10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2025; 21(13):5691-5703. doi:10.7150/ijbs.117211 This issue Cite
Review
Pathophysiology, development, and mortality of major non-communicable diseases in metabolic dysfunction-associated steatotic liver disease: A comprehensive review
1. Department of Biomedical Informatics, Korea University College of Medicine, Seoul, Republic of Korea.
2. Biomedical Research Center, Korea University Guro Hospital, Seoul, Republic of Korea.
3. Department of Biomedical Science, CHA University, Pocheon, Republic of Korea.
4. Department of Liver Surgery, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
#These authors contributed equally.
Received 2025-5-9; Accepted 2025-8-20; Published 2025-9-3
Abstract
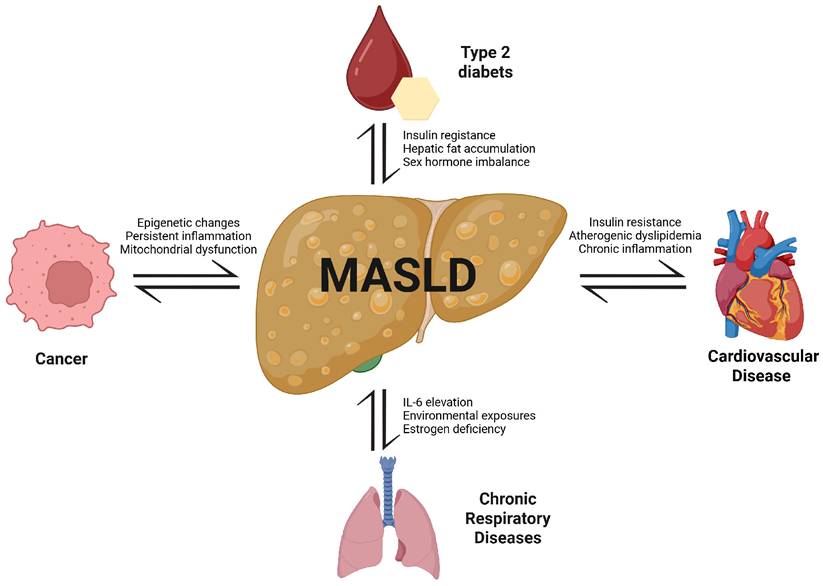
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as nonalcoholic fatty liver disease and metabolic dysfunction-associated fatty liver disease, has emerged as a critical contributor to the global burden of non-communicable diseases (NCDs). Beyond its hepatic manifestations, MASLD is pathophysiologically connected to broader metabolic dysfunction, including insulin resistance, obesity, type 2 diabetes, and cardiovascular complications. This review critically examines the bidirectional associations between MASLD and major NCDs, including type 2 diabetes, cardiovascular disease, chronic respiratory disease, and cancer, focusing on shared mechanisms such as chronic inflammation, insulin resistance, oxidative stress, lipotoxicity, and epigenetic alterations. Furthermore, we explore disease-specific mortality patterns and mortality-related factors in MASLD patients across NCD domains. This review underscores the need for comprehensive and multidisciplinary strategies that address not only metabolic control but also systemic inflammation, immunometabolic dysregulation, and epigenetic alterations. Such integrative approaches are essential to mitigating the multisystem burden of MASLD and reducing mortality from its associated NCDs.
Introduction
Non-communicable diseases (NCDs), including cardiovascular disease (CVD), cancer, type 2 diabetes (T2D), and chronic respiratory diseases, have emerged as major global health concerns (1). These diseases, linked by shared pathophysiological mechanisms such as metabolic dysfunction, chronic inflammation, and oxidative stress, are integral in understanding the connection between metabolic disorders and the onset of NCDs, particularly highlighting metabolic-associated steatosis and liver disease (MASLD) as a key factor contributing to the escalating global NCD burden (2).
Previously known as non-alcoholic fatty liver disease (NAFLD), MASLD, characterized by excessive hepatic fat accumulation without significant alcohol consumption, is increasingly seen not just as a liver-specific issue but as a condition deeply intertwined with systemic metabolic and inflammatory disturbances (3). Notably, MASLD has a strong association with major NCDs, including CVD, cancer, T2D, and chronic respiratory diseases, and it can contribute to multiple extrahepatic complications.
In addition to MASLD, steatotic liver diseases can be categorized based on alcohol consumption as metabolic-associated alcohol-related liver disease (MetALD) and alcohol-related liver disease (ALD). Given that alcohol consumption influences the incidence, risk, and mortality rates of major NCDs and related complications, considerable research has been conducted to examine the differences in end-stage liver disease and mortality risks among MASLD, MetALD, and ALD (4, 5).
However, the pathophysiological interactions between MASLD and individual NCDs, as well as the associated mortality risks, remain inadequately understood. Therefore, this review aims to explore the pathophysiological links between MASLD and NCDs such as CVD, cancer, T2D, and chronic respiratory diseases, and to assess their impact on cause-specific mortality (CVD-, cancer-, T2D-, and respiratory disease-related mortality) and overall mortality.
Specifically, this review addresses: (1) The shared pathophysiological mechanisms underpinning MASLD and major NCDs, (2) The interplay between MASLD progression and the development of NCDs, and (3) The impact of MASLD on cause-specific mortality, including CVD-, cancer-, T2D-, and chronic respiratory disease-related deaths, as well as all-cause mortality. By providing a detailed analysis of these topics, this review aims to clarify MASLD's central role in the context of NCDs and identify opportunities for integrated prevention and management strategies. By clarifying the central role of MASLD in the pathophysiology of NCDs, this review aims to contribute to the development of integrated prevention and management strategies.
Shared pathophysiology between MASLD and NCDs
MASLD is a multifactorial disorder involving complex interactions among metabolic dysregulation, hepatic lipid accumulation, oxidative stress, and chronic inflammation. The disease process is initiated by sustained caloric excess and insulin resistance, which disrupt glucose and lipid homeostasis (Figure 1). Importantly, MASLD affects multiple organs through metabolic and inflammatory pathways shared with NCDs, with the gut-liver, liver-heart, and liver-pancreas axes serving as key inter-organ links that underscore its role as a central mediator of cardiometabolic and extrahepatic complications (6).
In insulin-resistant states, hepatic gluconeogenesis persists despite hyperinsulinemia. Concurrently, increased lipolysis in adipose tissue elevates free fatty acids (FFAs), which are transported to the liver. These FFAs promote de novo lipogenesis through the upregulation of sterol regulatory element-binding protein-1c (SREBP-1c) and carbohydrate-responsive element-binding protein (ChREBP) (7). Insulin resistance also reduces adiponectin secretion, further amplifying hepatic fat accumulation in a vicious cycle (Figure 2) (8). As hepatic lipid accumulation increases, mitochondrial dysfunction and endoplasmic reticulum (ER) stress impairs β-oxidation and increases the generation of reactive oxygen species (ROS), leading to lipid peroxidation and hepatocyte injury. The release of damage-associated molecular patterns (DAMPs) activates Kupffer cells and hepatic stellate cells, triggering NF-κB and NLRP3 inflammasome signaling, and including cytokines, such as TNF-α, IL-6, and IL-1β (9). Notably, chronic airway inflammation observed in diseases such as chronic obstructive pulmonary disease (COPD) and asthma has also been associated with elevated IL-6 levels (10), and emerging evidence suggests that MASLD-related activation of neutrophils and macrophages may similarly contribute to emphysema pathogenesis by releasing proteolytic enzymes that overwhelm alpha-1 antitrypsin (A1AT) regulation, a mechanism implicated in alveolar damage and disease progression in COPD (11-13). Furthermore, insulin resistance, a hallmark of MASLD, has been shown to double the risk of hepatocellular carcinoma (HCC) and increase its mortality by approximately 50% (14). While closely linked to T2D, insulin resistance also independently drives hepatic carcinogenesis through hyperinsulinemia and enhanced IGF-1 signaling, which activates the PI3K/AKT and MAPK pathways via insulin receptor substrate (IRS), promoting proliferation and inhibiting apoptosis (15-17). This oncogenic mechanism is observed even in the absence of overt T2D (18), although the coexistence of T2D in MASLD patients further amplifies the risk of HCC (19).
MASLD is also associated with dysregulated lipid metabolism. Patients often exhibit atherogenic dyslipidemia characterized by elevated small dense low-density lipoprotein (sdLDL), increased very low-density lipoprotein (VLDL) secretion, and decreased high-density lipoprotein (HDL) cholesterol (20). Epigenetic changes, such as aberrant DNA methylation of insulin signaling genes (e.g., IRS1, AKT2), histone modifications, and altered microRNAs (e.g., miR-122, miR-34a), further drive disease progression (21-23). Telomere shortening, p53 suppression, and loss of chromatin remodeling proteins (e.g., ARID1A) have also been identified in MASLD-associated HCC, linking genomic instability to carcinogenesis (24).
