral administration of highly-purified isomers of tannic acid exerts anti-COVID-19 activity against both the omicron and delta variants [60]. A phase III clinical trial will be carried out in 2022 (NCT04911777).
Tannic acid acts as a potent dual inhibitor of the cell surface protease TMPRSS2 and the viral main protease (Mpro/3CLpro) to inhibit the SARS-CoV-2 activity [65-70].
Conclusion
Substantial evidence supports the ameliorating functions of tannins to treat diseases involving metabolic dysregulation and protect against cancer through opposing the damage caused by oxidative stresses and inflammatory insults. A large body of studies highlights the therapeutic potential of tannins, particularly tannic acid, in preventing and treating of SARS-CoV-2 infection. Interestingly, a high-efficiency particulate air (HEPA) filter coated with tannic acid has been developed which showed rapid and highly efficient capability to capture the influenza A virus, revealing a new application of tannic acid to constrain virus spread [75]. Thus, it is expected that tannin derivatives and natural source products can be exploited to develop safe and promising strategies in responding to globally emerging new viruses or other pathogens.
As the world is evolving into the post-pandemic era of COVID-19 with potential unknown variants arising in the horizon, tapping the natural sources for safe and effective medication is an attractive resort. As summarized, a body of studies support that incorporating daily diet with the source food of anti-viral activity can have protective or ameliorating effects to curb viral infection. Thus, it is of high public interests to entail a clinically defined dieting practice for disease control. For example, the known concentrations of the different tannins required for viral inhibition can be used as a guidance to develop the formulae consisting of quality-defined natural products such as tea and fruits for daily consuming by the general population. Furthermore, given the potency of the tannin derivatives in curtailing SARS-CoV-2 infection in vitro, compounds derived from modified tannins can be exploited for the development of anti-COVID drugs. Finally, it is worth exploring to determine whether combination of the tannin-based drugs with the FDA-approved COVID-19 medicines can enhance the therapeutic efficacy. The progression in these directions may also be applied to other viruses and help us prepare to fight the arising health-threatening pathogens in the future.
Acknowledgements
We acknowledge the support from the following grants: (1) MOST 109-2327-B-039-003 and MOST 110-2639-B-039-001-ASP (to M.C.-H); (2) NHRI-EX110-11012BI and NHRI-EX111-11012BI (to S.C.-W); (3) This study was supported in part by the Research Center for Cancer Biology of the China Medical University and the Center for Molecular Medicine of the China Medical University Hospital.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C. et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474-84
2. Lipsitch M, Krammer F, Regev-Yochay G, Lustig Y, Balicer RD. SARS-CoV-2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nat Rev Immunol. 2022;22:57-65
3. Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E. et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532-46
