10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2025; 21(13):5842-5858. doi:10.7150/ijbs.120980 This issue Cite
Review
Metabolic Syndrome-Associated Erectile Dysfunction: Multiple Vascular Endothelial Dysfunction Mechanisms and Potential Therapeutic Targets
1. Department of Andrology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China.
2. Guo Jun Qihuang Scholar Inheritance Studio, Ningxia Hui Autonomous Region Hospital of Traditional Chinese Medicine, Ningxia Hui Autonomous Region Academy of Traditional Chinese Medicine, Ningxia Hui Autonomous Region, China.
3. Department of Urology, Princess Alexandra Hospital, University of Queensland, Brisbane, QLD, Australia.
4. AndroUrology Centre, Brisbane, QLD, Australia.
Received 2025-7-4; Accepted 2025-8-19; Published 2025-9-12
Abstract
Metabolic syndrome (MetS) causes vascular structural abnormalities, nerve damage, hormonal level changes and other lesions, which promote the occurrence and development of erectile dysfunction (ED). Penile vascular endothelial dysfunction is an important pathological feature of MetS-associated ED, and has received increasing attention in recent years. MetS negatively affects penile cavernous vascular function through the synergistic effects of insulin resistance, dyslipidemia, hypertension and obesity. The multiple pathological process may lead to impaired endothelium-dependent vasodilation, progressive fibrosis and reduced penile vascular blood flow reserve. This review summarized several common mechanisms of penile vascular endothelial dysfunction in MetS-associated ED, deeply discussed the roles of common pathological manifestations of MetS such as glucose metabolism disorder, hypertension, dyslipidemia and obesity on penile vascular endothelium, and explored treatments targeting these mechanisms in order to provide potential therapeutic targets and strategies in patients with MetS-associated ED.
Keywords: metabolic syndrome, erectile dysfunction, endothelial dysfunction, diabetes, hypertension
1. Introduction
Erectile dysfunction (ED) refers to the inability to achieve and/or maintain a sufficient erection to have satisfactory sexual life. It is not life-threatening, but can cause sexual life disorders and seriously affect the quality of relationships between patients and their sexual partners [1]. It has been reported that the overall global prevalence was 13.1-71.2% [2], From a global study, 65% of men were not very satisfied with their erection hardness [3]. Due to the differences in the regions, races, ages and cultural backgrounds of the study populations, the prevalence rates vary. The causes of ED mainly fall into three categories: psychological, organic and mixed. Most organic ED patients have vascular ED caused by the hemodynamic disorder, which is associated with endothelial dysfunction, arterial insufficiency, and/or venous occlusive dysfunction [4]. ED is an early warning sign of cardiovascular disease [5], and it is also strongly associated with metabolic diseases [6]. A number of metabolic disorders, including dyslipidemia, glucose intolerance, insulin resistance, and obesity, play important roles in the induction of ED, which is particularly prevalent in many middle-aged and elderly patients [7].
Metabolic syndrome (MetS) is a cluster of metabolic abnormalities that starts with insulin resistance and continues with the abdominal obesity, diabetes mellitus (DM), dyslipidemia, and hypertension [8]. In United States, it affects approximately 35-39% of the population [9], and according to the data of the International Diabetes Federation, the prevalence of MetS was highest in the Eastern Mediterranean Region (36.6%) and lowest in the Africa (23.1%) [10]. MetS can increase the risk of a number of diseases and adverse events, with ED being relatively prominent in male reproductive health [11]. The Massachusetts Male Aging Study revealed an evolution of ED developing concurrently with the development of the Mets [12]. Although many clinical trials suggested that patients with MetS have more propensity for ED, it is difficult for these cross-sectional studies or meta-analysis to elucidate how MetS induce ED. Recently, an Indian population study showed that ED prevalence rose with more MetS components, and flow-mediated dilation was significantly lower in ED patients (5.1 ± 1.1%) vs. non-ED (10.9 ± 3.3%) [13]. Another study compared 100 men with MetS to an age- and body mass index-matched control group, results showed that the MetS group had an increased prevalence of ED (26.7% vs 13%), and a sixfold reduction in endothelial function score [14]. Endothelial dysfunction may be the key mechanism in the pathogenesis of MetS-associated ED, and focusing on vascular endothelial function in the corpus cavernosum (CC) of MetS-associated ED holds significant promise for the discovery of drug targets and addressing the therapeutic challenges of organic ED. Previous outdated literature reviews did not focus on penile vascular endothelial function [15,16], or only focused one manifestation of MetS [17]. Therefore, it is still necessary to conduct a comprehensive and in-depth review to discuss the penis vascular endothelial damage related to MetS, and provide reasonable suggestions for the discovery and development of drug targets through summarizing the relevant mechanisms [18-20].
