10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(7):2607-2621. doi:10.7150/ijbs.93806 This issue Cite
Review
Glycosylation Targeting: A Paradigm Shift in Cancer Immunotherapy
1. Department of Oncology, the Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an, Shaanxi, China.
2. Key Laboratory of Resource Biology and Biotechnology in Western China, Ministry of Education, College of Life Sciences, Northwest University, Xi'an, Shaanxi, China.
Received 2024-1-2; Accepted 2024-4-18; Published 2024-4-22
Abstract
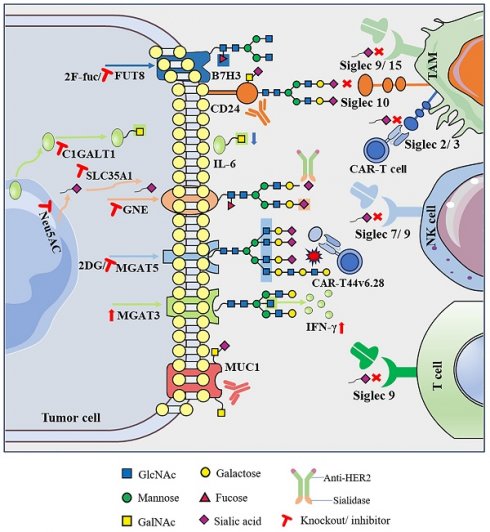
Immunotherapy has shown great potential in cancer treatment. However, even with the intervention of techniques such as immune checkpoint inhibitor therapy, tumors can still achieve immune escape, leading to a low response rate. Abnormal glycosylation is a widely recognized hallmark of cancer. The development of a complex “glyco-code” on the surface of tumor cells can potentially influence the immune system's ability to monitor tumors and can impact the anti-tumor immune response. Therefore, abnormal glycosylation has emerged as a promising target for immunotherapy. Many recent studies have shown that targeted glycosylation can reshape the tumor microenvironment (TME) and promote the immune response, thereby improving the response to immunotherapy. This review summarizes how glycosylation affects anti-tumor immune function in the TME and synthesizes the latest research progress on targeted glycosylation in immunotherapy. It is hoped that by elucidating the basic laws and biological connotations of glycosylation, this review will enable researcher to thoroughly analyze the mechanism of its influence on the immune metabolic regulation network, which will provide a theoretical tool for promoting the clinical application of glycosylation codes.
Introduction
In recent years, with continuous innovation in tumor treatment, fruitful results have been achieved, from traditional treatment methods (surgery, chemotherapy, and radiotherapy) to targeted drugs, and we have now entered a new era of immunotherapy. Unlike traditional treatments, immunotherapy utilizes cytokines, chemokines, and immune cells to reshape the tumor microenvironment (TME), thereby improving the anti-tumor efficacy and preventing recurrence [1]. The development of monoclonal antibodies (mAbs), immune checkpoint inhibitors (ICIs), adoptive cell transfer therapy (ACT), and tumor vaccines has shown surprising therapeutic prospects for some cancers. In particular, the success of ICIs, represented by PD-1/PD-L1 mAbs, and of ACT, represented by chimeric antigen receptor T (CAR-T) cell therapy, in the field of anti-tumor therapy represents a new stage in tumor immunotherapy. However, owing to the influence of primary or acquired drug resistance caused by tumor immune escape, the vast majority of patients do not benefit from immunotherapy [2]. Therefore, conducting an in-depth exploration of the immune escape mechanism and developing new strategies to overcome immune escape are the only ways to enhance the response efficiency of immunotherapy and make it more widely used.
Glycosylation is the most common and complex post-translational modification (PTM) that requires the coordination of different glycosyltransferases, glycosidases, nucleotide sugar transporters, and appropriate substrates [3]. In recent years, changes in the surface glycosylation patterns of cancer cells have received extensive attention. Abnormal glycosylation is a marker for the occurrence and development of malignant tumors and plays an important role in regulating key carcinogenic processes (including malignant transformation, invasion, metastasis, angiogenesis, and immune escape) [4, 5]. Notably, the regulation of tumor immunity by abnormal glycosylation not only leads to immunosuppression by changing the recognition of tumor cells by the immune system but also induces immune escape by affecting the binding of cell-surface glycosylation receptors to ligands [6]. Tumor-associated glycosylation has been widely studied as a biomarker. The most common abnormal glycosylation modifications are O-glycan truncation, increased N-glycan branching, and changes in sialylation and fucosylation [4]. For example, the interaction between sialoglycan and Siglec receptors contributes to the formation of an immunosuppressive TME by inducing the tumor-promoting phenotype of tumor-associated macrophages (TAMs), inhibiting the activation of natural killer (NK) cells and neutrophils, reducing dendritic cell (DC) maturation and antigen presentation, and inhibiting T cell responses [7]. In addition, most immune checkpoint molecules are glycoproteins whose structure and biological functions are largely affected by glycosylation. For instance, N-glycosylation can reduce the proteasomal degradation of PD-L1 and maintain its interaction with PD-1, thereby inhibiting T cell immune escape [8], whereas O-GlcNAcylation can facilitate tumor immune evasion by suppressing the lysosomal degradation of PD-L1 [9].
Many recent studies have shown that targeted glycosylation can improve tumor immunotherapy, offering a new clinical perspective for advancing cancer treatment [10]. Hence, this study aims to review and summarize the research progress on the role of glycosylation in regulating tumor immunity and affecting immunotherapy and to provide objective reasoning and direction for further research on the prospect of targeting glycosylation to improve the efficacy of immunotherapy.
O-glycosylation and immunotherapy
O-glycosylation is a PTM in which glycans are covalently bound to the hydroxyl groups of serine (Ser), threonine (Thr), or hydroxylysine (Hyl) in the polypeptide chain to form O-glycosidic bonds [11]. O-glycosylation structures are complex and diverse, including O-GlcNAc modification; O-xylose-linked glycosaminoglycans; O-mannosylation of dystroglycan, cadherins, and protocadherins; O-fucosylation and O-glucosylation of Notch receptors; O-galactosylation of collagen; and, finally, the O-glycosylation modification of O-linked N-acetylgalactosamine (O-GalNAc), which is most abundant on membrane and secretory proteins [12].
GalNAc modification is initiated by up to 20 different polypeptide GalNAc-transferase isozymes (ppGalNAc-Ts) with different but partially overlapping substrate specificities [13]. The initiation and extension of O-GalNAc glycosylation produces a single GalNAc (Tn antigen), which is further extended into different core structures as the backbone of complex O-GalNAc glycans [14]. O-GalNAc modification affects protein folding, stability, transport, and protein interactions and participates in inflammatory responses, pathogenic microbial immune escape, cell adhesion, migration, apoptosis, and other key physiological and pathological processes [11]. In tumor cells, changes in glycosylation sites and/or glycan structures often lead to the exposure of protein epitopes. In addition to the exposed polypeptide sequences, abnormal glycosylation can result in the production of novel glycans that subsequently become tumor-associated carbohydrate antigens [15]. Elevated expression of truncated O-glycans, such as tumor-associated Tn and sTn, is often observed in malignant tumors such as breast cancer (BC) and colorectal cancer (CRC), which bind with c-type macrophage galactose-type lectin to evade immune surveillance, resulting in poor prognosis [16]. Related studies have also shown that the binding of tumor-associated sTn antigens to lectins expressed on immune cells inhibits DC maturation and affects antigen presentation, thereby hindering T cell stimulation and activation and allowing for immune escape [17]. In preclinical trials, a vaccine targeting sTn (Theratope) induced an effective antibody response and delayed BC growth in mice. However, in a large phase 3 clinical trial (NCT00003638) of patients with metastatic BC, Theratope did not affect the time to progression or overall survival (OS); nevertheless, the addition of Theratope to an endocrine therapy regimen may improve the clinical outcome of patients [18]. MUC1 is a transmembrane glycoprotein with extensive O-GalNAc modifications. In tumors, the long O-GalNAc branch of MUC1 is truncated to form a classic tumor antigen, making it an emerging target for immunotherapy [19]. MUC1 is overexpressed in various solid tumors, particularly BC, and has become the most relevant and important antigen in BC-targeted therapy. Studies have shown that the targeted O-GalNAc modification of MUC1 plays a role in changing the chemotherapy tolerance of BC and improving the efficacy of anti-tumor therapy [20]. Mucin antigen vaccines are being actively used to treat various solid tumors. An MUC1 glycoprotein-targeted DC vaccine (CVac) has been shown to be safe and well tolerated in patients with ovarian cancer (OC). Compared with the standard of care, patients in second clinical remission (CR2) showed improved progression-free survival (18 vs. 13 months) and prolonged OS (>42 vs. 26 months), suggesting that targeting MUC1 is worthy of further study (NCT01068509) [21]. In a phase 3 clinical trial of patients with non-small cell lung cancer (NSCLC), tecemotide, another vaccine against MUC1, combined with chemoradiotherapy showed no significant difference in OS compared to a placebo (NCT00409188) [22]. Other vaccines targeting MUC1, such as ETBX-061, TG4010, and ImMucin, as well as other vaccines targeting glycosylation, are also in different clinical trial stages (Table 1). The Tn glycoform of MUC1 (Tn-MUC1) is a promising target for CAR therapy, and several CAR therapies targeting Tn-MUC1 are currently in preclinical research. Anti-Tn-MUC1 CAR-T cells reportedly showed specific targeted cytotoxicity and anti-tumor efficacy in T cell leukemia and pancreatic cancer xenograft models [23]. In addition to directly targeting the O-glycosylation modification of glycoproteins, the regulation of some glycosyltransferases can lead to changes in the glycan pattern of target proteins, and changes in the function of related proteins are beneficial to tumor cell progression. Glycosyltransferase targeting is expected to become a new means for tumor treatment. Core-1-β1,3-galactosyltransferase (C1GALT1) is the key enzyme controlling the initiation of O-GalNAc glycosylation; it not only directly promotes cell survival and invasion but also contributes to tumor-mediated immune escape. When the expression of C1GALT1 is inhibited in head and neck cancer, it leads to a reduction in immunosuppressive macrophages and an enhanced killing effect of effector T cells, thereby reshaping the TME [24].