4. Yoo J-H. Antivirals for coexistence with COVID-19: brief review for general physicians. J Korean Med Sci. 2021;36:e298
5. Chotpitayasunondh T, Fischer TK, Heraud JM, Hurt AC, Monto AS, Osterhaus A. et al. Influenza and COVID-19: what does co-existence mean? Influenza Other Respir Viruses. 2021;15:407-12
6. Olender SA, Perez KK, Go AS, Balani B, Price-Haywood EG, Shah NS. et al. Remdesivir for severe coronavirus disease 2019 (COVID-19) versus a cohort receiving standard of care. Clin Infect Dis. 2021;73:e4166-e74
7. Lamb YN. Nirmatrelvir plus Ritonavir: first approval. Drugs. 2022;82:585-91
8. Syed YY. Molnupiravir: first approval. Drugs. 2022;82:455-60
9. Bhattacharyya RP, Hanage WP. Challenges in inferring intrinsic severity of the SARS-CoV-2 omicron variant. N Engl J Med. 2022;386:e14
10. Dyer O. Covid-19: FDA expert panel recommends authorising molnupiravir but also voices concerns. BMJ. 2021;375:n2984
11. Zhou S, Hill CS, Sarkar S, Tse LV, Woodburn BMD, Schinazi RF. et al. β-d-N4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells. J Infect Dis. 2021;224:415-9
12. Strobelt R, Adler J, Paran N, Yahalom-Ronen Y, Melamed S, Politi B. et al. Imatinib inhibits SARS-CoV-2 infection by an off-target-mechanism. Sci Rep. 2022;12:5758
13. Lin Y-Z, Shen Y-C, Wu W-R, Wang W-J, Wang Y-L, Lin C-Y. et al. Imatinib (STI571) inhibits the expression of angiotensin-converting enzyme 2 and cell entry of the SARS-CoV-2-derived pseudotyped viral particles. Int J Mol Sci. 2021;22:6938
14. Li Z, Peng M, Chen P, Liu C, Hu A, Zhang Y. et al. Imatinib and methazolamide ameliorate COVID-19-induced metabolic complications via elevating ACE2 enzymatic activity and inhibiting viral entry. Cell Metab. 2022;34:424-40
15. The Lancet Regional Health-Europe. The vaccinated and unvaccinated need to coexist with tolerance and respect. Lancet Reg Health Eur. 2022;13:100326
16. Pataro IML, Oliveira JF, Morato MM, Amad AAS, Ramos PIP, Pereira FAC. et al. A control framework to optimize public health policies in the course of the COVID-19 pandemic. Sci Rep. 2021;11:13403
17. Al-Bsheish M, Jarrar Mt, Scarbrough A. A public safety compliance model of safety behaviors in the age of the COVID-19 pandemic. Inquiry. 2021;58:00469580211031382
18. Mallakpour S, Azadi E, Hussain CM. Protection, disinfection, and immunization for healthcare during the COVID-19 pandemic: role of natural and synthetic macromolecules. Sci Total Environ. 2021;776:145989
19. Ali S, Alam M, Khatoon F, Fatima U, Elasbali AM, Adnan M. et al. Natural products can be used in therapeutic management of COVID-19: probable mechanistic insights. Biomed Pharmacother. 2022;147:112658
20. Alzaabi MM, Hamdy R, Ashmawy NS, Hamoda AM, Alkhayat F, Khademi NN. et al. Flavonoids are promising safe therapy against COVID-19. Phytochem Rev. 2022;21:291-312
21. Khanbabaee K, van Ree T. Tannins: classification and definition. Nat Prod Rep. 2001;18:641-9
22. Maugeri A, Lombardo GE, Cirmi S, Süntar I, Barreca D, Laganà G. et al. Pharmacology and toxicology of tannins. Arch Toxicol. 2022;96:1257-77
23. Watrelot AA, Norton EL. Chemistry and reactivity of tannins in Vitis spp.: a review. Molecules. 2020;25:2110