2. Metabolic Syndrome-Induced Erectile Dysfunction: Penile Vascular Damage
Studies have highlighted the increasing importance of MetS, which is characterized by central obesity, impaired glucose metabolism, hypertriglyceridemia, low high-density lipoprotein cholesterol (HDL-C), and hypertension, in the pathogenesis and exacerbation of male reproductive issues [21,22], particularly in sexual dysfunction [23]. MetS and male sexual dysfunction can occur independently but are often interconnected. Demir et al investigated the relationship between MetS and ED, and they found 74% of the 89 MetS patients had ED [24]. Also, the severity of ED was directly correlated with MetS [25]. Gamidov et al divided ED patients into Mets group and no Mets group, and results showed that ED appeared earlier in the Mets group (43.46 ± 9.87 vs. 50.38 ± 13.35 years, p < 0.05) and lasted longer (6.36 ± 4.13 vs. 3.55 ± 3.27 years, p < 0.05) [26]. Also, half of the patients in the MetS group had severe ED, while severe ED was two times less common in the no MetS group [26]. In fact, the mechanisms involved in MetS-associated ED are multifaceted [27]. In MetS, ED was mainly arteriogenic, with 36.36% of cases showing subnormal androgen levels and 42.2% exhibiting neurogenic disorders [26]. Arteriogenic ED patients presented penile hemodynamics alterations, with mean peak systolic velocity significantly lower comparatively to no MetS patients [28]. Koca et al also used the penile color Doppler ultrasonography to detected the penile vascular condition in ED patients with or without Mets, and they found that there was a significant correlation between veno-occlusive dysfunction and Mets (left end-diastolic velocity 3.7 ± 3.3 cm/s vs. 2.7 ± 3.1 cm/s, p = 0.014; right end-diastolic velocity 3.8 ± 3.4 cm/s vs. 2.8 ± 3.3 cm/s, p = 0.016) [29].
3. Metabolic Syndrome-Induced Penile Vascular Damage: Endothelial Dysfunction
Erection is triggered by the stimulation of efferent autonomic nerves through either local sensory input or central psychogenic signals. This leads to the expansion of cavernosal and helicine arteries, enhancing blood flow into the lacunar spaces, alongside nitric oxide (NO)-induced relaxation of trabecular smooth muscle. The vascular endothelial function of the penis plays a crucial role in facilitating this process, and the vascular endothelium, a single-cell layer lining blood vessels, also responds to both biochemical and mechanical signals by generating various mediators [30]. Endothelial cells produce various factors that regulate cellular adhesion, smooth muscle reactivity, proliferation, inflammation, and atherogenesis. Endothelial cells in a quiescent state promote anticoagulation, resist cellular adhesion, and facilitate vasodilation, whereas activated endothelial cells adopt procoagulant, adhesive, and vasoconstrictive functions. Degradation of endothelial integrity results in functional impairment, including disrupted NO secretion, reduced antiatherogenic effects, etc, and it also plays a role in organic ED.
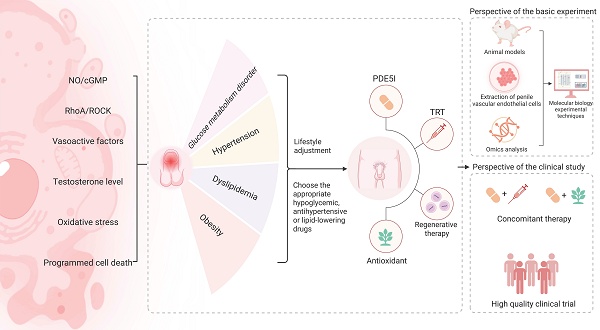
MetS exerts its detrimental effects on CC vascular function through the synergistic interplay of insulin resistance, dyslipidemia, hypertension, and obesity [31]. This multifaceted pathogenesis can result in impaired endothelium-dependent vasodilation, progressive fibrosis, and diminished blood flow reserve within the penile vasculature. While the specific mechanisms underlying endothelial dysfunction in CC may vary among the different pathological characteristics of MetS, but they converge on several common pathological pathways such as disturbances in both of the NO/Cyclic Guanosine Monophosphate (cGMP) and Ras homolog family member A (RhoA)/ Rho-associated coiled-coil containing protein kinase (ROCK) signaling pathways, imbalance of endothelium-derived vasoactive factors, alterations in sex hormone regulation, oxidative stress, and programmed cell death (Figure 1).