GlcNAcylation is an enzymatic process directed by N-acetyl-D-glucosamine (GlcNAc) glycosyltransferase (OGT), which transfers GlcNAc to proteins (Ser and Thr residues) in the cytoplasm and nucleus, and its removal is regulated by O-GlcNAcase (OGA) [25, 26]. O-GlcNAcylation controls the development, activation, and differentiation of various T cell subsets at different stages through the dynamic coordination of OGT and OGA, which is essential for regulating the activation of homeostatic and mature B cells, as well as efficient germinal centers and antibody responses; regulating macrophage inflammation and antiviral responses; promoting the function of activated neutrophils; and inhibiting the activity of NK cells [27].
Clinical trials of targeted tumor glycosylation vaccines in phase 2 and subsequent research stages.
| Vaccine | Target | Type | Conditions | Phase | ClinicalTrials.gov ID | Study Start | Study Status | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | Tecemotide | MUC1 | Vaccine | Lung neoplasmsNon-small cell lung carcinoma | 2 | NCT00157209 | 2000.08 | Completed | |
| Non-small cell lung carcinomaLung neoplasms | 2 | NCT00157196 | 2005.04 | Completed | |||||
| Non-small cell lung cancer | 3 | NCT00409188 | 2007.01 | Completed | |||||
| Multiple myeloma | 2 | NCT01094548 | 2008.01 | Completed | |||||
| Non-small cell lung cancer | 1/2 | NCT00960115 | 2008.12 | Completed | |||||
| Lung cancer | 2 | NCT00828009 | 2011.01 | Completed | |||||
| Colon carcinomaRectum carcinoma | 2 | NCT01462513 | 2011.08 | Completed | |||||
| Prostate cancer | 2 | NCT01496131 | 2011.10 | Completed | |||||
| Rectal cancer | 2 | NCT01507103 | 2012.02 | Completed | |||||
| 2 | ETBX-061 | MUC1 | Vaccine | Triple-negative breast cancer | 1/2 | NCT03387085 | 2018.03 | Active, not recruiting | |
| Head and neck cancerHead and neck neoplasms | 1/2 | NCT04247282 | 2020.06 | Completed | |||||
| 3 | TG4010 | MUC1 | Vaccine | Non-small cell lung carcinoma | 2/3 | NCT00415818 | 2005.12 | Completed | |
| Recurrent Stage I-IV non-small cell lung cancer | 2 | NCT02823990 | 2016.12 | Completed | |||||
| 4 | CVac | MUC1 | Vaccine | Epithelial ovarian cancer | 2 | NCT01068509 | 2010.07 | Completed | |
| 5 | ImMucin | MUC1 | Vaccine | Multiple myeloma | 1/2 | NCT01232712 | 2010.09 | Completed | |
| 6 | BEC2 | GD3 | Vaccine | Non-small cell lung carcinoma | 3 | NCT00037713 | 1998.09 | Completed | |
| Lung cancer | 3 | NCT00006352 | 1999.09 | Completed | |||||
| 7 | GD2L/GD3L-KLH/OPT-821 | GD2/GD3 | Bivalent vaccine | Neuroblastoma | 1/2 | NCT00911560 | 2009.05 | Active, not recruiting | |
| 8 | GM2/GD2L/GD3L-KLH/OPT-821 | GM2/GD2/GD3 | Trivalent vaccine | Sarcoma | 2 | NCT01141491 | 2010.06 | Completed | |
| 9 | Racotumomab | NeuGcGM3 | Vaccine | Advanced non-small cell lung cancer | 2 | NCT01240447 | 2009.09 | Completed | |
| Neuroblastoma | 2 | NCT02998983 | 2016.11 | Completed | |||||
| 10 | OBI-822 | Globo-H | Vaccine | Triple-negative breast cancer | 3 | NCT03562637 | 2018.12 | Recruiting | |
| 11 | OPT-822/OPT-821 | Globo-H | Vaccine | Metastatic breast cancer | 2 | NCT01516307 | 2011.12 | Completed | |
| Triple-negative breast cancer | 3 | NCT03562637 | 2018.12 | Recruiting | |||||
| 12 | OBI-833/OBI-821 | Globo-H | Vaccine | Esophageal cancer | 2 | NCT05376423 | 2022.06 | Recruiting | |
| Non-small cell lung cancer | 2 | NCT05442060 | 2022.07 | Recruiting | |||||
| 13 | STn-KLH | sTn | Vaccine | Breast cancer | 3 | NCT00003638 | 1999.01 | Completed | |
| Breast neoplasms | 2 | NCT00046371 | 2002.08 | Completed | |||||
Note: 1. Data source: https://www.clinicaltrials.gov/;
2. Clinical trials whose status was shown to be terminated and unknow were not included.
Recent studies have shown that PTM of the PD-L1 protein can regulate its stability and interactions with PD-1, thereby affecting anti-tumor immunotherapy of various solid tumors. Further elucidation of the molecular mechanisms that regulate PD-L1 expression is expected to provide new intervention targets for tumor treatment. OGT in exosomes derived from esophageal carcinoma stem cells (ECSCs) can be taken up by adjacent CD8+ T cells and increase the expression of PD-1 in CD8+ T cells, thereby inhibiting the killing of ECSCs by CD8+ T cells. When OSMI-1 was used to inhibit OGT activity, the self-renewal and immune escape abilities of ECSCs were inhibited [28]. The lysosomal degradation of PD-L1 is mediated by intracellular endosomal sorting complexes required for transport (ESCRT). Hepatocyte growth factor-regulated tyrosine kinase substrate, a key protein in the ESCRT complex, exhibits a high degree of O-GlcNAc glycosylation that hinders the degradation of PD-L1 via the lysosomal pathway, thereby inhibiting the killing effect of T cells towards tumor cells. The combined use of OSMI-4 (another OGT inhibitor) and PD-L1 mAb can restore tumor immunity and synergistically inhibit the growth of liver cancer and melanoma in fully immunized mice, providing new ideas for PD-L1 mediated ICI therapy [9].
In summary, O-glycosylation is essential for tumor immune regulation, and its related mechanisms require further investigation. New, efficient, and safe anticancer strategies targeting O-glycosylation require continuous development.
N-glycosylation and immunotherapy
The core of the N-linked glycan comprises two sequential GlcNAcs and three mannose molecules, which can be further extended and modified by various glycosyltransferases and glycosidases to form complex, hybrid, and high-mannose glycans [29]. The increase in N-glycan branches is involved in regulating the interaction between tumor cells and the matrix and in promoting the migration of tumor cells, which is considered one of the characteristics of cancer [30].
CAR-T cell therapy targeting N-glycans has great potential for tumor immunotherapy. In this therapy, T cells from patients are genetically engineered in vitro, and CAR is introduced as a navigation system to activate T cells so that they can specifically recognize and attack tumor cells accurately and efficiently and improve the immune response [31]. As a burgeoning anti-tumor immunotherapy, the noteworthy clinical efficacy of CAR-T cell therapy in certain malignant hematological tumors deserves acknowledgment, but the scope of clinical trials on solid tumors remains considerably limited [32]. In addition to the challenges associated with antigen escape, off-target effects, and the transport and infiltration of CAR-T cells, the immunosuppressive nature of the TME may be an important factor [33]. To improve the effects of cell immunotherapy and enhance the resistance of T cells to tumor cell immunosuppression, researchers have focused on the metabolic reprogramming of immune cells. The influence of N-glycans on reprogramming cannot be underestimated and requires meticulous attention. Researchers have found that N-glycans impede the effectiveness of CAR-T cells in targeting solid tumors by disrupting the formation of immune synapses, diminishing transcriptional activation, reducing cytokine production, and lowering cytotoxicity [34]. 2-Deoxy-D-glucose (2DG), an analog of glucose, regulates N-glycosylation and inhibits N-glycan biosynthesis [35]. After 2DG treatment, the N-glycan barrier on T cells is broken, leading to enhanced efficacy of T cell-based cancer immunotherapy [36]. The anti-tumor effect of CAR-T cells was significantly enhanced when the expression of N-glycosylation was weakened by the knockout of the glycosyltransferase MGAT5 in pancreatic cancer cells. Furthermore, combining this approach with 2DG increases the anti-tumor efficacy of CAR-T cells. This combination represents a promising strategy for overcoming the limitations of CAR-T cells in the treatment of solid tumors [34]. In addition, N-glycosylation disorders are associated with immunotherapy resistance. Advanced CRC frequently exhibits primary immune escape traits, highlighting its immune resistance characteristics and explaining its poor response to IFN-y therapy or ICI [37]. The glycosyltransferase MGAT3 catalyzes the formation of bisecting GlcNAc linked to mannose in the pentasaccharide core. This specific N-glycan structure is considered a metastatic suppressor that affects cell adhesion and migration. Decreased expression of MGAT3 is linked to IFN-γ resistance in CRC [38]. Increasing the expression of MGAT3 can restore the sensitivity of CRC to IFN-γ or ICI treatment, representing a novel strategy to address immune-resistant CRC [39].
