ISSN: 1449-2288
Int J Biol Sci 2023; 19(10):3209-3225. doi:10.7150/ijbs.82266 This issue Cite
Research Paper
A Novel Isotope-labeled Small Molecule Probe CC12 for Anti-glioma via Suppressing LYN-mediated Progression and Activating Apoptosis Pathways
1. PhD Program for Cancer Molecular Biology and Drug Discovery, College of Medical Science and Technology, Taipei Medical University, Taipei 110, Taiwan; and Academia Sinica, Taipei 115, Taiwan, R.O.C.
2. Graduate Institute for Cancer Biology & Drug Discovery, College of Medical Science and Technology, Taipei Medical University, Taipei 110, Taiwan, R.O.C.
3. Department of Radiation Oncology, Show Chwan Memorial Hospital, Changhua 500, Taiwan, R.O.C.
4. Department of Radiation Oncology, Chang Bing Show Chwan Memorial Hospital, Lukang, Changhua 505, Taiwan, R.O.C.
5. Department of Medical Imaging and Radiological Sciences, Central Taiwan University of Science and Technology, Taichung 406, Taiwan, R.O.C.
6. Medical administrative center, Show Chwan Memorial Hospital, Changhua 500, Taiwan.
7. UPMC Hillman Cancer Center, University of Pittsburgh, Pittsburgh, PA 15260, USA.
8. Department of Pathology, University of Pittsburgh, Pittsburgh, PA 15260, USA.
9. Department of Biological Science and Technology, China Medical University, Taichung 406, Taiwan, R.O.C.
10. Organ Transplantation Center, China Medical University Hospital, Taichung 404, Taiwan, R.O.C.
11. Cell Therapy Center, China Medical University Hospital, Taichung 404, Taiwan, R.O.C.
12. Department of Pharmacology, School of Medicine, College of Medicine, Taipei Medical University, Taipei 110, Taiwan, R.O.C.
13. School of Post-baccalaureate Chinese Medicine, College of Chinese Medicine, China Medical University, Taichung 404, Taiwan, R.O.C.
14. Master Program in Graduate Institute of Cancer Biology and Drug Discovery, Taipei Medical University, Taipei 110, Taiwan, R.O.C.
*These authors contributed equally to this work.
Abstract
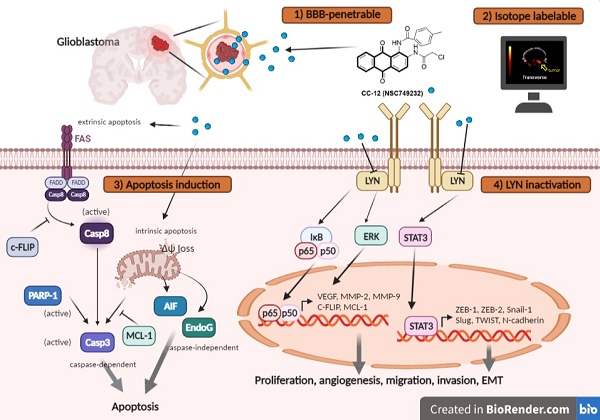
Background: Glioblastoma multiforme (GBM) is the most lethal malignancy in brain, which is surrounded by the blood-brain barrier (BBB), which limits the efficacy of standard treatments. Developing an effective drug that can penetrate the blood-brain barrier (BBB) remains a critical challenge in the fight against GBM. CC12 (NSC749232) is an anthraquinone tetraheterocyclic homolog with a lipophilic structure that may facilitate penetration of the brain area.
Methods: We used temozolomide sensitive and resistance GBM cells and animal model to identify the CC12 delivery, anti-tumor potential and its underlying mechanism.
Results: Importantly, toxicity triggered by CC12 was not associated with the methyl guanine-DNA methyl transferase (MGMT) methylation status which revealed a greater application potential compared to temozolomide. Alexa F488 cadaverine-labelled CC12 successfully infiltrated into the GBM sphere; in addition, 68Ga-labeled CC12 was also found in the orthotopic GBM area. After passing BBB, CC12 initiated both caspase-dependent intrinsic/extrinsic apoptosis pathways and apoptosis-inducing factor, EndoG-related caspase-independent apoptosis signaling in GBM. RNA sequence analysis from The Cancer Genome Atlas indicated that LYN was overexpressed in GBM is associated with poorer overall survival. We proved that targeting of LYN by CC12 may diminish GBM progression and suppress it downstream factors such as signal transduction and activator of extracellular signal-regulated kinases (ERK)/transcription 3 (STAT3)/nuclear factor (NF)-κB. CC12 was also found to participate in suppressing GBM metastasis and dysregulation of the epithelial-mesenchymal transition (EMT) through inactivation of the LYN axis.
Conclusion: CC12, a newly developed BBB-penetrating drug, was found to possess an anti-GBM capacity via initiating an apoptotic mechanism and disrupting LYN/ERK/STAT3/NF-κB-regulated GBM progression.
Keywords: Glioblastoma, Anthraquinone, CC12, LYN, Metastasis, Epithelial-mesenchymal transition

 Global reach, higher impact
Global reach, higher impact