3.1 Nitric oxide/cyclic guanosine monophosphate pathway
When sexual stimulation activates the CC, the non-adrenergic non-cholinergic nerve endings and L-arginine within the endothelial cells can promote the release of NO from nitric oxide synthase (NOS) (such as endothelial NOS and neuronal NOS) [32]. NO diffuses to the CC smooth muscle cells, activating soluble guanylate cyclase, which catalyzes the generation of guanosine triphosphate to produce the second messenger, cGMP. cGMP reduces intracellular calcium concentration via protein kinase G, leading to smooth muscle relaxation while compressing the veins to block reflux and maintain erection. We have exhaustively elucidated the mechanisms of NO/cGMP-mediated ED and the multiple pathways that cause penile vascular endothelial dysfunction by affecting NO/cGMP [33]. NO is the main vasorelaxant released from the endothelial lining of the sinusoids and important associated vessels such as the cavernosal and helicine arteries. The perivascular smooth muscle relaxation dependence of the NO produced by endothelial NOS is regulated by insulin. Insulin-resistant condition contributes to factor the decreased availability of NO in the endothelium, and it may be caused by reductions in the enzyme endothelial NOS, a lack of substrate or cofactors for endothelial NOS, and alterations in intracellular signaling [34]. NOS uncoupling not only results in reduced synthesis of NO, but enhances the capability of the enzyme to produce reactive oxygen species (ROS), and finally to be an important cause of MetS-associated ED and some other cardiovascular diseases [35].
Multiple vascular endothelial dysfunction mechanisms in MetS-associated ED. Abbreviations: MetS, metabolic syndrome; ED, erectile dysfunction; NO, nitric oxide; cGMP, cyclic guanosine monophosphate; eNOS, endothelial nitric oxide synthase; sGC, soluble guanylate cyclase; VEGF, vascular endothelial growth factor; ET-1, endothelin-1; VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intercellular cell adhesion molecule-1; RhoA, ras homolog family member A; ROCK, rho-associated coiled-coil containing protein kinase; GTP, guanosine triphosphatase; ROS, reactive oxygen species. Created in BioRender. Wang, H. (2025) https://BioRender.com/mcwyvzs.
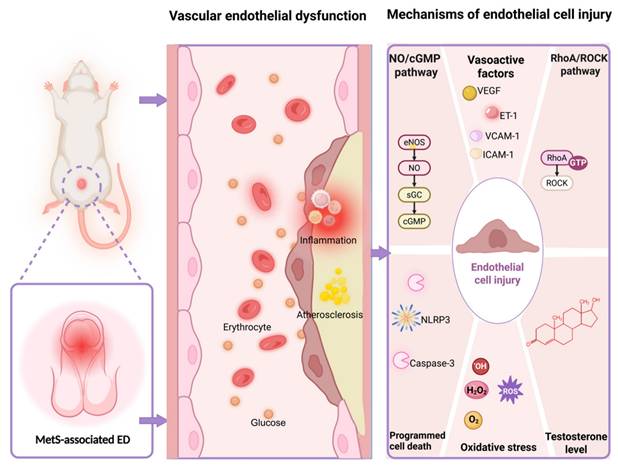
3.2 Ras homolog family member A/Rho-associated coiled-coil containing protein kinase pathway
RhoA/ROCK pathway plays a significant role in MetS-associated ED. In addition to reducing NO release and impairing vasodilation, RhoA/ROCK pathway regulates the vascular endothelium of the CC by modulating endothelial cell contraction and disrupting endothelial barrier function. Activated RhoA enhances ROCK activity, promoting phosphorylation of myosin light chain and increasing vascular tension [36]. ROCK regulates actin cytoskeleton reorganization by phosphorylating LIM kinase and cofilin, leading to increased endothelial cell gaps, elevated vascular permeability, and infiltration of inflammatory factors, thereby exacerbating endothelial damage [36]. ROCK also suppresses the expression of tight junction proteins such as zona occludens-1 and occludin, impairing endothelial barrier function [37]. Metabolic abnormalities in MetS (such as dyslipidemia and insulin resistance) activate RhoA/ROCK and then directly affect the endothelial cell. Also, in the state of metabolic abnormalities, activated RhoA/ROCK pathway may also damage the endothelial function in CC through other pathways such as promoting the release of inflammatory factors and increasing the inflammatory response in vascular endothelial cells [38], as well as persistently impairing endothelial-dependent vasodilation to reduce endothelial NOS activation by inhibiting the insulin receptor substrate-1/phosphoinositide 3-kinase/protein kinase B pathway [39]. Meanwhile, ROCK can enhance the transduction of transforming growth factor-β1/suppressor of mothers against decapentaplegic homolog signaling [40], thereby inducing endothelial-to-mesenchymal transition, increasing fibrosis markers, and structural remodeling of the microvasculature in the CC [41].