Environmental exposures, including endocrine-disrupting chemicals (EDCs) and air pollutants, such as PM2.5, contribute to hepatic steatosis and inflammation through oxidative stress, nuclear receptor interference, and gut-liver axis disruption (25, 26). The gut microbiota also influences MASLD pathophysiology via its metabolites, such as short-chain fatty acids (e.g., butyrate, acetate) that act as histone deacetylase (HDAC) inhibitors and modulate gene expression and immune responses. Microbial shifts affect the DNA methylation of genes involved in lipid metabolism and inflammation (e.g., APOA4, adiponectin, resistin), and modulate miRNA profiles related to hepatocyte apoptosis, fibrosis, and insulin sensitivity (e.g., miR-34a, miR-21, miR-582-3p) (27).
These mechanisms may vary by sex. Estrogen, SHBG, and ERα regulate lipid metabolism and immunity differently by sex (28). Epidemiological evidence shows that women with MASLD are at a higher risk of developing cirrhosis, while men have higher risks of hepatic decompensation, hepatocellular carcinoma (HCC; hazard ratio [HR], 2.59), cardiovascular disease (HR, 1.40), chronic kidney disease (HR, 1.16), and non-sex-specific cancers (HR, 1.32) (29). Similarly, asthma exhibits sex-dependent differences, with hormonal imbalance (e.g., excess estrogen in postmenopausal women) increasing disease burden (30). In men, testosterone enhances insulin sensitivity, and its deficiency has been linked to T2D onset in the context of MASLD (31). These hormonal imbalances may further exacerbate the metabolic burden of MASLD by promoting the development of NCDs.
MASLD and the development of T2D
T2D is driven by a combination of insulin resistance, metabolic dysfunction, and chronic inflammation. Although these pathophysiological features are common to many NCDs, MASLD distinctly contributes to T2D progression by disrupting hepatic glucose metabolism and impairing pancreatic islet homeostasis (32).
Bidirectional associations between MASLD and major NCDs. MASLD is centrally linked to the pathogenesis and progression of type 2 diabetes, cardiovascular diseases, chronic respiratory diseases, and cancer. Shared mechanisms include insulin resistance, chronic inflammation, dyslipidemia, hormonal imbalance, environmental exposures, and mitochondrial dysfunction. These pathways not only promote MASLD development but also exacerbate comorbid NCDs, creating a cycle of mutual reinforcement and increased mortality risk.
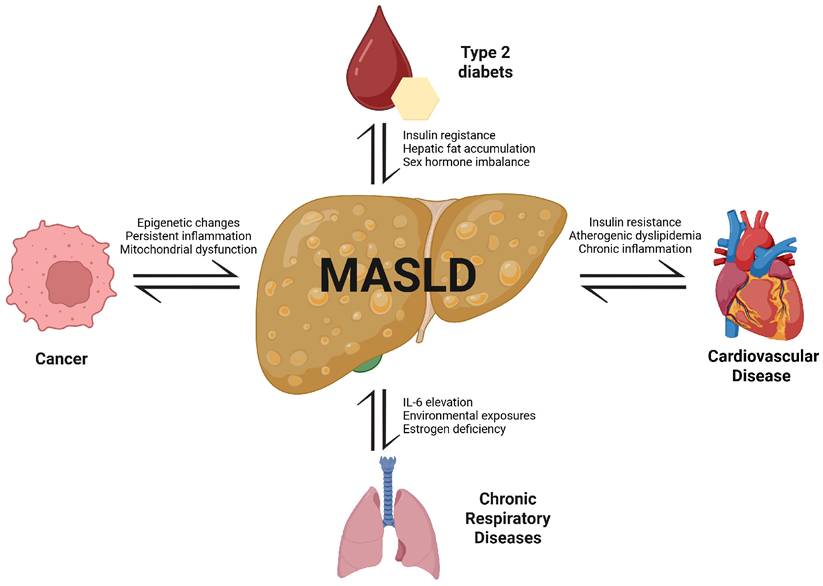
Mechanistic pathway linking insulin resistance to MASLD via adiponectin-AMPK signaling. Insulin resistance leads to decreased adiponectin levels, which suppresses AMPK activation in hepatocytes. This impairs ATP synthesis and enhances de novo lipogenesis, resulting in increased hepatic fat accumulation (fatty liver). Concurrently, hepatic insulin resistance disrupts glucose homeostasis, contributing to elevated blood glucose levels and further exacerbating metabolic dysfunction.
The primary mechanism through which MASLD contributes to T2D development is hepatic insulin resistance (33). Accumulation of excess lipids in hepatocytes impairs insulin signaling, reducing the liver's responsiveness to insulin. As a result, gluconeogenesis proceeds unchecked, leading to increased hepatic glucose production and fasting hyperglycemia (34, 35). In response to insulin resistance, pancreatic β-cells temporarily compensate by increasing insulin secretion. However, prolonged exposure to lipotoxicity and glucotoxicity induces β-cell dysfunction and eventual insulin insufficiency, culminating in T2D onset (36).
Inflammatory cytokines elevated in MASLD, particularly IL-1β, contribute to β-cell toxicity and impaired insulin secretion, thereby compounding metabolic dysfunction and insulin resistance (37, 38). Beyond hepatic lipid accumulation, recent evidence highlights the pivotal role of the gut microbiota in modulating liver inflammation, oxidative stress, and systemic insulin sensitivity. Dysbiosis-related microbial components such as lipopolysaccharide (LPS), peptidoglycans, and flagellin can translocate from the gut to the liver, activate innate immune signaling, and exacerbate hepatic injury and insulin resistance. The absence of protective microbial metabolites such as indole-3-propionic acid (IPA) has been shown to worsen fibrosis and metabolic dysregulation, particularly in germ-free models or under environmental insults like smoking or high-fat diets (39).
MASLD-related epigenetic reprogramming, including methylation of insulin signaling genes (e.g., IRS1, AKT2) and altered miRNA expression, impairs glucose utilization and promotes mitochondrial dysfunction (40, 41). Accelerated epigenetic aging has also been observed, marked by DNA methylation changes and elevated inflammatory chemokines such as CXCL10, CXCL11, and enRAGE, all of which increase vulnerability to T2D (42, 43).
MASLD and the development of CVD
Development of CVD is affected by various factors, such as air pollution, antibiotic use, body composition and weight, physical activity, cholecystectomy, and COPD (44-50). While MASLD is traditionally viewed as a hepatic manifestation of metabolic syndrome, hepatic steatosis itself with or without cardiometabolic risk factors has been identified as an independent predictor of cardiovascular morbidity and mortality (51-53), with the relative risk further increased in individuals with MetALD and ALD (54).
A key mechanism linking MASLD to CVD is vascular insulin resistance, which impairs endothelial function and promotes atherogenesis. In MASLD patients, lipotoxicity and chronic low-grade inflammation contribute to dysregulation of lipid metabolism, resulting in an atherogenic profile characterized by elevated small dense LDL, increased VLDL secretion, and reduced HDL cholesterol levels. These abnormalities accelerate plaque formation and heighten the risk of myocardial infarction, stroke, and heart failure (35). Under normal physiological conditions, insulin facilitates the degradation of apolipoprotein B (apoB), regulating plasma triglyceride levels. However, MASLD-induced insulin resistance disrupts this pathway, leading to increased triglyceride accumulation and altered lipoprotein composition, which further aggravate endothelial dysfunction and vascular inflammation (55).