24. Fraga-Corral M, Otero P, Cassani L, Echave J, Garcia-Oliveira P, Carpena M. et al. Traditional applications of tannin rich extracts supported by scientific data: chemical composition, bioavailability and bioaccessibility. Foods. 2021;10:251
25. Smeriglio A, Barreca D, Bellocco E, Trombetta D. Proanthocyanidins and hydrolysable tannins: occurrence, dietary intake and pharmacological effects. Br J Pharmacol. 2017;174:1244-62
26. Fraga-Corral M, García-Oliveira P, Pereira AG, Lourenço-Lopes C, Jimenez-Lopez C, Prieto MA. et al. Technological application of tannin-based extracts. Molecules. 2020;25:614
27. Miele S, Tegli S, Izquierdo CG, Cerboneschi M, Bargiacchi E. Hydrolysable tannins in agriculture. Tannins-structural properties, biological properties and current knowledge: IntechOpen. 2019
28. Tuominen A, Sundman T. Stability and oxidation products of hydrolysable tannins in basic conditions detected by HPLC/DAD-ESI/QTOF/MS. Phytochem Anal. 2013;24:424-35
29. Reitzer F, Berber E, Halgand J, Ball V, Meyer F. Use of gelatin as tannic acid carrier for its sustained local delivery. Pharm Front. 2020;2:e200002
30. Di Gaspero M, Ruzza P, Hussain R, Honisch C, Biondi B, Siligardi G. et al. The secondary structure of a major wine protein is modified upon interaction with polyphenols. Molecules. 2020;25:1646
31. Seddon TJ, Downey MO. Comparison of analytical methods for the determination of condensed tannins in grape skin. Aust J Grape Wine Res. 2008;14:54-61
32. Fitri A, Obitsu T, Sugino T. Effect of ensiling persimmon peel and grape pomace as tannin-rich byproduct feeds on their chemical composition and in vitro rumen fermentation. Anim Sci J. 2021;92:e13524
33. Valentová H, Skrovánková S, Panovská Z, Pokorný J. Time-intensity studies of astringent taste. Food Chem. 2002;78:29-37
34. S.Mohammed H. Inhibitory effect of tannic acid extracted from grape seeds and pomegranate peels on some microorganisms. Mesop J Agric. 2008;36:12-8
35. Prommajak T, Leksawasdi N, Rattanapanone N. Tannins in fruit juices and their removal. CMU J Nat Sci. 2020;19:76
36. Duda-Chodak A, Tarko T. Antioxidant properties of different fruit seeds and peels. Acta Sci Pol Technol Aliment. 2007;6:29-36
37. Obradovic D. Grape-derived tannins and their application. Aust N.Z. Grapegrow Winemak. 2006:66-73
38. Herderich M, Birse M, Dambergs R, Holt H, Iland P, Lattey K. et al. Grape and wine tannins-an overview on current research, emerging applications, and future challenges. Advances in tannin and tannin management: proceeding of ASVO seminar. 2005:4-10
39. Harbertson JF, Hodgins RE, Thurston LN, Schaffer LJ, Reid MS, Landon JL. et al. Variability of tannin concentration in red wines. Am J Enol Vitic. 2008;59:210-4
40. Tsuchiya T, Fukui Y, Izumi R, Numano K, Zeida M. Effects of oligomeric proanthocyanidins (OPCs) of red wine to improve skin whitening and moisturizing in healthy women - a placebo-controlled randomized double-blind parallel group comparative study. Eur Rev Med Pharmacol Sci. 2020;24:1571-84
41. Kitada M, Ogura Y, Monno I, Koya D. Supplementation with red wine extract increases insulin sensitivity and peripheral blood mononuclear Sirt1 expression in nondiabetic humans. Nutrients. 2020;12:3108
42. Ueda K, Kawabata R, Irie T, Nakai Y, Tohya Y, Sakaguchi T. Inactivation of pathogenic viruses by plant-derived tannins: strong effects of extracts from persimmon (Diospyros kaki) on a broad range of viruses. PLoS One. 2013;8:e55343