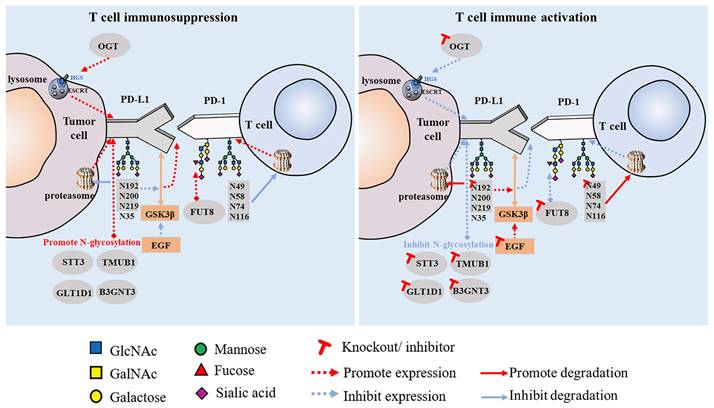
Increasing research has shown that glycosylation plays a vital role in the interaction between PD-1 and PD-L1. This is an important mechanism of tumor cell immune escape that significantly affects the efficacy of immunotherapy. Linking the glycosylation pathway to the strict regulation of PD-1/PD-L1 and further elucidating the molecular mechanisms related to glycosylation may provide clues for the discovery of immunotherapeutic targets for tumor therapy and new strategies to improve the efficacy of cancer immunotherapy. PD-1 is reportedly highly N-glycosylated at the N49, N58, N74, and N116 sites in T cells, which is essential for maintaining PD-1 stability and membrane expression. The glycosylation of PD-1, especially at the N58 site, plays an important role in mediating PD-L1 interaction. The mAb STM418, targeting the PD-1 N58 glycosylation site, showed higher PD-1 affinity than the previously FDA-approved nivolumab and pembrolizumab antibodies, as well as enhanced T cell anti-tumor immunity [40]. The N-glycosylation of PD-L1 (N192, N200, and N219) initiates T cell immunosuppression by inhibiting 26S proteasome-mediated protein degradation and further stabilizes PD-L1 by antagonizing the interaction between PD-L1 and GSK3β [8]. Glycosylated PD-L1 inhibits T cell activity in the TME, whereas non-glycosylated PD-L1 reduces immunosuppressive activity owing to its inability to bind to PD-1. Epidermal growth factor not only stabilizes PD-L1 by inhibiting GSK3β-β-TrCP-mediated degradation but also promotes the N-glycosylation of PD-L1 at N192 and N200 sites in triple-negative breast cancer (TNBC) cells by upregulating glycosyltransferase B3GNT3, thus promoting the binding of PD-L1 and PD-1 and leading to T cell failure [41]. Glycosylation can also regulate the expression of PD-L1 in cancer stem cells (CSCs) [42]. STT3 (catalytic subunit of the oligosaccharyltransferase complex) is upregulated by epithelial-mesenchymal transition (EMT) through the β-catenin signaling axis, thereby increasing the N-glycosylation of PD-L1, promoting the accumulation of PD-L1 in CSCs, and inducing immune escape. By targeting the EMT/β-catenin/STT3/PD-L1 axis, etoposide can further inhibit immune escape and induce the apoptosis of CSCs [43]. In B-cell non-Hodgkin lymphoma (B-NHL), glycosyltransferase 1 domain-containing 1 (GLT1D1) enhances the stability of PD-L1 through N-glycosylation, thereby promoting immunosuppression and tumor growth, and is a potential target for B-NHL treatment [44]. PD-L1 also has other regulatory factors, such as TGF-β1, which promotes the N-glycosylation of PD-L1 by activating the c-jun/STT3A (an isoform of STT3) signaling pathway, thereby facilitating the immune escape of nasopharyngeal carcinoma [45]. Another study found that transmembrane and ubiquitin-like domain-containing protein 1 (TMUB1) is a regulator of PTM of PD-L1 in tumor cells that enhances the N-glycosylation and stability of PD-L1 by recruiting STT3A, thereby promoting PD-L1 maturation and tumor immune escape [46]. When these positive PD-L1 regulators are inhibited, anti-tumor immunity can be enhanced (Figure 1).
These findings collectively suggest that the N-glycosylation of PD-L1 plays a crucial role in regulating its expression and stability, influencing tumor immune escape, and modulating the effectiveness of ICIs. Targeting molecular strategies that regulate the PTM of PD-L1 has significant clinical translational value.
Effect of PD-1/PD-L1 glycosylation on T cell immune activity.
Sialylation and immunotherapy
Sialylation, the process of adding sialic acid residues at the termini of glycans, is a crucial modification that plays diverse roles in cell recognition, cell adhesion, and intercellular signal transduction [47]. Tumor cells often exhibit elevated levels of sialic acid on their surfaces compared with normal cells. Owing to the significant changes in the structure and content of glycoproteins and glycolipids on the surface of cancer cells, a large amount of sialic acid may detach from the surface of cancer cells and enter the blood, resulting in an increase in serum sialic acid levels [48]. Abnormal sialylation is considered a hallmark of cancer and directly affects the interaction between tumor cells and the TME, especially the interaction with sialic acid-binding immunoglobulin-type lectins (Siglecs) in the regulation of immune cell function [49]. The sialoglycan-siglectin axis contributes to the formation of an immunosuppressive TME. This axis induces a tumor-promoting phenotype in TAMs, inhibits the activation of NK cells and neutrophils, reduces DC maturation and antigen presentation, and hampers T cell responses [50, 51]. As a result, numerous studies have explored novel avenues for cancer treatment by strategically targeting the sialoglycan-Siglec immune axis. These studies aimed to disrupt immunosuppressive signals within the TME, offering potential therapeutic strategies to enhance anti-tumor immune responses.
Previous studies have demonstrated that the administration of sialidase to induce desialylation significantly enhances NK cell-mediated cytotoxicity and inhibits tumor progression in leukemia and cervical cancer [52]. In melanoma, knockdown of the sialic acid transporter SLC35A1 can suppress the expression of sialoglycans, slow the growth rate of tumors, and promote the presence of greater quantities of effector immune cells within the TME, creating a milieu conducive to tumor immune control [53]. Moreover, intratumoral injection of the selective sialoglycan biosynthesis inhibitor Ac53FaxNeu5Ac effectively interferes with the expression of sialoglycans. This intervention inhibits the growth of melanoma and 9464D neuroblastoma in mice. Notably, it also induces a shift in the composition of immune cells within the TME, favoring immune-promoting properties [54]. Additional evidence supports the notion that antibody-sialidase conjugates represent a promising strategy to modulate anti-tumor immunity. In one approach, researchers coupled Vibrio cholerae sialidase with trastuzumab; the resulting conjugate, denoted T-Sia, demonstrated the ability to desialylate HER2+ BC cells in vitro, resulting in the removal of Siglec ligands and ultimately enhancing the efficacy of NK cells in killing cancer cells [55]. Based on this, researchers developed a second-generation HER2 antibody-sialidase conjugate, referred to as T-Sia 2. This novel conjugate demonstrated the effective and selective removal of a variety of sialoglycans from BC cells and blocked sialoglycan-Siglec interactions. Moreover, in mice with in vivo transplanted homologous HER2+ BC cells, desialylation induced by T-Sia 2 was shown to decelerate tumor growth and enhance immune cell infiltration and activation within the TME [56]. Recent studies have expanded our understanding of the mechanisms underlying tumor sialylation-mediated immunosuppression. High sialylation of tumor cells reportedly promotes the polarization of TAMs to an immunosuppressive phenotype by interacting with Siglec-E. Conversely, therapeutic desialylation can polarize TAMs to an immune-promoting phenotype. Notably, desialylation therapy exhibits a synergistic effect when combined with ICIs, suggesting its potential to enhance the efficacy of ICIs or alleviate drug resistance. These findings provide a robust foundation for the ongoing clinical development of sialoglycan-Siglec-targeting agents and their combination with PD-1 and CTLA-4 blocking immunotherapies [57].