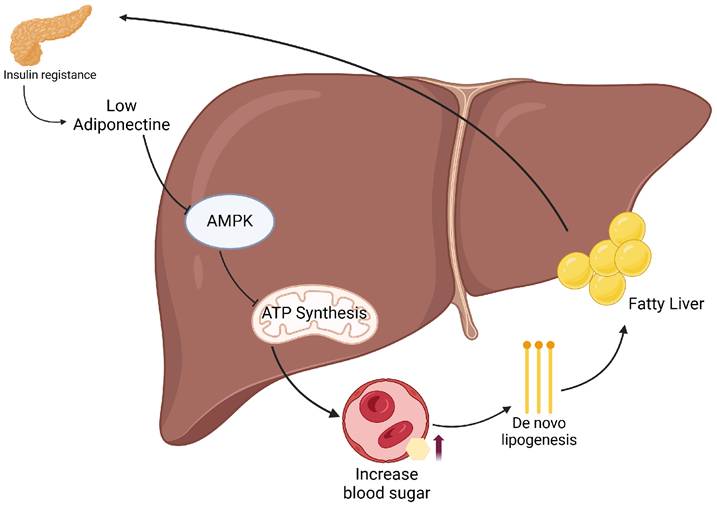
Overactivation of the renin-angiotensin-aldosterone system (RAAS) and heightened sympathetic nervous system activity, frequently observed in MASLD, contribute to increased vascular tone, sodium retention, and blood pressure elevation, thereby compounding cardiovascular risk (37). Hyperglycemia contributes to RAAS activation by increasing sodium-glucose reabsorption via SGLT2 and inducing oxidative stress, while insulin resistance enhances proximal sodium reabsorption, reduces sodium delivery to the macula densa, and stimulates renin release; these processes collectively activate the RAAS, upregulate components (such as angiotensinogen, ACE, and the AT1 receptor), and impair insulin signaling and nitric oxide bioavailability, thereby promoting endothelial dysfunction and elevating CVD risk in MASLD (Figure 3) (56-58). Moreover, persistent hyperglycemia in MASLD contributes to oxidative stress and impairs endothelial nitric oxide (NO) bioavailability, reducing vasodilatory capacity and promoting vascular stiffness (35). The intersection between MASLD and chronic kidney disease (CKD) also plays a role in CVD development. MASLD patients exhibit increased susceptibility to CKD, and the resultant decline in renal function exacerbates fluid imbalance and systemic inflammation, further elevating cardiovascular burden (59).
Hyperglycemia and the renin-angiotensin-aldosterone system (RAAS) in MASLD. Hyperglycemia promotes the upregulation of RAAS components, including angiotensinogen, ACE, angiotensin II, and the AT1 receptor. Angiotensin II stimulates aldosterone secretion and transactivation of the EGF receptor, while insulin signaling through the IRS-1/PI3K/AKT/eNOS pathway contributes to vasodilation. The interplay between these pathways may contribute to metabolic and vascular dysregulation in MASLD.
To date, the epigenetic mechanisms underlying the onset of CVD in patients with MASLD remain not fully understood. A study from Belgium found that gradual changes in DNA methylation is associated with progression of MASLD and epigenetic age acceleration (60). In MASLD, dysregulated DNA methylation disrupts phosphatidylcholine synthesis, which is critical for the maintenance of HDL and VLDL levels, thereby impairing metabolic homeostasis in a manner resembling atherogenic dyslipidemia, which is a well-established risk factor for CVD (61). Together, these epigenetic alterations may mechanistically link MASLD to increased CVD susceptibility.
MASLD and the development of chronic respiratory diseases (COPD and asthma)
Chronic respiratory diseases such as COPD and asthma are increasingly recognized to share overlapping mechanisms with MASLD, including low-grade systemic inflammation, oxidative stress, and immunosenescence (62, 63). While traditionally regarded as distinct conditions, emerging evidence suggests that metabolic dysfunction driven by MASLD can negatively impact pulmonary physiology. Specifically, hepatic metabolic disturbances may contribute to systemic hypoxia and redox imbalance, ultimately compromising respiratory function and increasing susceptibility to pulmonary complications (63).
One mechanism through which MASLD affects COPD progression is by promoting pulmonary vascular dysfunction. Impaired NO bioavailability and elevated peripheral vascular resistance, frequently observed in MASLD, can disrupt pulmonary circulation and contribute to pulmonary hypertension. This hemodynamic alteration increases right ventricular workload and oxygen delivery deficits in COPD patients, compounding the burden of cardiopulmonary impairment (62).
MASLD also exacerbates chronic inflammation that fuels structural changes in the respiratory tract. Elevated levels of proinflammatory cytokines such as IL-6 and TNF-α are frequently observed in MASLD patients (64). These cytokines enhance airway remodeling and promote inflammatory cell recruitment, thereby accelerating the decline in lung function and increasing the frequency of COPD exacerbations (65). Beyond cytokine signaling, MASLD-related activation of innate immune cells, particularly neutrophils and macrophages, may contribute to emphysema pathogenesis by releasing proteolytic enzymes that disrupt A1AT regulation, a well-established mechanism in COPD (11, 12). While direct evidence is limited, this pathway offers a plausible immunometabolic link between MASLD and emphysematous lung changes. Recent studies also suggest that MASLD-related gut dysbiosis may contribute to pulmonary inflammation through the gut-lung axis, thereby exacerbating respiratory complications via immune and redox imbalance (39).
Another crucial impact of MASLD lies in its contribution to immune and metabolic dysfunction. By increasing susceptibility to chronic hypoxia and oxidative stress, MASLD accelerates immune aging and further deteriorates systemic metabolic regulation (63, 64). During acute COPD exacerbations, venous stasis and a hypercoagulable state increase the risk of pulmonary embolism (PE), and the accompanying hyperinsulinemia and vascular dysfunction in MASLD patients further exacerbate this risk (37, 66).
In asthma, MASLD is similarly implicated in amplifying airway inflammation. Due to heightened baseline inflammation, individuals with MASLD may experience more frequent and severe asthma exacerbations (67). Moreover, recent large-scale analyses have identified a significant association between MASLD and eosinophilic esophagitis (EoE), which is a chronic Th2-driven inflammatory disease that commonly develops in patients with asthma (68). Although the evidence is lacking, the increased risk of EoE may be associated with MASLD-driven asthma, which awaits future studies to confirm. Epigenetic modifications associated with MASLD, particularly aberrant DNA methylation, may further exacerbate airway inflammation by upregulating proinflammatory gene expression (69).
MASLD and the development of cancer
Cancer development in patients with MASLD is driven by a combination of metabolic dysfunction, chronic inflammation, and epigenetic alterations, which collectively establish a tumor-promoting microenvironment (18). MASLD has been strongly associated with HCC, pancreatic cancer, colorectal cancer, and breast cancer, with hyperinsulinemia and activation of the IGF-1 signaling pathway playing central roles in tumor growth and metastasis (70). A pooled analysis of 18 cohort studies with conflicting results found that MASLD is associated with a higher risk of gastric, colorectal, thyroid, pancreatic, urinary system, biliary duct, skin, breast, and female genital cancers (71).
Chronic hepatic fat accumulation in MASLD leads to sustained hepatocellular injury, which impairs DNA repair mechanisms and increases genomic instability, thereby elevating the risk of tumorigenesis (72). Moreover, persistent oxidative stress and inflammation further contribute to hepatic tissue damage, fostering fibrosis and cirrhosis and ultimately raising the likelihood of HCC development (17). In MASLD, stressed hepatocytes release damage-associated molecular patterns, cytokines, and acute phase proteins that activate surrounding immune cells, contributing to a shift from hepatic immunotolerance to inflammation and driving progression toward fibrosis and HCC (73).
While HCC remains the most extensively studied cancer in MASLD, accumulating evidence suggests that systemic metabolic alterations in MASLD, such as hyperglycemia, insulin resistance, and inflammation, also contribute to the development of extrahepatic cancers (17). These abnormalities are particularly relevant in colorectal and pancreatic cancers, where chronic metabolic stress facilitates tumor proliferation and accelerates disease progression (70). In MASLD patients with coexisting T2D, insulin resistance within the colonic epithelium further promotes tumor growth and exacerbates cancer advancement (74). Notably, a recent cohort study suggested that improved glycemic regulation and weight management may reduce both liver and extrahepatic cancers (75). In addition, epigenetic modifications associated with MASLD also play a pivotal role in elevating cancer risk. Aberrant DNA methylation silences key tumor suppressor genes, including p16, p21, p27, and p53, thereby enhancing cellular proliferation, metastatic potential, and resistance to therapy factors that collectively contribute to poor cancer prognosis (76).
Recent evidence also highlights the influence of gut microbiota on tumor immunity. Dysbiosis-induced translocation of microbial products to the liver may trigger proinflammatory signaling and disrupt gut-liver immune homeostasis, potentially contributing to carcinogenesis, particularly in hepatocellular and colorectal cancers (39). In addition, metabolism-induced immune suppression in MASLD patients further facilitates tumor development (77, 78). Obesity, insulin resistance, and hepatic steatosis compromise immune surveillance, impairing the body's ability to eliminate malignant cells and increasing the risk of cancer initiation and metastasis (17).