43. Gülçin İ, Huyut Z, Elmastaş M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arab J Chem. 2010;3:43-53
44. Liu F, Liu X, Chen F, Fu Q. Mussel-inspired chemistry: a promising strategy for natural polysaccharides in biomedical applications. Prog Polym Sci. 2021;123:101472
45. Mori T, Rezai-Zadeh K, Koyama N, Arendash GW, Yamaguchi H, Kakuda N. et al. Tannic acid is a natural β-secretase inhibitor that prevents cognitive impairment and mitigates Alzheimer-like pathology in transgenic mice. J Biol Chem. 2012;287:6912-27
46. Alechinsky L, Favreau F, Cechova P, Inal S, Faye P-A, Ory C. et al. Tannic acid improves renal function recovery after renal warm ischemia-reperfusion in a rat model. Biomolecules. 2020;10:439
47. Ma D, Zheng B, Du H, Han X, Zhang X, Zhang J. et al. The mechanism underlying the protective effects of tannic acid against isoproterenol-induced myocardial fibrosis in mice. Front Pharmacol. 2020 11
48. Jin W, Xue Y, Xue Y, Han X, Song Q, Zhang J. et al. Tannic acid ameliorates arsenic trioxide-induced nephrotoxicity, contribution of NF-κB and Nrf2 pathways. Biomed Pharmacother. 2020;126:110047
49. Zhao K, Huang Z, Lu H, Zhou J, Wei T. Induction of inducible nitric oxide synthase increases the production of reactive oxygen species in RAW264.7 macrophages. Biosci Rep. 2010;30:233-41
50. Yeo J, Lee J, Yoon S, Kim WJ. Tannic acid-based nanogel as an efficient anti-inflammatory agent. Biomater Sci. 2020;8:1148-59
51. Nag S, Manna K, Saha M, Das Saha K. Tannic acid and vitamin E loaded PLGA nanoparticles ameliorate hepatic injury in a chronic alcoholic liver damage model via EGFR-AKT-STAT3 pathway. Nanomedicine (Lond). 2020;15:235-57
52. Naus PJ, Henson R, Bleeker G, Wehbe H, Meng F, Patel T. Tannic acid synergizes the cytotoxicity of chemotherapeutic drugs in human cholangiocarcinoma by modulating drug efflux pathways. J Hepatol. 2007;46:222-9
53. Nagesh PKB, Chowdhury P, Hatami E, Jain S, Dan N, Kashyap VK. et al. Tannic acid inhibits lipid metabolism and induce ROS in prostate cancer cells. Sci Rep. 2020;10:980
54. Sp N, Kang DY, Jo ES, Rugamba A, Kim WS, Park Y-M. et al. Tannic acid promotes TRAIL-induced extrinsic apoptosis by regulating mitochondrial ROS in human embryonic carcinoma cells. Cells. 2020;9:282
55. Ferreira-Pêgo C, Vidović BB, Oliveira NG, Fernandes AS, Costa JG. Chapter 21 - Fruit and vegetable juices and breast cancer. In: Preedy VR, Patel VB, editors. Cancer (Second Edition). San Diego: Academic Press. 2021:235-44
56. Lima FELd, Latorre MdRDdO, Costa MJdC, Fisberg RM. Diet and cancer in Northeast Brazil: evaluation of eating habits and food group consumption in relation to breast cancer. Cad Saude Publica. 2008;24:820-8
57. Ning Q, Wu D, Wang X, Xi D, Chen T, Chen G. et al. The mechanism underlying extrapulmonary complications of the coronavirus disease 2019 and its therapeutic implication. Signal Transduct Target Ther. 2022;7:57
58. Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine Storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708
59. Santos JC, Ribeiro ML, Gambero A. The impact of polyphenols-based diet on the inflammatory profile in COVID-19 elderly and obese patients. Front Physiol. 2021;11:612268