Siglecs are increasingly being identified as compelling targets for tumor immunotherapy, driven by an expanding body of research that unravels the intricate mechanisms through which interactions within the sialoglycan-Siglec axis influence immune escape within the TME. Siglecs are expressed in most immune cells and play crucial roles in regulating the activity and function of cells in the innate and adaptive immune systems [58]. Internal immune cells, especially macrophages, abundantly express various Siglec receptors, including Siglec-9, -10, -15, and others, which contribute to the transformation of TAMs to a cancer-promoting phenotype. Sialoglycan evades immune surveillance by binding to the inhibitory receptor Siglec-9 in the granulocyte-monocyte lineage; this is restored in a Siglec9/E-deficient mouse model, strengthening the ability of the innate immune system to effectively eliminate tumor cells [59]. Another study found that Siglec-9 in primary monocytes and macrophages could induce TAM polarization to promote tumor progression by binding to abnormally sialylated MUC1 glycans in the human BC cell line T47D [60]. A recent study has shown that in glioblastoma multiforme, Siglec-9 can function as an immune checkpoint molecule on the macrophage surface. This has significant implications for the efficacy of the TME and immunotherapy outcomes. Targeting Siglec-9 has been identified as a strategy to enhance the effectiveness of anti-PD-1/PD-L1 therapy [61]. The highly expressed inhibitory receptor Siglec-10 on TAMs interacts with the new “don't eat me” signaling molecule CD24, activates the SHP-1/SHP-2-mediated inhibitory signaling pathway, and inhibits the phagocytosis of tumor cells by macrophages, thereby exerting an immune escape effect [62]. A study published in Nature in 2019 showed that knockout of CD24 or Siglec-10 or the blocking of the CD24-Siglec-10 axis with mAbs significantly enhanced the phagocytosis of all human tumor cells expressing CD24 by macrophages [63]. Therefore, since CD24-Siglec-10 is an innate immune checkpoint essential for mediating anti-tumor immunity, it has become a very promising therapeutic target for tumor immunotherapy. Siglec-15, which is found on the surface of both tumor cells and M2 macrophages, can effectively inhibit T cell activation to suppress anti-tumor responses. Notably, Siglec-15 has been identified as a significant immunosuppressive factor in tumors that do not express PD-L1. The mAb NC318, designed to block Siglec-15, has shown promise in restoring the anti-tumor immune effect within the TME, and a clinical trial on the efficacy of NC318 in treating solid tumors is ongoing (NCT03665285). This approach may offer a viable therapeutic option for cancer patients in whom PD-L1 immunotherapy has proven ineffective [64]. Similarly, Siglec-7 and Siglec-9 are abundant in NK cells and inhibit NK cytotoxicity by interacting with sialoglycans [65]. In addition, inhibitory Siglecs are involved in T cell immunoregulation. Compared to peripheral T cells from healthy donors, Siglec-9 expression was significantly upregulated in tumor-infiltrating T cells from patients with NSCLC, CRC, and OC, and targeting the sialoglycan-Siglec-9 pathway enhanced anti-tumor immunity both in vitro and in vivo [66]. In addition, research on CAR-T cell therapy targeting these Siglec antigens is progressing. CAR-T cell therapy specifically targeting Siglec-2 (CD22) has demonstrated clinical effectiveness, particularly in patients with pre-B-cell acute lymphoblastic leukemia (B-ALL) who were resistant to CD19 CAR-T therapy [67]. Siglec-3 (CD33)-specific CAR-T cell therapy has shown efficacy in preclinical models of acute myeloid leukemia (AML) resistance and have begun clinical trial evaluation [68]. Siglec-6 is a particularly promising target because it is typically expressed in AML cell lines but not in normal hematopoietic stem and progenitor cells. The development of CAR-T cell therapy targeting Siglec-6 represents a recent breakthrough in AML treatment. Notably, this therapy is effective for treating AML without the need for subsequent allogeneic hematopoietic stem cell transplantation [69].
In recent years, the sialoglycan-Siglec axis has emerged as a potential new immune checkpoint to enhance cancer immunotherapy. The therapeutic potential of modulating this axis has been demonstrated in both related preclinical and clinical studies. Further exploration of the mechanism of sialoglycan-Siglec interaction in the TME will help to improve novel immunotherapy strategies and pave the way for further clinical applications.
Fucosylation and immunotherapy
Fucosylation, particularly core fucosylation, is one of the most widespread cancer-related changes in the N-glycan chain [70]. α-1,6-Fucosyltransferase (FUT8) is the sole known enzyme responsible for generating an α-1,6-fucosylated structure on the core residue of the N-glycan chain [71]. In 2017, Okada et al. employed CRISPR-Cas9-based knock out and whole-genome sequencing technology to screen FUT8 as a pivotal factor that regulates PD-1 expression on the cell surface. FUT8, by catalyzing PD-1 fucosylation, exerts control over PD-1 expression in T cells. The inhibition of FUT8 expression markedly diminishes core fucosylation modifications at N49 and N74 on PD-1. This reduction correlates with decreased PD-1 expression and augmented T cell activation, ultimately enhancing the efficacy of tumor eradication [72]. Similarly, a study by Zhang et al. on lung adenocarcinoma confirmed that blocking the core fucosylation of PD-1 represents a viable strategy to reduce PD-1 expression in future immunotherapies [73]. Furthermore, FUT8 regulates the core fucosylation of the highly glycosylated B7 homolog 3 protein (B7H3), contributing to its protein stability and immunosuppressive effects in TNBC. Knocking down FUT8 or using 2F-Fuc (a core fucosylation inhibitor) effectively rescued B7H3-mediated immunosuppression, indicating that targeting the FUT8-B7H3 axis, particularly in combination with PD-L1, has the potential to enhance the anti-tumor immune response in patients with TNBC [74].
Previous research findings have provided support for the inhibition of the fucosylation of glycoproteins, either as a standalone treatment or in combination with ICIs, as a promising therapeutic strategy for various cancers. Ongoing phase 1 clinical trials are investigating the fucosylation inhibitor SGN-2FF in patients with advanced solid tumors. Preliminary results revealed encouraging anti-tumor activity, underscoring the potential of fucosylation inhibition as a novel avenue for cancer treatment [75].
To enhance the efficacy of various ICIs, an increasing number of studies have focused on modifying the Fc segments, primarily through glycoengineering and site-directed mutagenesis. For instance, Rony et al. employed glycoengineering to eliminate the fucose subunit (aFuc-IgG1) from the linker glycan of the Fc segment of the PD-L1 antibody. This modification led to an increased binding capacity to activated FcγRIIIA. The glycoengineered PD-L1 antibody induced a shift in neutrophils from an immunosuppressive state to a proinflammatory state in MC38 tumor-bearing mice, resulting in heightened anti-tumor activity and a more robust immune response [76]. These findings suggest that defucosylation is a favorable factor for tumor immunotherapy. However, contradictions regarding the use of ACT have emerged. The efficacy of ACT in cancer treatment frequently falls short of the desired outcomes, primarily attributed to challenges associated with T cells failing to effectively home to tumor tissues [77]. Alatrash et al. found that fucosylation, when applied in vitro, has the potential to augment the homing ability of cytotoxic T lymphocytes to leukemic bone marrow and tumor tissues. This enhancement amplifies the efficacy of T cells in killing tumors, ultimately improving anti-tumor outcomes [78]. This underscores the complexity of fucosylation in tumor immunotherapy, challenging the simplistic classification as either favorable or unfavorable. Achieving optimal effects of targeted fucosylation requires a deeper understanding of its mechanisms of action in tumor immunology. Further exploration of these mechanisms is crucial to refine fucosylation-based strategies and maximize their therapeutic potential.
Conclusions and perspectives
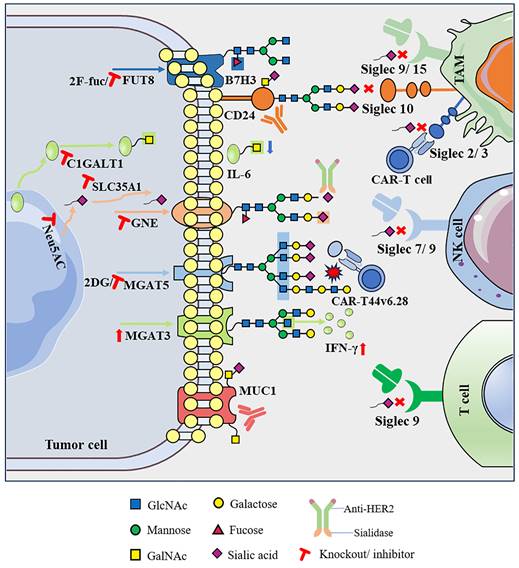
After consolidating the vast body of literature, this review presents compelling evidence that abnormal glycosylation on the surface of tumor cells, characterized by shorter O-glycans and more branched N-glycans, along with associated alterations in the sialylation and fucosylation of glycan terminal epitopes, exerts a significant impact on cancer progression and the characteristics of the TME. Abnormal glycosylation, considered a key feature of cancer, contributes to the formation of a complex “glyco-code” on the tumor cell surface. This glyco-code not only influences self-activation but also modulates interactions with immune cells within the TME, thereby playing a crucial role in shaping the dynamics of tumor immunity. Therefore, editing glycans on the cell surface to achieve glycan reconstruction and further modifying of other biomolecules can regulate cell recognition and communication functions, representing a new breakthrough in the field of immune response restoration. First, direct targeting of abnormal glycosylation on tumor cells to eliminate the glyco-code has shown promise in reducing immunosuppressive effects. For instance, the application of sialidase or sialoglycan synthesis inhibitors interferes with sialoglycan synthesis, promoting the immune control of tumors [54-56]. Second, disrupting abnormal glycosylation by modulating glycosyltransferases and glycosidases has significant therapeutic potential. For instance, knocking out MGAT5 to eliminate defective branched N-glycans transforms CAR-T cell therapy into a potent force akin to a “cannonball” targeting and attacking pancreatic cancer cells. Additionally, inhibiting the expression of the key enzyme C1GALT1, responsible for initiating GalNAc O-glycosylation, reshapes the TME in head and neck tumors, leading to improved immunotherapy efficacy [26, 36]. Third, intervention in the glycosylation-lectin pathway, specifically by directly blocking the binding of glycans to recognition molecules, as exemplified by the sialoglycan-Siglec interaction, is emerging as a potential novel immune checkpoint (Figure 2) [56].