Given the strong association between MASLD and cancer, early screening strategies targeting high-risk individuals are essential. Targeted anti-inflammatory therapies and metabolic regulation may serve to decelerate tumor progression and improve clinical outcomes in MASLD-related cancers (17, 78).
Mortality in patients with MASLD and T2D
In the past, CVD was the leading cause of death in patients with T2D. However, improvements in blood glucose control, broader use of antihypertensive medications and statins, and lifestyle modifications have contributed to a reduction in CVD-related mortality (79). Nevertheless, when MASLD coexists with T2D, the overall mortality risk remains elevated, and cancer and CKD have emerged as leading causes of death (79).
Cancer plays a significant role in mortality among individuals with both MASLD and T2D. While hyperinsulinemia and systemic inflammation in T2D promote oncogenesis, MASLD exacerbates these mechanisms (80). Epidemiological studies indicate markedly increased rates of HCC, pancreatic, colorectal, and breast cancers in this population (80). Specifically, when T2D was diagnosed prior to MASLD, the risk of HCC increased by approximately 1.96-fold (HR 1.96; 95% CI: 1.69-2.27) and pancreatic cancer by 1.25-fold (HR 1.25; 95% CI: 1.06-1.48), whereas the risks of renal cancer (HR 1.12; 95% CI: 0.98-1.29), colorectal cancer (HR 0.92; 95% CI: 0.80-1.07), and breast cancer (HR 0.98; 95% CI: 0.88-1.09) were not significantly increased (41).
Furthermore, in individuals with T2D who were subsequently diagnosed with MASLD, the risk of HCC surged dramatically, while significant increases were also observed for pancreatic cancer (HR 1.78; 95% CI: 1.12-2.84), renal cancer (HR 2.01; 95% CI: 1.24-3.26), and breast cancer (HR 1.43; 95% CI: 1.09-1.88) (41). However, the elevated risk of colorectal cancer (HR 1.21; 95% CI: 0.81-1.81) was not statistically significant (41). MASLD-associated hepatic lipid overload and inflammatory signaling promote genomic instability, contributing to tumorigenesis in this high-risk group (81). Additionally, a reduction in 5-hydroxymethylcytosine (5hmC) levels in insulin signaling-related genes such as IRS1 and AKT2 has been identified in MASLD, suggesting potential early biomarkers for cancer risk (82, 83).
Biological aging acceleration, as reflected by DNA methylation patterns, has emerged as a mortality risk factor. Elevated levels of inflammatory mediators including CXCL10, CXCL11, and enRAGE, alongside CD8+ T cell activation, suggest immune dysregulation and increased vulnerability in MASLD-T2D comorbidity (40, 82).
When MASLD and T2D coexist, the likelihood of developing CKD rises substantially, which in turn aggravates cardiovascular mortality risk. As renal function declines, systemic inflammation and fluid dysregulation intensify, heightening the risk of heart failure. In advanced CKD, escalating insulin resistance further complicates metabolic control, contributing to a steep increase in cardiovascular-related deaths (59).
Importantly, in vivo studies have provided mechanistic evidence linking MASLD to elevated mortality in the context of T2D. ALOX15 was shown to exacerbate hepatic inflammation and oxidative stress in diabetic mice, thereby worsening insulin resistance and MASLD progression (84). Additionally, in a rat model of early NAFLD, insulin resistance was found to cause liver microcirculatory dysfunction, including impaired hepatic perfusion, increased portal pressure, and reduced sinusoidal endothelial fenestration. These microvascular alterations likely amplify hypoxia and hepatocellular injury, exacerbating inflammation and progression toward cirrhosis (85). This mechanistic link highlights the pathological role of insulin resistance not only in metabolic dysfunction but also in hepatic hemodynamic deterioration, contributing to the elevated mortality risk in patients with MASLD and T2D (85). These findings support the notion that MASLD contributes to mortality not only through comorbidity burden but also through direct metabolic and inflammatory insults.
Evolutionarily, severe insulin resistance once posed a survival and reproductive disadvantage. However, advancements in modern healthcare have allowed individuals with these metabolic traits to live longer and pass on such predispositions. Consequently, vulnerabilities that might have been diminished by natural selection are now more prevalent, contributing to the global rise in MASLD, T2D, and their associated mortality (86).
Accordingly, proactive prevention and tailored treatment strategies are crucial for individuals with comorbid MASLD and T2D. Rather than focusing solely on glycemic control, therapeutic efforts should prioritize systemic metabolic regulation and suppression of chronic inflammation. Incorporating lifestyle interventions, pharmacologic agents, and early biomarker-based risk stratification may enhance long-term survival prospects in this high-risk population.
Mortality in patients with MASLD and CVD
CVD represents one of the most common causes of death in individuals with MASLD, primarily due to the convergence of metabolic dysregulation, atherosclerotic progression, and persistent inflammation (87). The link between MASLD and CVD is particularly robust. While hepatic fat accumulation has been identified as a feature of cardiometabolic syndrome and a predictor of cardiovascular mortality (88), the causal contribution of MASLD itself to cardiovascular death likely occurs through its impact on metabolic dysfunction and vascular injury.
Atherosclerotic progression is markedly accelerated in MASLD, translating into higher rates of cardiovascular death due to myocardial infarction, stroke, and heart failure (88). his is largely attributable to insulin resistance and lipid abnormalities, including elevated triglycerides and sdLDL, along with reduced HDL levels, which facilitate unstable plaque development and fatal vascular events (88).
Emerging in vivo studies provide mechanistic evidence linking MASLD with increased cardiovascular mortality. In a mouse model of MASLD, capillarization of liver sinusoidal endothelial cells (LSECs), characterized by loss of fenestrae and upregulation of capillarization markers such as CD31 and CD34, was shown to precede hepatic inflammation and was associated with elevated plasma LDL and triglyceride levels, supporting a causal role for LSEC dysfunction in systemic lipid dysregulation and cardiovascular disease progression (89). In this context, impaired hepatic clearance of chylomicron remnants due to LSEC capillarization may promote hyperlipidemia and atherosclerosis, key drivers of CVD-related mortality in MASLD (89).
Additionally, comorbid conditions such as obstructive sleep apnea syndrome (OSAS) and smoking may act as amplifiers of MASLD-induced cardiovascular damage. Periodic hypoxia in OSAS exacerbates hepatic steatosis via HIF2-α-mediated CD36 activation and increases systemic inflammation and insulin resistance in both mouse models and human studies, thereby compounding CVD risk (90). Smoking has similarly been shown to aggravate hepatic oxidative stress and gut-liver dysregulation in preclinical MASLD models, further promoting endothelial dysfunction and cardiometabolic complications (90).
Furthermore, MASLD may not only predispose individuals to CVD but also be worsened by CVD events. A recent in vivo study demonstrated that myocardial infarction (MI) in mice with MASH accelerates hepatic inflammation and fibrosis through Ly6Chi monocyte recruitment and elevated POSTN signaling, even though these mechanisms are typically associated with cardiac repair (91). These results suggest a bidirectional interaction, in which MASLD contributes to cardiovascular mortality while cardiac events simultaneously aggravate liver disease progression (91).
Persistent hypertension in MASLD patients, fueled by RAAS overactivity and sympathetic nervous system stimulation, contributes to vascular remodeling and arterial rigidity. These hemodynamic disturbances significantly raise the risk of cardiovascular mortality by intensifying end-organ damage and circulatory strain (37).
CKD acts as a compounding factor in the CVD mortality trajectory among MASLD patients. Declining renal function intensifies fluid overload and systemic inflammation, which together accelerate cardiovascular deterioration and contribute to higher fatality rates (59). To mitigate cardiovascular mortality in MASLD, prompt regulation of glycemic and lipid parameters is vital (88). Anti-inflammatory therapies also represent a crucial intervention, emphasizing the importance of integrated strategies aimed at curbing metabolic stress and vascular injury. Early screening and preventive interventions for cardiovascular disease in MASLD patients can significantly improve long-term survival outcomes.
Mortality in patients with MASLD and chronic respiratory diseases (COPD and asthma)
The coexistence of MASLD with chronic respiratory diseases such as COPD and asthma significantly heightens mortality risk, largely due to the systemic complications induced by hepatic metabolic dysregulation. These include persistent inflammation, oxidative damage, and impaired immune responses, which collectively compromise pulmonary function and elevate the risk of fatal respiratory events (92).