60. SyneuRx. Proof of Principle Study to evaluate the safety, PK, viral shedding and efficacy of Pentarlandir™ UPPTA for patients with early COVID-19. Clinical trialAvailable in https://clinicaltrials.gov/ct2/show/NCT04911777. 2021
61. Molino S, Pisarevsky A, Mingorance FL, Vega P, Stefanolo JP, Repetti J. et al. Natural tannin extracts supplementation for COVID-19 patients (TanCOVID): a structured summary of a study protocol for a randomized controlled trial. Trials. 2021;22:310
62. Zeinalian M. Evaluation of the protective effect of a combination drug including Apocynin, Niacin, and Tannin on prevention of cardiovascular and respiratory morbidities and mortality in COVID-19 infection: a clinical trial. TrialID: IRCT20200418047122N1. Iranian registry of clinical trials in https://en.irct.ir/trial/47268. 2020
63. Pisarevsky AA, Mingorance FL, Vega P, Stefanolo JP, Repetti JA, Ludueña G. et al. Fr578 Oral tannins reduce proinflammatory cytokines associated with diarrhea and pneumonia in hospitalized COVID-19 patients. Gastroenterology. 2021;160:S-371
64. Furukawa R, Kitabatake M, Ouji-Sageshima N, Suzuki Y, Nakano A, Matsumura Y. et al. Persimmon-derived tannin has antiviral effects and reduces the severity of infection and transmission of SARS-CoV-2 in a Syrian hamster model. Sci Rep. 2021;11:23695
65. Coelho C, Gallo G, Campos CB, Hardy L, Würtele M. Biochemical screening for SARS-CoV-2 main protease inhibitors. PLoS One. 2020;15:e0240079
66. Chen C-N, Lin CPC, Huang K-K, Chen W-C, Hsieh H-P, Liang P-H. et al. Inhibition of SARS-CoV 3C-like protease activity by theaflavin-3,3'-digallate (TF3). Evid Based Complement Alternat Med. 2005;2:607278
67. Nguyen TTH, Jung J-H, Kim M-K, Lim S, Choi J-M, Chung B. et al. The inhibitory effects of plant derivate polyphenols on the main orotease of SARS coronavirus 2 and their structure-activity relationship. Molecules. 2021;26:1924
68. Wang S-C, Chen Y, Wang Y-C, Wang W-J, Yang C-S, Tsai C-L. et al. Tannic acid suppresses SARS-CoV-2 as a dual inhibitor of the viral main protease and the cellular TMPRSS2 protease. Am J Cancer Res. 2020;10:4538-46
69. Haddad M, Gaudreault R, Sasseville G, Nguyen PT, Wiebe H, Van De Ven T. et al. Molecular interactions of tannic acid with proteins associated with SARS-CoV-2 infectivity. Int J Mol Sci. 2022;23:2643
70. Zhu G, Zhu C, Zhu Y, Sun F. Minireview of progress in the structural study of SARS-CoV-2 proteins. Curr Res Microb Sci. 2020;1:53-61
71. Zhu Y, Xie D-Y. Docking characterization and in vitro inhibitory activity of flavan-3-ols and dimeric proanthocyanidins against the main protease activity of SARS-Cov-2. Front Plant Sci. 2020 11
72. Tito A, Colantuono A, Pirone L, Pedone E, Intartaglia D, Giamundo G. et al. Pomegranate peel extract as an inhibitor of SARS-CoV-2 spike binding to human ACE2 receptor (in vitro): a promising source of novel antiviral drugs. Front Chem. 2021 9
73. Kicker E, Tittel G, Schaller T, Pferschy-Wenzig E-M, Zatloukal K, Bauer R. SARS-CoV-2 neutralizing activity of polyphenols in a special green tea extract preparation. Phytomedicine. 2022;98:153970
74. Khalifa I, Zhu W, Mohammed HHH, Dutta K, Li C. Tannins inhibit SARS-CoV-2 through binding with catalytic dyad residues of 3CL(pro): an in silico approach with 19 structural different hydrolysable tannins. J Food Biochem. 2020: e13432.