In this new era of immunotherapy, it is crucial to emphasize enhanced response rates in patients with cancer. Most immune checkpoint molecules are glycoproteins, and their structures and biological functions are largely affected by glycosylation. Several previous studies have shown that abnormal glycosylation on the surface of immune checkpoint molecules can regulate their stability and enable tumors to evade immune surveillance. Among the extensively studied immune checkpoint molecules, PD-1 and PD-L1 undergo various types of glycosylation, including but not limited to O-GlcNAc glycosylation, which impedes lysosomal pathway-mediated PD-L1 degradation, and N-glycosylation, which inhibits PD-L1 degradation mediated by the proteasome pathway. Additionally, FUT8 catalyzes PD-1 core fucosylation, stabilizing PD-1 expression on T cells and initiating T cell immunosuppression [8, 9, 69]. Some new drugs for glycosylation are undergoing in vitro and in vivo trials, whereas others have entered clinical trials. STM108 mAb targets the N192 and N200 glycosylation sites of PD-L1 and can block the interaction of PD-L1/PD-1 to promote the internalization and degradation of PD-L1. The STM108 antibody-drug conjugate, coupled with the potent anti-mitotic drug monomethyl auristatin E (MMAE), induced strong anti-tumor activity in in vivo and in vitro TNBC models [41]. Similarly, STM418, MW11-h317, and MAb059c mAbs targeting the N58 glycosylation site of PD-1 can also effectively inhibit the binding of PD-1 to PD-L1 and enhance the T cell-mediated anti-tumor immune response, and these are still in the pre-clinical trial stage [40, 79, 80]. SEA-TGT, an antibody targeting non-fucosylated T cell immunoreceptor with Ig and ITIM domains (TIGIT) as a new immunosuppressive molecule, was found to show significantly enhanced anti-tumor activity in previous clinical trials [81]. Phase 1 clinical trials have been initiated to test the safety and efficacy of SEA-TGT in combination with anti-PD-1 antibodies in patients with advanced solid tumors and specific lymphomas [82]. The glycosylation of immune checkpoint molecules has become a high-profile target for tumor immunotherapy; in particular, preclinical trials targeting the N-glycosylation of PD-1/PD-L1 have shown preliminary achievements in tumor immunotherapy. However, with in-depth study of O-glycosylation, fucosylation, sialylation, and other glycosylation processes, there is a need to develop new effective drug targets for tumor immunotherapy. In addition, abnormal glycosylation by different mechanisms leads to the formation of tumor-associated carbone antigens (TACAs), including mucin-related truncated O-glycans (Tn, sTn), gangliosides (GD2, GD3, fucosyl-GM1, GM2, and GM3), and the globular serine glycan Globo-H. Novel mAbs, antibody-drug conjugates, bispecific T cell engagers, and vaccines targeting these TACAs have entered different clinical trial stages (Tables 1 and 2), showing strong therapeutic potential in synergy with other anti-tumor strategies [83-85]. Therefore, a profound exploration of the mechanism of glycosylation in the malignant process of tumors and the continuous development of anti-tumor drugs based on glycosylation are expected to provide new targets and theoretical support for personalized treatment of tumor patients.
Targeting abnormal glycosylation restores the anti-tumor immune activity of immune cells.
However, owing to the complexity and diversity of glycosylation modifications, glycosylation analysis faces significant challenges. In recent years, research on glycosylation and related fields has shown the following main development trends: (1) Development and application of glycan synthesis, analysis, and editing technology: continuous innovation in mass spectrometry instrumentation coupled with the optimization of analytical and bioinformatics methods and the synthesis of standard glycoprotein/glycopeptides, which has facilitated remarkable progress. Through the integration of multi-omics datasets encompassing glycomic, genomic, and proteomic information, we can now achieve more comprehensive and accurate detection of glycosylation modifications at immune checkpoints. This holistic approach enhances our understanding of glycoprotein function and structure, thereby offering robust support for drug development and clinical treatments. (2) In-depth exploration of the molecular mechanism of abnormal glycosylation in tumors: tumor-associated abnormal glycosylation is a characteristic of cancer and plays an important role in key pathological steps such as tumor development and progression. As the regulation of abnormal glycosylation in tumor immunity has received extensive attention, an in-depth study of the molecular mechanism of glycosylation-mediated immune escape and the discovery of new effective drug targets are expected to promote the development of next-generation tumor immunotherapy. (3) Clinical transformation of tumor glycosylation drugs: At present, a considerable number of targeted tumor glycosylation drugs are in preclinical and clinical trials at different stages, showing preliminary therapeutic potential. Further efforts are needed to elucidate the mechanism of action, optimize drug molecules, and evaluate their safety and efficacy to successfully transform this strategy into a new type of tumor immunotherapy for clinical application.
Clinical trials of targeted tumor glycosylation drugs in phase 2 and subsequent research stages.
| Drug Candidate | Target | Drug Type | Conditions | Phase | ClinicalTrials.gov ID | Study Start | Study Status | |
|---|---|---|---|---|---|---|---|---|
| 1 | Moxetumomab pasudotox | CD22 | ADC | Hairy cell leukemia | 2 | NCT00923013 | 2008.10 | Active, not recruiting |
| Non-Hodgkin lymphomaChronic lymphocytic leukemia | 2 | NCT01030536 | 2010.02 | Completed | ||||
| Hairy cell leukemia | 3 | NCT01829711 | 2013.04 | Completed | ||||
| 2 | Inotuzumab ozogamicin | CD22 | ADC | B-cell lymphoma | 1/2 | NCT00299494 | 2006.05 | Completed |
| B-cell lymphoma | 2 | NCT00867087 | 2009.06 | Completed | ||||
| Lymphoma | 2 | NCT00868608 | 2009.07 | Completed | ||||
| Acute lymphoblastic leukemia | 2 | NCT01134575 | 2010.06 | Completed | ||||
| B acute lymphoblastic leukemia with t(9;22)(q34.1;q11.2); BCR-ABL1;B acute lymphoblastic leukemia, Philadelphia chromosome negativeBurkitt-like lymphoma with 11q aberrationHigh grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangementsHigh grade B-cell lymphoma, not otherwise specifiedRecurrent B acute lymphoblastic leukemiaRecurrent Burkitt lymphomaRefractory B acute lymphoblastic leukemiaRefractory Burkitt lymphoma | 1/2 | NCT01371630 | 2011.08 | Recruiting | ||||
| Acute lymphocytic leukemia | 2 | NCT01363297 | 2011.08 | Completed | ||||
| Acute lymphoblastic leukemia | 3 | NCT01564784 | 2012.08 | Completed | ||||
| Hematopoietic and lymphoid cell neoplasm | 1/2 | NCT01664910 | 2012.10 | Completed | ||||
| Diffuse large B-cell lymphoma | 1/2 | NCT01562990 | 2012.12 | Completed | ||||
| Diffuse large B-cell lymphoma | 2 | NCT01679119 | 2013.10 | Completed | ||||
| B acute lymphoblastic leukemia with t(9;22)(q34.1;q11.2); BCR-ABL1Blast phase chronic myelogenous leukemia, BCR-ABL1 PositiveBlasts more than 5 percent of bone marrow nucleated cellsPhiladelphia chromosome-positive, BCR-ABL1-positive chronic myelogenous leukemiaRecurrent chronic myelogenous leukemia, BCR-ABL1 positiveRefractory chronic myelogenous leukemia, BCR-ABL1 positive | 1/2 | NCT02311998 | 2015.04 | Completed | ||||
| B acute lymphoblastic leukemiaB lymphoblastic leukemia | 2 | NCT02877303 | 2016.11 | Recruiting | ||||
| Recurrent B acute lymphoblastic leukemia Recurrent B lymphoblastic leukemiaRefractory B acute lymphoblastic leukemiaRefractory B lymphoblastic lymphoma | 2 | NCT02981628 | 2017.06 | Active, not recruiting | ||||