Metabolic imbalances inherent to MASLD increase susceptibility to sustained hypoxic conditions and oxidative stress, both of which progressively undermine respiratory capacity and contribute to long-term pulmonary decline (64). Notably, mitochondrial oxidative phosphorylation (OXPHOS), enhanced by free fatty acid metabolism such as palmitate-induced β-oxidation, leads to the excessive production of ROS, including superoxide anions and hydrogen peroxide (93). These ROS exacerbate oxidative stress in MASLD, contributing to systemic insulin resistance and amplifying JNK signaling, a key modulator of inflammatory and apoptotic pathways (93). The elevated oxidative burden not only impairs hepatic insulin signaling but also damages pulmonary endothelial and epithelial cells, further deteriorating respiratory function in patients with coexisting MASLD and chronic respiratory conditions (93).
In vivo studies have begun to elucidate mechanistic links between MASLD and pulmonary pathology. A recent mouse study demonstrated that MASLD induced by a high-fat diet resulted in not only hepatic inflammation and fibrosis, but also significant structural damage in the lung, including increased pulmonary fibrosis and elevated expression of pro-inflammatory mediators (94). Treatment with quercetin, a flavonoid with anti-inflammatory properties, attenuated both hepatic and pulmonary injury, suggesting that MASLD-induced systemic inflammation directly contributes to respiratory disease progression (94).
In individuals with COPD, the presence of MASLD heightens the risk of pulmonary hypertension and cor pulmonale. This is largely driven by MASLD-induced reductions in NO bioavailability and elevated peripheral vascular resistance, both of which compromise pulmonary hemodynamics, restrict oxygen transport, and cumulatively increase cardiopulmonary strain, factors that significantly elevate long-term mortality risk in COPD patients (37).
Chronic low-grade inflammation driven by MASLD serves as a key accelerator of COPD progression. Elevated circulating levels of IL-6 and TNF-α, commonly observed in MASLD patients, contribute to worsening alveolar damage and persistent airway inflammation. Evidence further suggests that COPD patients with coexisting MASLD experience more frequent acute exacerbations, higher hospitalization rates, and increased risk of respiratory-related mortality (64).
The coexistence of metabolic dysfunction and immunosenescence in MASLD patients heightens vulnerability to oxidative stress and impairs host defense mechanisms. This immune compromise increases susceptibility to respiratory infections, thereby playing a significant role in the elevated mortality observed in COPD patients with MASLD (92). While MASLD may not act as a direct cause of death in chronic respiratory diseases, it likely exacerbates disease progression and outcomes through metabolic and inflammatory stress that compromises pulmonary physiology. Acute exacerbations of COPD are associated with an elevated risk of PE, which is further intensified in MASLD patients due to the presence of hyperinsulinemia and vascular dysfunction. This compounding effect may increase the vulnerability to acute cardiovascular events and related mortality in individuals with both conditions (95).
The presence of MASLD may contribute to increased asthma-related mortality by amplifying systemic inflammation and exacerbating airway vulnerability. As asthma is defined by persistent airway inflammation and heightened responsiveness to stimuli, the systemic inflammatory burden seen in MASLD may intensify disease severity and lead to more frequent and life-threatening exacerbations (96). Epigenetic alterations linked to MASLD, such as aberrant DNA methylation, may heighten inflammatory gene expression and exacerbate bronchial inflammation, thereby contributing to asthma-related mortality (97).
Severe asthma attacks and subsequent respiratory failure, often requiring mechanical ventilation, are the primary drivers of asthma-related mortality. In individuals with MASLD, hyperglycemia and systemic metabolic derangements impair immune surveillance, increasing vulnerability to infections and thereby elevating the risk of life-threatening asthma exacerbations (98). Thus, early screening and proactive interventions are essential for patients with coexisting MASLD and chronic respiratory diseases. Anti-inflammatory treatments and metabolic regulation strategies are crucial for reducing mortality and preserving lung function. Additionally, integrated management of respiratory function, vascular health, and immune status plays a vital role in improving the survival outcomes of MASLD patients.
Mortality in patients with MASLD and cancer
Cancer-related mortality in MASLD patients has been on the rise, largely driven by factors that also underlie carcinogenesis, namely, metabolic imbalance, chronic inflammation, and epigenetic dysregulation (18, 99).
Rather than acting solely as an indirect contributor, MASLD may also exert direct effects that increase cancer mortality, as demonstrated by emerging in vivo evidence. A recent study revealed that CD8⁺ T cell exhaustion within the tumor microenvironment of MASLD-associated HCC leads to immune evasion and resistance to immune checkpoint blockade therapies. In particular, PD-1⁺CD8⁺ T cells exhibited reduced cytotoxicity, and their depletion accelerated tumor growth in MASLD mouse models, suggesting an intrinsic impairment in tumor surveillance driven by hepatic steatosis and inflammation (100). This finding underscores the functional link between MASLD and diminished anti-tumor immunity, which may worsen cancer prognosis (100).
In addition, suppression of ACMSD, a key modulator of NAD⁺ biosynthesis, has been shown to reduce γH2AX expression and improve mitochondrial function, thereby attenuating DNA damage, steatohepatitis, and hepatocarcinogenesis in MASLD models (101). Suppression of ACMSD has been shown to restore NAD⁺ levels and reduce γH2AX expression in MASLD models (101). Recent evidence further indicates that NAD⁺ depletion impairs DNA repair pathways, including RAD51 and γH2AX expression, providing mechanistic insight into how mitochondrial dysfunction and genomic instability contribute to MASLD-associated carcinogenesis (102).
Recent data also suggest that MASLD-associated hepatocarcinogenesis is exacerbated by genomic instability, including DNA repair failure, telomere shortening, and p53 pathway impairment, which have been observed in both clinical biopsies and in vivo mouse models (18, 72). MASLD is also known to heighten the risk of several malignancies, particularly HCC, pancreatic, colorectal, and breast cancers, with hyperinsulinemia and sustained IGF-1 pathway activation contributing not only to tumorigenesis but also to more aggressive tumor behavior and poorer prognostic outcomes (70, 103).
A major contributor to elevated cancer mortality in MASLD is the cumulative hepatocellular damage driven by steatosis and chronic oxidative stress. This ongoing injury compromises DNA repair capacity and promotes genomic instability, which not only increases the likelihood of tumor initiation but also accelerates HCC progression and reduces treatment efficacy (72). In advanced stages, cirrhosis, often a consequence of long-standing inflammation and fibrosis emerges as a pivotal determinant of poor survival in MASLD-associated liver cancer (104).
The increased cancer mortality associated with MASLD extends beyond hepatocellular carcinoma to include colorectal and pancreatic malignancies. In these cancers, metabolic disruptions, particularly chronic hyperglycemia, persistent inflammation, and insulin resistance, drive more aggressive tumor phenotypes and contribute to faster disease progression and reduced survival (97, 104). Among MASLD patients with comorbid T2D, insulin resistance within the colonic epithelium has been linked to enhanced tumor proliferation and metastatic potential, underscoring the compounded oncogenic risk in this population (105).
Beyond biological factors, socioeconomic disparities also significantly influence cancer mortality in MASLD. Patients with limited access to healthcare services are more likely to face delays in cancer diagnosis, leading to later-stage detection and diminished treatment outcomes. These systemic barriers contribute to the widening gap in survival rates among MASLD patients from underserved populations (106). This pattern is consistent with broader evidence indicating that poverty, inadequate education, and lack of insurance are associated with lower screening uptake, less access to standard-of-care therapies, and poorer overall survival, especially among racial and ethnic minorities (107). Even after adjusting for cancer stage, disparities in access to treatment, differences in physician recommendations, and challenges in patient, provider communication further contribute to unequal outcomes. Thus, the intersection of MASLD with socioeconomic inequality amplifies the burden of cancer mortality in vulnerable populations (107).
Given the elevated cancer risk in MASLD patients, expanding early detection efforts, especially for liver and colorectal cancers is vital for improving survival. Intensified screening protocols, coupled with targeted therapies aimed at reducing inflammation and restoring metabolic balance, may enhance treatment responsiveness and long-term outcomes in high-risk MASLD populations (108). Furthermore, addressing systemic barriers through public health initiatives, community outreach, and equitable access to screening and treatment resources should be prioritized to close the survival gap in MASLD-associated cancers (107).
In summary, early intervention and treatment strategies are essential to reduce cancer-related mortality in MASLD patients. Future research should not only focus on identifying specific therapeutic targets for MASLD-associated cancers but also on developing precision medicine and public health approaches that address both biological risk and social vulnerability (107).
Conclusions and Future Directions
This review highlights the pivotal role of MASLD as a driver of NCDs and mortality, extending beyond hepatic pathology to systemic metabolic dysfunction. The findings underscore that MASLD not only exacerbates liver-related outcomes, such as cirrhosis and HCC, but also significantly increases the risk of CVD and T2D (109).