75. Kim S, Chung J, Lee SH, Yoon JH, Kweon D-H, Chung W-J. Tannic acid-functionalized HEPA filter materials for influenza virus capture. Sci Rep. 2021;11:979
76. Ref'at AA, Takruri HR, Al-Sayyed H. Tannin contents of selected plants used in Jordan. Jordan J Agric Sci. 2008;4:265-74
77. Sharma K, Kumar V, Kaur J, Tanwar B, Goyal A, Sharma R. et al. Health effects, sources, utilization and safety of tannins: a critical review. Toxin Rev. 2019:1-13
78. Natella F, Belelli F, Gentili V, Ursini F, Scaccini C. Grape seed proanthocyanidins prevent plasma postprandial oxidative stress in humans. J Agric Food Chem. 2002;50:7720-5
79. Sivaprakasapillai B, Edirisinghe I, Randolph J, Steinberg F, Kappagoda T. Effect of grape seed extract on blood pressure in subjects with the metabolic syndrome. Metabolism. 2009;58:1743-6
80. Zern TL, Wood RJ, Greene C, West KL, Liu Y, Aggarwal D. et al. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J Nutr. 2005;135:1911-7
81. McKay DL, Chen CY, Zampariello CA, Blumberg JB. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem. 2015;168:233-40
82. Takahagi S, Harada N, Kamegashira A, Suzuki S, Shindo H, Kanatani H. et al. Randomized double-blind cross-over trial of bath additive containing tannic acid in patients with atopic dermatitis. J Cutan Immunol Allergy. 2020;3:56-61
83. Ren A, Zhang W, Thomas HG, Barish A, Berry S, Kiel JS. et al. A tannic acid-based medical food, Cesinex(®), exhibits broad-spectrum antidiarrheal properties: a mechanistic and clinical study. Dig Dis Sci. 2012;57:99-108
84. Cao AH, Wang J, Gao HQ, Zhang P, Qiu J. Beneficial clinical effects of grape seed proanthocyanidin extract on the progression of carotid atherosclerotic plaques. J Geriatr Cardiol. 2015;12:417-23
85. Losso JN, Finley JW, Karki N, Liu AG, Prudente A, Tipton R. et al. Pilot study of the tart cherry juice for the treatment of insomnia and investigation of mechanisms. Am J Ther. 2018;25:e194-e201
86. Wahner-Roedler DL, Bauer BA, Loehrer LL, Cha SS, Hoskin TL, Olson JE. The effect of grape seed extract on estrogen levels of postmenopausal women: a pilot study. J Diet Suppl. 2014;11:184-97
87. Dodd GF, Williams CM, Butler LT, Spencer JP. Acute effects of flavonoid-rich blueberry on cognitive and vascular function in healthy older adults. Nutr Healthy Aging. 2019;5:119-32
88. Bondonno NP, Bondonno CP, Blekkenhorst LC, Considine MJ, Maghzal G, Stocker R. et al. Flavonoid-rich apple improves endothelial function in individuals at risk for cardiovascular disease: a randomized controlled clinical trial. Mol Nutr Food Res. 2018 62
89. Razavi SM, Gholamin S, Eskandari A, Mohsenian N, Ghorbanihaghjo A, Delazar A. et al. Red grape seed extract improves lipid profiles and decreases oxidized low-density lipoprotein in patients with mild hyperlipidemia. J Med Food. 2013;16:255-8
90. Nantz MP, Rowe CA, Muller C, Creasy R, Colee J, Khoo C. et al. Consumption of cranberry polyphenols enhances human γδ-T cell proliferation and reduces the number of symptoms associated with colds and influenza: a randomized, placebo-controlled intervention study. Nutr J. 2013;12:161
91. Delimont NM, Fiorentino NM, Kimmel KA, Haub MD, Rosenkranz SK, Lindshield BL. Long-term dose-response condensed tannin supplementation does not affect iron status or bioavailability. Curr Dev Nutr. 2017;1:e001081
92. Russo M, Coppola V, Giannetti E, Buonavolontà R, Piscitelli A, Staiano A. Oral administration of tannins and flavonoids in children with acute diarrhea: a pilot, randomized, control-case study. Ital J Pediatr. 2018;44:64
93. Gato N, Kadowaki A, Hashimoto N, Yokoyama S, Matsumoto K. Persimmon fruit tannin-rich fiber reduces cholesterol levels in humans. Ann Nutr Metab. 2013;62:1-6
Author contact
![]() Corresponding authors: Mien-Chie Hung, Email: mhungcmu.edu.tw; Shao-Chun Wang, Email: scpwangcmu.edu.tw
Corresponding authors: Mien-Chie Hung, Email: mhungcmu.edu.tw; Shao-Chun Wang, Email: scpwangcmu.edu.tw

 Global reach, higher impact
Global reach, higher impact