| Acute lymphocytic leukemia | 1/2 | NCT03104491 | 2017.07 | Recruiting | ||||
| Acute lymphoblastic leukemia-Ph-negative CD22+ B-cell precursor | 2 | NCT03249870 | 2017.12 | Active, not recruiting | ||||
| Acute lymphoblastic leukemiaB acute lymphoblastic leukemiaRecurrent B acute lymphoblastic leukemia | 2 | NCT03441061 | 2018.02 | Recruiting | ||||
| Precursor cell lymphoblastic leukemia | 2 | NCT03460522 | 2018.05 | Recruiting | ||||
| Acute lymphoblastic leukemia Hyperbilirubinemia | 2 | NCT03564678 | 2018.05 | Recruiting | ||||
| Acute lymphoblastic leukemia | 2 | NCT03913559 | 2019.05 | Recruiting | ||||
| B acute lymphoblastic leukemia, Philadelphia chromosome negativeRecurrent B acute lymphoblastic leukemiaRefractory B acute lymphoblastic leukemia | 2 | NCT03739814 | 2019.05 | Recruiting | ||||
| Leukemia, precursor B-cell lymphoblasticLeukemia-lymphoma;Acute lymphoblastic leukemia | 4 | NCT03677596 | 2019.07 | Completed | ||||
| Acute lymphoblastic leukemiaB acute lymphoblastic leukemiaLymphocytic neoplasmLymphoma | 2 | NCT03856216 | 2019.10 | Recruiting | ||||
| B acute lymphoblastic leukemiaB lymphoblastic leukemiaCentral nervous system leukemiaMixed phenotype acute leukemiaTesticular leukemia | 3 | NCT03959085 | 2019.10 | Recruiting | ||||
| Leukemia, acute lymphoblastic | 3 | NCT04307576 | 2020.07 | Recruiting | ||||
| B acute lymphoblastic leukemia with t(9;22)(q34.1;q11.2); BCR-ABL1 | 3 | NCT04530565 | 2021.01 | Recruiting | ||||
| Lymphoblastic leukemiaAcute lymphoblastic leukemiaPh+ acute lymphoblastic leukemia | 2 | NCT04747912 | 2021.03 | Recruiting | ||||
| Acute lymphocytic leukemia | 2 | NCT05456698 | 2022.08 | Not yet recruiting | ||||
| ALLMRD-positive | 2 | NCT05940961 | 2022.08 | Recruiting | ||||
| Acute lymphoblastic leukemia | 4 | NCT05687032 | 2023.02 | Not yet recruiting | ||||
| B acute lymphoblastic leukemia B lymphoblastic lymphoma | 2 | NCT05303792 | 2023.02 | Recruiting | ||||
| Acute lymphoblastic leukemia | 2 | NCT05748171 | 2023.05 | Recruiting | ||||
| Leukemia | 2 | NCT05645718 | 2023.07 | Recruiting | ||||
| Acute lymphoblastic leukemia | 1/2 | NCT06087419 | 2023.10 | Not yet recruiting | ||||
| Relapsed/refractory B-cell acute lymphocytic leukemia | 1/2 | NCT06287229 | 2024.08 | Not yet recruiting | ||||
| 3 | Epratuzumab | CD22 | ADC | LeukemiaLymphoma | 1/2 | NCT00004107 | 1998.02 | Completed |
| LeukemiaLymphoma | 1/2 | NCT00004084 | 1998.04 | Completed | ||||
| Non-Hodgkin's lymphomaLymphoma, B-Cell | 1/2 | NCT00061425 | 2000.08 | Completed | ||||
| NHLB-cell NHLNon-Hodgkin's Lymphoma | 1/2 | NCT00421395 | 2002.08 | Completed | ||||
| Recurrent childhood acute lymphoblastic leukemia | 2 | NCT00098839 | 2005.02 | Completed | ||||
| Lymphoma | 2 | NCT00301821 | 2006.01 | Completed | ||||
| Non-Hodgkin's lymphoma | 2 | NCT00044902 | 2007.12 | Completed | ||||
| Lymphoma | 2 | NCT00553501 | 2008.03 | Completed | ||||
| Leukemia | 2 | NCT00945815 | 2010.09 | Completed | ||||
| B ALL;CD22+ expressionRefractory B-ALL | 2 | NCT01219816 | 2010.11 | Completed | ||||
| Acute lymphoblastic leukemia (ALL) | 3 | NCT01802814 | 2014.05 | Completed | ||||
| Blasts 5 percent or more of bone marrow nucleated cellsCD22 positivePhiladelphia chromosome positiveRecurrent B acute lymphoblastic leukemiaRefractory B acute lymphoblastic leukemia | 1/2 | NCT03698552 | 2018.08 | Recruiting | ||||
| 4 | Gemtuzumab ozogamycin | CD33 | ADC | Leukemia, myelocytic, acute | 3 | NCT00962767 | 2002.05 | Completed |
| Leukemia, myelocytic, acute | 3 | NCT00136084 | 2002.08 | Completed | ||||
| Leukemia | 3 | NCT00052299 | 2002.09 | Completed | ||||
| Leukemia | 3 | NCT00049517 | 2002.12 | Completed | ||||
| Acute myeloid leukemia | 3 | NCT00476541 | 2004.01 | Completed | ||||
| Leukemia | 3 | NCT00085709 | 2004.07 | Completed | ||||
| Leukemia;Myelodysplastic syndromes | 3 | NCT00121303 | 2005.01 | Completed | ||||
| Leukemia | 3 | NCT00372593 | 2006.08 | Completed | ||||
| Leukemia; myelodysplastic syndromes | 2/3 | NCT00454480 | 2006.08 | Completed | ||||
| Leukemia | 3 | NCT00492856 | 2007.06 | Completed | ||||
| Acute myeloid leukemia | 3 | NCT00860639 | 2007.10 | Completed | ||||
| Acute myeloid leukemia | 3 | NCT00927498 | 2007.12 | Completed | ||||
| Acute myeloid leukemia | 2/3 | NCT00909168 | 2008.03 | Completed | ||||
| Acute myeloid leukemia | 3 | NCT00893399 | 2010.05 | Completed | ||||
| Acute myeloid leukemia | 2/3 | NCT02473146 | 2015.11 | Completed | ||||
| 5 | Lintuzumab | CD33 | ADC | Leukemia | 2 | NCT00002609 | 1994.08 | Completed |
| LeukemiaMyelodysplastic syndromesNeutropenia | 2 | NCT00002800 | 1996.07 | Completed | ||||
| Leukemia | 2 | NCT00016159 | 2000.01 | Completed | ||||
| Acute myeloid leukemia | 2 | NCT00528333 | 2007.09 | Completed | ||||
| 6 | Abagovomab | MUC1 | mAbs | Ovarian cancerFallopian tube neoplasmsPeritoneal neoplasms | 1/2 | NCT00103545 | 2003.07 | Completed |
| 7 | BM7PE | MUC1 | ADC | Colorectal cancer metastatic | 1/2 | NCT04550897 | 2020.08 | Recruiting |
| 8 | Oregovomab | MUC16 | mAbs | Ovarian neoplasms | 2 | NCT01616303 | 2012.06 | Completed |
| Pancreatic adenocarcinomaResectable pancreatic carcinomaStage I-III pancreatic cancer | 2 | NCT01959672 | 2013.09 | Completed | ||||
| Carcinoma, ovarian epithelialOvarian neoplasmsOvarian cancerOvarian serous adenocarcinomaFallopian tube neoplasmsFallopian tube adenocarcinomaFallopian tube serous adenocarcinomaPeritoneal cancerPeritoneal carcinomaPeritoneal neoplasms | 3 | NCT04498117 | 2020.08 | Active, not recruiting | ||||
| 9 | REGN-4018 | MUC16/CD3 | BiTE | Recurrent ovarian cancerRecurrent fallopian tube cancerRecurrent primary peritoneal cancerRecurrent endometrial cancer | 1/2 | NCT03564340 | 2018.05 | Recruiting |
| Ovarian cancerFallopian tube cancerPrimary peritoneal cancer | 1/2 | NCT04590326 | 2020.12 | Recruiting | ||||
| 10 | REGN-5668 | MUC16/CD28 | BiTE | Ovarian cancerFallopian tube cancerPrimary peritoneal cancer | 1/2 | NCT04590326 | 2020.12 | Recruiting |
| 11 | Dinutuximab | GD2 | mAbs | High-risk neuroblastoma | 3 | NCT01041638 | 2009.12 | Completed |
| Neuroblastoma, recurrent | 2 | NCT02258815 | 2010.08 | Completed | ||||
| Neuroblastoma | 1/2 | NCT01592045 | 2012.08 | Completed | ||||
| GanglioneuroblastomaRecurrent neuroblastoma | 2 | NCT01767194 | 2013.02 | Completed | ||||
| Metastatic malignant neoplasm in the lungMetastatic osteosarcomaRecurrent osteosarcoma | 2 | NCT02484443 | 2016.02 | Completed | ||||
| 12 | Naxitamab | GD2 | mAbs | Recurrent osteosarcoma | 2 | NCT02502786 | 2015.07 | Recruiting |
| Neuroblastoma | 2 | NCT03363373 | 2018.04 | Recruiting | ||||
| Neuroblastoma recurrent | 2 | NCT05754684 | 2022.01 | Recruiting | ||||
| High-risk neuroblastoma | 2 | NCT05489887 | 2022.09 | Recruiting | ||||
| Neuroblastoma | 4 | NCT05421897 | 2022.10 | Recruiting | ||||
| Neuroblastoma | 2 | NCT06013618 | 2023.06 | Recruiting | ||||
| Ewing sarcoma | 2 | NCT05968768 | 2023.10 | Recruiting | ||||
| Neuroblastoma | 4 | NCT06047535 | 2023.10 | Active, not recruiting | ||||
| Anatomic stage IV breast cancer AJCC v8HER2-negative breast carcinoma | 1/2 | NCT06026657 | 2024.03 | Recruiting | ||||
| 13 | Ecromeximab | GD3 | mAbs | Metastatic melanomaCutaneous melanoma | 2 | NCT00679289 | 2008.03 | Completed |
| 14 | BMS-986012 | FucGM1 | mAbs | Small cell lung cancer | 1/2 | NCT02247349 | 2014.11 | Completed |
| Extensive-stage small cell lung cancer | 2 | NCT04702880 | 2021.03 | Recruiting | ||||
| 15 | PankoMab-GEX™ | Tn | mAbs | Ovarian epithelial cancer recurrentFallopian tube cancerPrimary peritoneal cancer | 2 | NCT01899599 | 2013.09 | Completed |
Note: 1. Data source: https://www.clinicaltrials.gov/;2. Clinical trials whose statuses were shown to be terminated and unknown were not included;3. The clinical trials of gemtuzumab ozogamycin are numerous, with only phase 3 and subsequent clinical trials listed in the table.