From a clinical perspective, the literatures emphasize the importance of addressing insulin resistance to mitigate the progression of MASLD and its related NCDs (110). In addition, recent studies have identified that comprehensive lifestyle interventions, including Mediterranean dietary patterns, caloric restriction, and structured physical activity (e.g., moderate aerobic exercise or high-intensity interval training), not only reduce hepatic fat and inflammation but also improve insulin sensitivity and cortisol regulation(111, 112).
Future research should prioritize longitudinal studies, such as multi-center cohort studies or randomized controlled trials, which investigate the long-term risk of NCDs and mortality in MASLD patients. Stratified approaches targeting high-risk groups, such as individuals with advanced fibrosis or elevated FIB-4 scores, as well as stratification based on genetic and phenotypic characteristics, will also be essential for tailoring interventions and improving mortality outcomes (110, 113). By integrating MASLD into broader NCD prevention and management frameworks, healthcare providers may better address the high yet often overlooked burden of NCDs in patients with MASLD.
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2024-00441029, RS-2025-00523629).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Diseases GBD, Injuries C. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2024;403(10440):2133-61
2. Ferenc K, Jarmakiewicz-Czaja S, Sokal-Dembowska A, Stasik K, Filip R. Common denominator of MASLD and some non-communicable diseases. Current Issues in Molecular Biology. 2024;46(7):6690-709
3. Jia S, Ye X, Kong Y, Wang Z, Wu J. Association of High-Density Lipoprotein Cholesterol-Based Inflammatory Markers With MASLD and Significant Liver Fibrosis in US Adults: Insights From NHANES 2017-2020. Clin Transl Gastroenterol. 2025
4. Kalligeros M, Vassilopoulos A, Vassilopoulos S, Victor DW, Mylonakis E, Noureddin M. Prevalence of steatotic liver disease (MASLD, MetALD, and ALD) in the United States: NHANES 2017-2020. Clinical Gastroenterology and Hepatology. 2024;22(6):1330-2 e4
5. Chen YT, Chen TI, Yang TH, Yin SC, Lu SN, Liu XR. et al. Long-term Risks of Cirrhosis and Hepatocellular Carcinoma Across Steatotic Liver Disease Subtypes. Am J Gastroenterol. 2024;119(11):2241-50
6. Luo XY, Ying SQ, Cao Y, Jin Y, Jin F, Zheng CX. et al. Liver-based inter-organ communication: A disease perspective. Life Sci. 2024;351:122824
7. Ezhilarasan D. Mechanism of Semaglutide in MASLD Treatment: Where Is the Master Key? J Gastroenterol Hepatol. 2025
8. Fang H, Judd RL. Adiponectin Regulation and Function. Compr Physiol. 2018;8(3):1031-63
9. Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes research and clinical practice. 2005;69(1):29-35
10. Grubek-Jaworska H, Paplińska M, Hermanowicz-Salamon J, Białek-Gosk K, Dąbrowska M, Grabczak E. et al. IL-6 and IL-13 in induced sputum of COPD and asthma patients: correlation with respiratory tests. Respiration. 2012;84(2):101-7
11. Shrestha S, Jeon JH, Hong CW. Neutrophils in MASLD and MASH. BMB Rep. 2025;58(3):116-23
12. Larsson K. Inflammatory markers in COPD. Clin Respir J. 2008;2(Suppl 1):84-7
13. Finkelstein R, Fraser RS, Ghezzo H, Cosio MG. Alveolar inflammation and its relation to emphysema in smokers. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1666-72
14. Zheng H, Sechi LA, Navarese EP, Casu G, Vidili G. Metabolic dysfunction-associated steatotic liver disease and cardiovascular risk: a comprehensive review. Cardiovascular Diabetology. 2024;23(1):346
15. Kineman RD, Del Rio-Moreno M, Waxman DJ. Liver-specific actions of GH and IGF1 that protect against MASLD. Nat Rev Endocrinol. 2025;21(2):105-17
16. Zhang CH, Zhou BG, Sheng JQ, Chen Y, Cao YQ, Chen C. Molecular mechanisms of hepatic insulin resistance in nonalcoholic fatty liver disease and potential treatment strategies. Pharmacol Res. 2020;159:104984
17. Ma Y, Wang J, Xiao W, Fan X. A review of MASLD-related hepatocellular carcinoma: progress in pathogenesis, early detection, and therapeutic interventions. Frontiers in Medicine. 2024;11:1410668
18. Wang X, Zhang L, Dong B. Molecular mechanisms in MASLD/MASH related HCC. Hepatology. 2024:10.1097.
19. Meroni M, Longo M, Dongiovanni P. Cardiometabolic risk factors in MASLD patients with HCC: the other side of the coin. Front Endocrinol (Lausanne). 2024;15:1411706
20. Kalligeros M, Henry L, Younossi ZM. Metabolic dysfunction-associated steatotic liver disease and its link to cancer. Metabolism. 2024:156004.
21. Maude H, Sanchez-Cabanillas C, Cebola I. Epigenetics of hepatic insulin resistance. Frontiers in Endocrinology. 2021;12:681356
22. Caputo V, Tarantino G, Santini SJ, Fracassi G, Balsano C. The Role of Epigenetic Control of Mitochondrial (Dys)Function in MASLD Onset and Progression. Nutrients. 2023 15(22)
23. Desalegn H, Farias R, Hudson D, Idalsoaga F, Cabrera D, Diaz LA. et al. Prevention and control of risk factors in metabolic and alcohol-associated steatotic liver disease. Metabolism and Target Organ Damage. 2024;4(3):25
24. Danpanichkul P, Suparan K, Dutta P, Kaeosri C, Sukphutanan B, Pang Y. et al. Disparities in metabolic dysfunction-associated steatotic liver disease and cardiometabolic conditions in low and lower middle-income countries: a systematic analysis from the global burden of disease study 2019. Metabolism. 2024;158:155958
25. Stefan N, Yki-Järvinen H, Neuschwander-Tetri BA. Metabolic dysfunction-associated steatotic liver disease: heterogeneous pathomechanisms and effectiveness of metabolism-based treatment. The Lancet Diabetes & Endocrinology. 2025;13(2):134-48
26. Moolla Y, Ramsuran V. An Insight into the Genetic Predisposition of Metabolic Dysfunction-Associated Steatotic Liver Disease in Africa. Liver International Communications. 2024;5(4):e70006
27. Ha S, Wong VW-S, Zhang X, Yu J. Interplay between gut microbiome, host genetic and epigenetic modifications in MASLD and MASLD-related hepatocellular carcinoma. Gut. 2025;74(1):141-52
28. Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA. et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70(4):1457-69
29. Yan T, Zhang X, Wong T, Cheung R, Nguyen MH. Sex Differences in Adverse Liver and Nonliver Outcomes in Steatotic Liver Disease. JAMA Network Open. 2024;7(12):e2448946-e
30. Assaggaf H, Felty Q. Gender, estrogen, and obliterative lesions in the lung. International Journal of Endocrinology. 2017;2017(1):8475701
31. Ciarambino T, Crispino P, Guarisco G, Giordano M. Gender differences in insulin resistance: new knowledge and perspectives. Current Issues in Molecular Biology. 2023;45(10):7845-61
32. Fujii H, Kawada N, Nafld JSGo. The role of insulin resistance and diabetes in nonalcoholic fatty liver disease. International journal of molecular sciences. 2020;21(11):3863
33. Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59(2):713-23
34. Nieto-Vazquez I, Fernández-Veledo S, Krämer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M. Insulin resistance associated to obesity: the link TNF-alpha. Archives of physiology and biochemistry. 2008;114(3):183-94
35. Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(9):2104-12
36. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840-6
37. Andreozzi F, Laratta E, Sciacqua A, Perticone F, Sesti G. Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circulation research. 2004;94(9):1211-8
38. Gora IM, Ciechanowska A, Ladyzynski P. NLRP3 inflammasome at the interface of inflammation, endothelial dysfunction, and type 2 diabetes. Cells. 2021;10(2):314
39. Mignini I, Galasso L, Piccirilli G, Calvez V, Termite F, Esposto G. et al. Interplay of Oxidative Stress, Gut Microbiota, and Nicotine in Metabolic-Associated Steatotic Liver Disease (MASLD). Antioxidants. 2024;13(12):1532
40. Pinzón-Cortés JA, Perna-Chaux A, Rojas-Villamizar NS, Díaz-Basabe A, Polanía-Villanueva DC, Jácome MF. et al. Effect of diabetes status and hyperglycemia on global DNA methylation and hydroxymethylation. Endocrine connections. 2017;6(8):708-25
41. Riley DR, Hydes T, Hernadez G, Zhao SS, Alam U, Cuthbertson DJ. The synergistic impact of type 2 diabetes and MASLD on cardiovascular, liver, diabetes-related and cancer outcomes. Liver International. 2024;44(10):2538-50
42. Sabbatinelli J, Giuliani A, Kwiatkowska KM, Matacchione G, Belloni A, Ramini D. et al. DNA Methylation-derived biological age and long-term mortality risk in subjects with type 2 diabetes. Cardiovascular diabetology. 2024;23(1):250
43. Ascaso P, Palanca A, Martinez-Hervás S, Sanz MJ, Ascaso JF, Piqueras L. et al. Peripheral blood levels of CXCL10 are a useful marker for diabetic polyneuropathy in subjects with type 2 diabetes. International Journal of Clinical Practice. 2021;75(8):e14302
44. Kim HJ, Oh YH, Park SJ, Song J, Kim K, Choi D. et al. Combined Effects of Air Pollution and Changes in Physical Activity With Cardiovascular Disease in Patients With Dyslipidemia. J Am Heart Assoc. 2024;13(23):e035933
45. Kang JH, Park SJ, Jeong S, Park YJ, Kim HJ, Song J. et al. Association between antibiotic use and cardiovascular diseases in metabolic dysfunction-associated steatotic liver disease: A nationally representative retrospective cohort study. Hepatol Res. 2024
46. Kim JS, Song J, Choi S, Kim SM, Park YJ, Park SJ. et al. Association between body composition and subsequent cardiovascular diseases among 5-year breast cancer survivors. Nutr Metab Cardiovasc Dis. 2024;34(7):1787-97
47. Lee SK, Lim Y, Jeong S, Han HW. COVID-19-related cardiovascular disease risk due to weight gain: a nationwide cohort study. Eur J Med Res. 2024;29(1):2
48. Jeong S, Lee G, Choi S, Kim KH, Chang J, Kim K. et al. Association of physical activity with stroke among long-term colorectal cancer survivors. J Cancer Surviv. 2022;16(2):366-73
49. Park S, Jeong S, Park SJ, Song J, Kim SM, Chang J. et al. Associations of cholecystectomy with metabolic health changes and incident cardiovascular disease: a retrospective cohort study. Sci Rep. 2024;14(1):3195
50. Lee SJ, Yoon SS, Lee MH, Kim HJ, Lim Y, Park H. et al. Health-Screening-Based Chronic Obstructive Pulmonary Disease and Its Effect on Cardiovascular Disease Risk. J Clin Med. 2022 11(11)
51. Govindarajan G, Whaley-Connell A, Mugo M, Stump C, Sowers JR. The cardiometabolic syndrome as a cardiovascular risk factor. The American journal of the medical sciences. 2005;330(6):311-8
52. Lim Y, Jeong S, Hong M, Han HW. Non-Alcoholic Fatty Liver Disease, Atherosclerosis, and Cardiovascular Disease in Asia. Rev Cardiovasc Med. 2023;24(6):173
53. Oh YH, Jeong S, Park SJ, Ahn JC, Park SM. Reversal of nonalcoholic fatty liver disease reduces the risk of cardiovascular disease among Korean. Medicine (Baltimore). 2023;102(44):e35804
54. Yin S-C, Chen Y-T, Chang W-T, Chen T-I, Yang T-H, Liu X-R. et al. Attributable burden of steatotic liver disease on cardiovascular outcomes in Asia. JHEP Reports. 2025;7(9):101479
55. Haas ME, Attie AD, Biddinger SB. The regulation of ApoB metabolism by insulin. Trends in Endocrinology & Metabolism. 2013;24(8):391-7
56. Underwood PC, Adler GK. The renin angiotensin aldosterone system and insulin resistance in humans. Curr Hypertens Rep. 2013;15(1):59-70
57. Yang X, Qi Y, Hao J, Wei H, Li Z, Xu M. et al. Effects of oral antidiabetic agents on the renin-angiotensin-aldosterone system. Eur J Clin Pharmacol. 2025;81(6):801-13
58. Mkhize BC, Mosili P, Ngubane PS, Sibiya NH, Khathi A. Diet-induced prediabetes: Effects on the activity of the renin-angiotensin-aldosterone system in selected organs. J Diabetes Investig. 2022;13(5):768-80
59. Said S, Hernandez GT. The link between chronic kidney disease and cardiovascular disease. Journal of nephropathology. 2014;3(3):99
60. Van Dijck E, Van Laere S, Logie E, Timmermans S, Fransen E, Ibrahim J. et al. Gradual DNA methylation changes reveal transcription factors implicated in metabolic dysfunction-associated steatotic liver disease progression and epigenetic age acceleration. Clin Epigenetics. 2025;17(1):138
61. Zahoor I, Mir GJ, Lone NA, Ashraf NU. Crosstalk between Epigenetics and Autophagy in Metabolic Dysfunction-Associated Steatotic Liver Disease. J Obes Metab Syndr. 2025;34(3):253-67
62. Repine JE, Bast A, Lankhorst I, Group OSS. Oxidative stress in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 1997;156(2):341-57
63. Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respiratory research. 2006;7:1-10
64. Wang Y, Xu J, Meng Y, Adcock IM, Yao X. Role of inflammatory cells in airway remodeling in COPD. International journal of chronic obstructive pulmonary disease. 2018:3341-8
65. Rincon M, Irvin CG. Role of IL-6 in asthma and other inflammatory pulmonary diseases. International journal of biological sciences. 2012;8(9):1281
66. Kim V, Goel N, Gangar J, Zhao H, Ciccolella DE, Silverman EK. et al. Risk factors for venous thromboembolism in chronic obstructive pulmonary disease. Chronic Obstructive Pulmonary Diseases: Journal of the COPD Foundation. 2014;1(2):239
67. Holt P, Macaubas C, Stumbles P, Sly P. The role of allergy in the development of asthma. Nature. 1999;402(Suppl 6760):12-7
68. Kohli I, Sohal A, Patel J, Roytman M. Analysis of the Association between Eosinophilic Esophagitis and MASLD: Retrospective, Observational, Cohort Analysis of the National Inpatient Sample 2016-2020. Journal of Gastrointestinal & Liver Diseases. 2024 33(3)
69. Legaki E, Arsenis C, Taka S, Papadopoulos NG. DNA methylation biomarkers in asthma and rhinitis: are we there yet? Clinical and translational allergy. 2022;12(3):e12131
70. Mantovani A, Lonardo A, Stefan N, Targher G. Metabolic dysfunction-associated steatotic liver disease and extrahepatic gastrointestinal cancers. Metabolism. 2024:156014.