In conclusion, as we have uncovered the pivotal regulatory role of abnormal glycosylation modifications in tumor cells and the intricate mechanisms linking them to immune responses, a comprehensive understanding of the significance of the glyco-code will serve as the foundation for a more refined approach to cancer immunotherapy. This presents new hope for individuals suffering from cancer, pointing towards innovative strategies that may revolutionize the cancer treatment landscape.
Abbreviations
TME: tumor microenvironment; mAbs: monoclonal antibodies; ICIs: immune checkpoint inhibitors; ACT: adoptive cell transfer therapy; CAR-T: chimeric antigen receptor T; PTM: post-translational modification; TAMs: tumor-associated macrophages; NK cells: natural killer cells; DC: dendritic cell; Siglecs: sialic acid-binding immunoglobulin-type lectins; OC: ovarian cancer; NSCLC: non-small cell lung cancer; GalNAc: N-acetylgalactosamine; TACAs: tumor-associated carbohydrate antigens; BC: breast cancer; TNBC: triple negative breast cancer; AML: acute myeloid leukemia; CRC: colorectal cancer; GlcNAc: N-acetyl-D-glucosamine; ECSCs: esophageal carcinoma stem cells; ESCRT: endosomal sorting complexes required for transport; 2DG: 2-Deoxy-D-glucose; CSCs: cancer stem cells; EMT: epithelial-mesenchymal transition; B-NHL: B-cell Non-Hodgkin's lymphoma; MMAE: monomethyl auristatin E; TIGIT: T cell immunoreceptor with Ig and ITIM domains; ADC: antibody-drug conjugates; BiTE: Bispecific T cell engager.
Acknowledgements
This study was supported by the National Science Foundation of China (No. 32371336), the Science Foundation for Distinguished Young Scholars of Shaanxi Province (2021JC-39), and the Shaanxi Innovation Team Project (2023-CX-TD-58). We would like to thank Editage (www.editage.cn) for English language editing.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. The Journal of clinical investigation. 2015;125:3335-7
2. Huang Q, Lei Y, Li X, Guo F, Liu M. A Highlight of the Mechanisms of Immune Checkpoint Blocker Resistance. Frontiers in cell and developmental biology. 2020;8:580140
3. Eichler J. Protein glycosylation. Current biology: CB. 2019;29:R229-r31
4. Raglow Z, McKenna MK, Bonifant CL, Wang W, Pasca di Magliano M, Stadlmann J. et al. Targeting glycans for CAR therapy: The advent of sweet CARs. Molecular therapy: the journal of the American Society of Gene Therapy. 2022;30:2881-90
5. Guo Y, Jia W, Yang J, Zhan X. Cancer glycomics offers potential biomarkers and therapeutic targets in the framework of 3P medicine. Frontiers in endocrinology. 2022;13:970489
6. RodrÍguez E, Schetters STT, van Kooyk Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nature reviews Immunology. 2018;18:204-11
7. Wang J, Manni M, Bärenwaldt A, Wieboldt R, Kirchhammer N, Ivanek R. et al. Siglec Receptors Modulate Dendritic Cell Activation and Antigen Presentation to T Cells in Cancer. Frontiers in cell and developmental biology. 2022;10:828916
8. Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW. et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nature communications. 2016;7:12632
9. Zhu Q, Wang H, Chai S. O-GlcNAcylation promotes tumor immune evasion by inhibiting PD-L1 lysosomal degradation. Proceedings of the National Academy of Sciences of the United States of America. 2023;120:e2216796120
10. Chiang AWT, Baghdassarian HM, Kellman BP, Bao B, Sorrentino JT, Liang C. et al. Systems glycobiology for discovering drug targets, biomarkers, and rational designs for glyco-immunotherapy. Journal of biomedical science. 2021;28:50
11. Schjoldager KT, Narimatsu Y. Global view of human protein glycosylation pathways and functions. Nature reviews Molecular cell biology. 2020;21:729-49
12. Wandall HH, Nielsen MAI. Global functions of O-glycosylation: promises and challenges in O-glycobiology. The FEBS journal. 2021;288:7183-212
13. Xu Z, Li X, Zhou S, Xie W, Wang J, Cheng L. et al. Systematic identification of the protein substrates of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase-T1/T2/T3 using a human proteome microarray. Proteomics. 2017 17
14. Pinto D, Parameswaran R. Role of Truncated O-GalNAc Glycans in Cancer Progression and Metastasis in Endocrine Cancers. Cancers. 2023 15
15. Nath S, Mukherjee P. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends in molecular medicine. 2014;20:332-42
16. Beatson R, Maurstad G, Picco G, Arulappu A, Coleman J, Wandell HH. et al. The Breast Cancer-Associated Glycoforms of MUC1, MUC1-Tn and sialyl-Tn, Are Expressed in COSMC Wild-Type Cells and Bind the C-Type Lectin MGL. PloS one. 2015;10:e0125994
17. Carrascal MA, Severino PF, Guadalupe Cabral M, Silva M, Ferreira JA, Calais F. et al. Sialyl Tn-expressing bladder cancer cells induce a tolerogenic phenotype in innate and adaptive immune cells. Molecular oncology. 2014;8:753-65
18. Lee DH, Choi S, Park Y, Jin HS. Mucin1 and Mucin16: Therapeutic Targets for Cancer Therapy. Pharmaceuticals (Basel). 2021 14
19. Müller S, Alving K, Peter-Katalinic J, Zachara N, Gooley AA, Hanisch FG. High density O-glycosylation on tandem repeat peptide from secretory MUC1 of T47D breast cancer cells. The Journal of biological chemistry. 1999;274:18165-72
20. Xi X, Wang J, Qin Y, Huang W, You Y, Zhan J. Glycosylated modification of MUC1 maybe a new target to promote drug sensitivity and efficacy for breast cancer chemotherapy. Cell death & disease. 2022;13:708
21. Gray HJ, Benigno B, Berek J, Chang J, Mason J, Mileshkin L. et al. Progression-free and overall survival in ovarian cancer patients treated with CVac, a mucin 1 dendritic cell therapy in a randomized phase 2 trial. Journal for immunotherapy of cancer. 2016;4:34
22. Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M. et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. The Lancet Oncology. 2014;15:59-68
23. Posey AD Jr, Schwab RD, Boesteanu AC, Steentoft C, Mandel U, Engels B. et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity. 2016;44:1444-54
24. Lin MC, Chuang YT, Wu HY, Hsu CL, Lin NY, Huang MC. Targeting tumor O-glycosylation modulates cancer-immune-cell crosstalk and enhances anti-PD-1 immunotherapy in head and neck cancer. Molecular oncology. 2023
25. Haltiwanger RS, Holt GD, Hart GW. Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-N-acetylglucosamine:peptide beta-N-acetylglucosaminyltransferase. The Journal of biological chemistry. 1990;265:2563-8
26. Dong DL, Hart GW. Purification and characterization of an O-GlcNAc selective N-acetyl-beta-D-glucosaminidase from rat spleen cytosol. The Journal of biological chemistry. 1994;269:19321-30
27. Chang YH, Weng CL, Lin KI. O-GlcNAcylation and its role in the immune system. Journal of biomedical science. 2020;27:57
28. Yuan Y, Wang L, Ge D, Tan L, Cao B, Fan H. et al. Exosomal O-GlcNAc transferase from esophageal carcinoma stem cell promotes cancer immunosuppression through up-regulation of PD-1 in CD8(+) T cells. Cancer letters. 2021;500:98-106
29. Radovani B, Gudelj I. N-Glycosylation and Inflammation; the Not-So-Sweet Relation. Frontiers in immunology. 2022;13:893365
30. Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nature reviews Cancer. 2015;15:540-55
31. Lim WA, June CH. The Principles of Engineering Immune Cells to Treat Cancer. Cell. 2017;168:724-40
32. Abreu TR, Fonseca NA, Gonçalves N, Moreira JN. Current challenges and emerging opportunities of CAR-T cell therapies. Journal of controlled release: official journal of the Controlled Release Society. 2020;319:246-61
33. Beavis PA, Slaney CY, Kershaw MH, Gyorki D, Neeson PJ, Darcy PK. Reprogramming the tumor microenvironment to enhance adoptive cellular therapy. Seminars in immunology. 2016;28:64-72
34. Greco B, Malacarne V. Disrupting N-glycan expression on tumor cells boosts chimeric antigen receptor T cell efficacy against solid malignancies. Science translational medicine. 2022;14:eabg3072
35. Zhang D, Li J, Wang F, Hu J, Wang S, Sun Y. 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer letters. 2014;355:176-83
36. Sasawatari S, Okamoto Y, Kumanogoh A, Toyofuku T. Blockade of N-Glycosylation Promotes Antitumor Immune Response of T Cells. Journal of immunology (Baltimore, Md: 1950). 2020;204:1373-85
37. Pfizenmaier K, Bartsch H, Scheurich P, Seliger B, Ucer U, Vehmeyer K. et al. Differential gamma-interferon response of human colon carcinoma cells: inhibition of proliferation and modulation of immunogenicity as independent effects of gamma-interferon on tumor cell growth. Cancer research. 1985;45:3503-9
38. Taniguchi N, Kizuka Y. Glycans and cancer: role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics. Advances in cancer research. 2015;126:11-51
39. Krug J, Rodrian G, Petter K, Yang H, Khoziainova S, Guo W. et al. N-glycosylation Regulates Intrinsic IFN-γ Resistance in Colorectal Cancer: Implications for Immunotherapy. Gastroenterology. 2023;164:392-406.e5
40. Sun L, Li CW, Chung EM, Yang R. Targeting Glycosylated PD-1 Induces Potent Antitumor Immunity. Cancer research. 2020;80:2298-310
41. Li CW, Lim SO, Chung EM, Kim YS, Park AH, Yao J. et al. Eradication of Triple-Negative Breast Cancer Cells by Targeting Glycosylated PD-L1. Cancer cell. 2018;33:187-201.e10
42. Zheng L, Yang Q, Li F, Zhu M, Yang H, Tan T. et al. The Glycosylation of Immune Checkpoints and Their Applications in Oncology. Pharmaceuticals (Basel, Switzerland). 2022 15
43. Hsu JM, Xia W, Hsu YH, Chan LC, Yu WH, Cha JH. et al. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nature communications. 2018;9:1908
44. Liu X, Zhang Y, Han Y, Lu W, Yang J, Tian J. et al. Overexpression of GLT1D1 induces immunosuppression through glycosylation of PD-L1 and predicts poor prognosis in B-cell lymphoma. Molecular oncology. 2020;14:1028-44
45. Ma XM, Luo YF, Zeng FF, Su C, Liu X, Li XP. et al. TGF-β1-Mediated PD-L1 Glycosylation Contributes to Immune Escape via c-Jun/STT3A Pathway in Nasopharyngeal Carcinoma. Frontiers in oncology. 2022;12:815437
46. Shi C, Wang Y, Wu M, Chen Y, Liu F, Shen Z. et al. Promoting anti-tumor immunity by targeting TMUB1 to modulate PD-L1 polyubiquitination and glycosylation. Nature communications. 2022;13:6951
47. Munkley J. Aberrant Sialylation in Cancer: Therapeutic Opportunities. Cancers. 2022 14
48. Murugesan G, Weigle B, Crocker PR. Siglec and anti-Siglec therapies. Current opinion in chemical biology. 2021;62:34-42
49. Pearce OM, Läubli H. Sialic acids in cancer biology and immunity. Glycobiology. 2016;26:111-28
50. van de Wall S, Santegoets KCM, van Houtum EJH, Büll C, Adema GJ. Sialoglycans and Siglecs Can Shape the Tumor Immune Microenvironment. Trends in immunology. 2020;41:274-85
51. Läubli H, Nalle SC. Targeting the Siglec-Sialic Acid Immune Axis in Cancer: Current and Future Approaches. Cancer immunology research. 2022;10:1423-32
52. Jandus C, Boligan KF, Chijioke O, Liu H, Dahlhaus M, Démoulins T. et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. The Journal of clinical investigation. 2014;124:1810-20
53. Perdicchio M, Cornelissen LA, Streng-Ouwehand I, Engels S, Verstege MI, Boon L. et al. Tumor sialylation impedes T cell mediated anti-tumor responses while promoting tumor associated-regulatory T cells. Oncotarget. 2016;7:8771-82
54. Büll C, Boltje TJ, Balneger N, Weischer SM, Wassink M, van Gemst JJ. et al. Sialic Acid Blockade Suppresses Tumor Growth by Enhancing T-cell-Mediated Tumor Immunity. Cancer research. 2018;78:3574-88
55. Xiao H, Woods EC, Vukojicic P, Bertozzi CR. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:10304-9
56. Gray MA, Stanczak MA, Mantuano NR, Xiao H, Pijnenborg JFA, Malaker SA. Targeted glycan degradation potentiates the anticancer immune response in vivo. Nature chemical biology. 2020;16:1376-84
57. Stanczak MA, Rodrigues Mantuano N, Kirchhammer N. Targeting cancer glycosylation repolarizes tumor-associated macrophages allowing effective immune checkpoint blockade. Science translational medicine. 2022;14:eabj1270
58. Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nature reviews Immunology. 2014;14:653-66
59. Läubli H, Pearce OM, Schwarz F, Siddiqui SS, Deng L, Stanczak MA. et al. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:14211-6
60. Beatson R, Tajadura-Ortega V, Achkova D, Picco G, Tsourouktsoglou TD, Klausing S. et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nature immunology. 2016;17:1273-81
61. Mei Y, Wang X, Zhang J, Liu D, He J, Huang C. et al. Siglec-9 acts as an immune-checkpoint molecule on macrophages in glioblastoma, restricting T-cell priming and immunotherapy response. Nature cancer. 2023;4:1273-91
62. Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science (New York, NY). 2009;323:1722-5
63. Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW. et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572:392-6
64. Wang J, Sun J, Liu LN, Flies DB, Nie X, Toki M. et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nature medicine. 2019;25:656-66
65. Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nature reviews Immunology. 2007;7:255-66
66. Stanczak MA, Siddiqui SS, Trefny MP, Thommen DS, Boligan KF, von Gunten S. et al. Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells. The Journal of clinical investigation. 2018;128:4912-23
67. Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S. et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nature medicine. 2018;24:20-8
68. Kim MY, Yu KR, Kenderian SS, Ruella M, Chen S, Shin TH. et al. Genetic Inactivation of CD33 in Hematopoietic Stem Cells to Enable CAR T Cell Immunotherapy for Acute Myeloid Leukemia. Cell. 2018;173:1439-53.e19
69. Jetani H, Navarro-Bailón A. Siglec-6 is a novel target for CAR T-cell therapy in acute myeloid leukemia. Blood. 2021;138:1830-42
70. Keeley TS, Yang S, Lau E. The Diverse Contributions of Fucose Linkages in Cancer. Cancers. 2019 11
71. García-García A, Ceballos-Laita L. Structural basis for substrate specificity and catalysis of α1,6-fucosyltransferase. Nature communications. 2020;11:973
72. Okada M, Chikuma S, Kondo T, Hibino S, Machiyama H, Yokosuka T. et al. Blockage of Core Fucosylation Reduces Cell-Surface Expression of PD-1 and Promotes Anti-tumor Immune Responses of T Cells. Cell reports. 2017;20:1017-28
73. Zhang N, Li M, Xu X, Zhang Y, Liu Y, Zhao M. et al. Loss of core fucosylation enhances the anticancer activity of cytotoxic T lymphocytes by increasing PD-1 degradation. European journal of immunology. 2020;50:1820-33
74. Huang Y, Zhang HL, Li ZL. FUT8-mediated aberrant N-glycosylation of B7H3 suppresses the immune response in triple-negative breast cancer. Nature communications. 2021;12:2672
75. Do KT, Chow LQM, Reckamp K, Sanborn RE, Burris H, Robert F. et al. First-In-Human, First-In-Class, Phase I Trial of the Fucosylation Inhibitor SGN-2FF in Patients with Advanced Solid Tumors. The oncologist. 2021;26:925-e1918
76. Cohen Saban N, Yalin A. Fc glycoengineering of a PD-L1 antibody harnesses Fcγ receptors for increased antitumor efficacy. Science immunology. 2023;8:eadd8005
77. Abastado JP. The next challenge in cancer immunotherapy: controlling T-cell traffic to the tumor. Cancer research. 2012;72:2159-61
78. Alatrash G, Qiao N, Zhang M, Zope M, Perakis AA, Sukhumalchandra P. et al. Fucosylation Enhances the Efficacy of Adoptively Transferred Antigen-Specific Cytotoxic T Lymphocytes. Clinical cancer research: an official journal of the American Association for Cancer Research. 2019;25:2610-20
79. Wang M, Wang J, Wang R, Jiao S, Wang S, Zhang J. Identification of a monoclonal antibody that targets PD-1 in a manner requiring PD-1 Asn58 glycosylation. Communications biology. 2019;2:392
80. Liu J, Wang G, Liu L, Wu R, Wu Y, Fang C. et al. Study of the interactions of a novel monoclonal antibody, mAb059c, with the hPD-1 receptor. Scientific reports. 2019;9:17830
81. Rousseau A, Parisi C, Barlesi F. Anti-TIGIT therapies for solid tumors: a systematic review. ESMO open. 2023;8:101184
82. Garralda E, Sanborn RE, Minchom AR, Davar D, Curigliano G, Ribrag V. et al. SGNTGT-001: A phase 1 study of SEA-TGT, an effector-function enhanced monoclonal antibody (mAb), in advanced malignancies (trial in progress). Journal of Clinical Oncology. 2021;39:TPS2657-TPS
83. Berois N, Pittini A, Osinaga E. Targeting Tumor Glycans for Cancer Therapy: Successes, Limitations, and Perspectives. Cancers. 2022 14
84. Chen X, Sandrine IK, Yang M, Tu J, Yuan X. MUC1 and MUC16: critical for immune modulation in cancer therapeutics. Frontiers in immunology. 2024;15:1356913
85. Matsumoto Y, Ju T. Aberrant Glycosylation as Immune Therapeutic Targets for Solid Tumors. Cancers. 2023 15
Author contact
![]() Corresponding authors: Feng Guan, guanfengedu.cn; Huafeng Kang, kanghuafeng1973com.
Corresponding authors: Feng Guan, guanfengedu.cn; Huafeng Kang, kanghuafeng1973com.

 Global reach, higher impact
Global reach, higher impact