71. Zhou BG, Jiang X, She Q, Ding YB. Association of MASLD with the risk of extrahepatic cancers: A systematic review and meta-analysis of 18 cohort studies. Eur J Clin Invest. 2024;54(11):e14276
72. Phoolchund AG, Khakoo SI. MASLD and the Development of HCC: Pathogenesis and Therapeutic Challenges. Cancers. 2024;16(2):259
73. Kostallari E, Schwabe RF, Guillot A. Inflammation and immunity in liver homeostasis and disease: a nexus of hepatocytes, nonparenchymal cells and immune cells. Cell Mol Immunol. 2025
74. Giovannucci E. Insulin and colon cancer. Cancer Causes & Control. 1995;6(2):164-79
75. Chen TI, Chen MH, Yin SC, Lin CJ, Lam TK, Huang CW. et al. Associations between metabolic syndrome and cholangiocarcinoma risk: A large-scale population-based cohort study. Hepatology. 2025
76. Sarkar S, Horn G, Moulton K, Oza A, Byler S, Kokolus S. et al. Cancer development, progression, and therapy: an epigenetic overview. International journal of molecular sciences. 2013;14(10):21087-113
77. Strauss L, Guarneri V, Gennari A, Sica A. Implications of metabolism-driven myeloid dysfunctions in cancer therapy. Cellular & molecular immunology. 2021;18(4):829-41
78. Doyle SL, Donohoe CL, Lysaght J, Reynolds JV. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proceedings of the Nutrition Society. 2012;71(1):181-9
79. Khalil CA, Roussel R, Mohammedi K, Danchin N, Marre M. Cause-specific mortality in diabetes: recent changes in trend mortality. European journal of preventive cardiology. 2012;19(3):374-81
80. Wongtrakul W, Charatcharoenwitthaya N, Charatcharoenwitthaya P. Metabolic dysfunction-associated steatotic liver disease and the risk of mortality in individuals with type 2 diabetes: a systematic review and meta-analysis. European Journal of Gastroenterology & Hepatology. 2024;36(4):351-8
81. Kubota N, Kubota T, Kadowaki T. Physiological and pathophysiological actions of insulin in the liver. Endocrine Journal. 2025;72(2):149-59
82. Chen P, Li Y, Dai Y, Wang Z, Zhou Y, Wang Y. et al. Advances in the Pathogenesis of Metabolic Liver Disease-Related Hepatocellular Carcinoma. Journal of Hepatocellular Carcinoma. 2024:581-94
83. Yang Y, Zeng C, Lu X, Song Y, Nie J, Ran R. et al. 5-Hydroxymethylcytosines in circulating cell-free DNA reveal vascular complications of type 2 diabetes. Clinical chemistry. 2019;65(11):1414-25
84. Yan W, Cui X, Guo T, Liu N, Wang Z, Sun Y. et al. ALOX15 Aggravates Metabolic Dysfunction-Associated Steatotic Liver Disease in Mice with Type 2 Diabetes via Activating the PPARγ/CD36 Axis. Antioxidants & Redox Signaling. 2025
85. Pasarín M, Abraldes JG, Rodríguez-Vilarrupla A, La Mura V, García-Pagán JC, Bosch J. Insulin resistance and liver microcirculation in a rat model of early NAFLD. Journal of hepatology. 2011;55(5):1095-102
86. Little BB, Pena Reyes ME, Malina RM. Natural selection and type 2 diabetes-associated mortality in an isolated indigenous community in the valley of Oaxaca, southern Mexico. American Journal of Physical Anthropology. 2017;162(3):561-72
87. Yanai H, Adachi H, Hakoshima M, Iida S, Katsuyama H. Metabolic-Dysfunction-Associated Steatotic Liver Disease—Its Pathophysiology, Association with Atherosclerosis and Cardiovascular Disease, and Treatments. International journal of molecular sciences. 2023;24(20):15473
88. Chew NW, Pan XH, Chong B, Chandramouli C, Muthiah M, Lam CS. Type 2 diabetes mellitus and cardiometabolic outcomes in metabolic dysfunction-associated steatotic liver disease population. Diabetes Research and Clinical Practice. 2024;211:111652
89. Minetti ET, Hamburg NM, Matsui R. Drivers of cardiovascular disease in metabolic dysfunction-associated steatotic liver disease: the threats of oxidative stress. Frontiers in Cardiovascular Medicine. 2024;11:1469492
90. Driessen S, Francque SM, Anker SD, Cabezas MC, Grobbee DE, Tushuizen ME. et al. Metabolic dysfunction associated steatotic liver disease and the heart. Hepatology. 2023:10.1097.
91. Xie W, Gan J, Zhou X, Tian H, Pan X, Liu W. et al. Myocardial infarction accelerates the progression of MASH by triggering immunoinflammatory response and induction of periosti. Cell metabolism. 2024;36(6):1269-86 e9
92. Zheng D, Liu X, Zeng W, Zhou W, Zhou C. Association of hepatic steatosis and liver fibrosis with chronic obstructive pulmonary disease among adults. Scientific Reports. 2024;14(1):10822
93. Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H. et al. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. Journal of Biological Chemistry. 2009;284(22):14809-18
94. Ahamed F, Eppler N, Jones E, Zhang Y. Understanding Macrophage Complexity in Metabolic Dysfunction-Associated Steatotic Liver Disease: Transitioning from the M1/M2 Paradigm to Spatial Dynamics. Livers. 2024;4(3):455-78
95. Halpin DM. Mortality of patients with COPD. Expert Review of Respiratory Medicine. 2024;18(6):381-95
96. Brannan JD, Lougheed MD. Airway hyperresponsiveness in asthma: mechanisms, clinical significance, and treatment. Frontiers in physiology. 2012;3:460
97. Miller RL, Rivera J, Lichtiger L, Govindarajulu US, Jung KH, Lovinsky-Desir S. et al. Associations between mitochondrial biomarkers, urban residential exposures and childhood asthma outcomes over 6 months. Environmental research. 2023;239:117342
98. D'Amato G, Vitale C, Molino A, Stanziola A, Sanduzzi A, Vatrella A. et al. Asthma-related deaths. Multidisciplinary respiratory medicine. 2016;11:1-5
99. Qi M, Yang X, Zhang F, Lin T, Sun X, Li Y. et al. ERG rearrangement is associated with prostate cancer-related death in Chinese prostate cancer patients. PloS one. 2014;9(2):e84959
100. Jeong B-K, Choi W-I, Choi W, Moon J, Lee WH, Choi C. et al. A male mouse model for metabolic dysfunction-associated steatotic liver disease and hepatocellular carcinoma. Nature communications. 2024;15(1):6506
101. Liu YJ, Kimura M, Li X, Sulc J, Wang Q, Rodríguez-López S. et al. ACMSD inhibition corrects fibrosis, inflammation, and DNA damage in MASLD/MASH. Journal of hepatology. 2025;82(2):174-88
102. Kim SJ, Hyun J. Altered lipid metabolism as a predisposing factor for liver metastasis in MASLD. Molecules and Cells. 2024;47(2):100010
103. Gupta A, Das A, Majumder K, Arora N, Mayo HG, Singh PP. et al. Obesity is independently associated with increased risk of hepatocellular cancer-related mortality: a systematic review and meta-analysis. American journal of clinical oncology. 2018;41(9):874-81
104. Vitellius C, Desjonqueres E, Lequoy M, Amaddeo G, Fouchard I, N'Kontchou G. et al. MASLD-related HCC: Multicenter study comparing patients with and without cirrhosis. JHEP Reports. 2024;6(10):101160
105. Habib S. Team players in the pathogenesis of metabolic dysfunctions-associated steatotic liver disease: The basis of development of pharmacotherapy. World Journal of Gastrointestinal Pathophysiology. 2024;15(4):93606
106. Hines RB, Markossian TW. Differences in late-stage diagnosis, treatment, and colorectal cancer-related death between rural and urban African Americans and whites in Georgia. The Journal of Rural Health. 2012;28(3):296-305
107. Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A. et al. Cancer disparities by race/ethnicity and socioeconomic status. CA: a cancer journal for clinicians. 2004;54(2):78-93
108. Zhao J-F, Zhou B-G, Lv Y, Teng Q-P, Wang X-M, Li X-Y. et al. Association between metabolic dysfunction-associated steatotic liver disease and risk of colorectal cancer or colorectal adenoma: an updated meta-analysis of cohort studies. Frontiers in Oncology. 2024;14:1368965
109. Michalopoulou E, Thymis J, Lampsas S, Pavlidis G, Katogiannis K, Vlachomitros D. et al. The Triad of Risk: Linking MASLD, Cardiovascular Disease and Type 2 Diabetes; From Pathophysiology to Treatment. J Clin Med. 2025 14(2)
110. Targher G, Byrne CD, Tilg H. MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut. 2024;73(4):691-702
111. Bagnato CB, Bianco A, Bonfiglio C, Franco I, Verrelli N, Carella N. et al. Healthy Lifestyle Changes Improve Cortisol Levels and Liver Steatosis in MASLD Patients: Results from a Randomized Clinical Trial. Nutrients. 2024;16(23):4225
112. Beygi M, Ahi S, Zolghadri S, Stanek A. Management of metabolic-associated fatty liver disease/metabolic dysfunction-associated steatotic liver disease: from medication therapy to nutritional interventions. Nutrients. 2024;16(14):2220
113. Dawod S, Brown K. Non-invasive testing in metabolic dysfunction-associated steatotic liver disease. Frontiers in Medicine. 2024;11:1499013
Author contact
![]() Corresponding author: Seogsong Jeong, MD, PhD, Department of Biomedical Informatics, Korea University College of Medicine. 73 Goryeodae-ro, Seongbuk-gu, Seoul, Republic of Korea, 02841. Email: seogsongjeongac.kr. Telephone: +82-2-3407-2097. ORCID: 0000-0003-4646-8998.
Corresponding author: Seogsong Jeong, MD, PhD, Department of Biomedical Informatics, Korea University College of Medicine. 73 Goryeodae-ro, Seongbuk-gu, Seoul, Republic of Korea, 02841. Email: seogsongjeongac.kr. Telephone: +82-2-3407-2097. ORCID: 0000-0003-4646-8998.

 Global reach, higher impact
Global reach, higher impact