3.3 Endothelium-derived vasoactive factors
Endothelial cells release various vasoactive factors in response to mechanical forces and neurohumoral mediators, actively regulating vascular tone, inflammatory responses, coagulation balance, and vascular remodeling under both physiological and pathological conditions. The vascular endothelium and cavernosal arteries are a source of vasorelaxing factors such as NO, prostaglandin I2, endothelium-derived hyperpolarizing factor (EDHF), the vasoconstrictor factors such as angiotensin II (Ang II) and endothelin-1 (ET-1), cell adhesion molecules such as Intercellular Cell Adhesion Molecule-1 (ICAM-1) and Vascular Cell Adhesion Molecule-1 (VCAM-1), growth factors such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), inflammatory factors, etc. These factors work together to improve CC smooth muscle function, which is essential for maintaining physiological homeostasis in the penile vascular bed in response to changes in blood flow, shear stress, and agonists [42]. Bełtowski et al found the impairment of both NO and EDHF in MetS rats, and this impairment probably results in unbalanced sympathetic nervous system stimulation and blood pressure elevation [43]. Some clinical studies have shown that excessive production of Ang II, ET-1 are associated with ED, especially those with organic ED [44,45]. In normo-weight subjects with MetS, there is a strong association of metabolic parameters with VCAM-1 and Asymmetric Dimethylarginine (ADMA) (a NOS inhibitor) [46], and the aggravation of insulin resistance is often accompanied by abnormal elevations of ICAM-1 and VCAM-1 [47], which decrease with the recovery of erectile function and effective treatment in ED patients [48]. Also, biochemical measures of endothelial cells activation have been evaluated in patients with ED, and increased ICAM-1, VCAM-1 and ET-1 concentrations may be associated with ED independently of coexisting cardiovascular risk factors and overt vascular damage [49].
3.4 Testosterone level
The relationship between endothelial function and testosterone is complex, involving both structural changes and cell signaling pathways [50]. Testosterone deficiency leads to endothelial dysfunction, and it may also be related to multiple mechanisms such as regulating endothelial progenitor cells (EPCs) and endothelium-derived vasoactive factors. EPCs serve as fundamental mediators in vascular repair, angiogenesis, and replacing damaged endothelial cells in blood vessels [51]. Foresta et al suggested that hypotestosteronemia was related to the reduction of circulating EPCs in young hypogonadotropic hypogonadal men [52], whereas the reduction of EPCs could be reversed by the testosterone replacement through a direct stimulatory effect on the bone marrow [53], and the androgen receptor/VEGF/cyclin A-mediated mechanism may be involved in this process [54]. Androgen deficiency regulates endothelial cells by affecting endothelial NOS activity, and then reducing the NO availability [55]. Testosterone replacement has potential in reducing ADMA concentrations and increasing NO production in idiopathic hypotrophic hypogonadism [56]. Another study by Babcock et al reported that men with low testosterone had higher plasma ET-1, which may be associated with worse brachial artery flow-mediated dilation [57]. And the elevation of ET-1 can also activate the RhoA/ROCK pathway in CC then may affect erectile function [58]. Testosterone circulating levels tend to a step-ward decrease with ageing as well as the presence of numerous co-morbidities such as MetS [59], and a low level of testosterone may exacerbate obesity, insulin resistance, dyslipidemia, and hypertension [60]. Testosterone replacement therapy (TRT) for MetS-associated ED patients can improve MetS conditions (e.g., insulin resistance) and endothelial function [61].
3.5 Oxidative stress
The excessive ROS production is strongly associated with endothelial dysfunction and also be recognized as a key contributor to erectile impairment. Uncoupled endothelial NOS, nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase, xanthine oxidase, and mitochondrial electron transport are the main sources of ROS. When endothelial NOS function is abnormal caused by pathological stimulating factors such as insulin resistance, impaired glucose tolerance and obesity, its catalytic process becomes "uncoupled," leading to the production of superoxide anion (O₂⁻) instead of NO. The uncoupled endothelial NOS generates O₂⁻, which reacts with NO to form ONOO⁻, reducing NO bioavailability while activating pro-inflammatory pathways and exacerbating vascular endothelial damage, marked by diminished vasodilation, elevated vascular resistance, inflammatory activation and reduction of penile blood flow, ultimately impairing erectile function [62]. ROS such as hydrogen peroxide serve as signaling molecules, stimulating the action of vasoactive agents like Ang II, ET-1, aldosterone, and prostanoids, while also influencing calcium homeostasis [63]. Furthermore, ROS can enhance the expression of pro-inflammatory chemokines and cytokines, leading to the recruitment and activation of immune and inflammatory cells. Clinical evidence showed that there was a lower plasma antioxidant enzyme activity and more biomarkers of oxidative injury in MetS patients compared with healthy subjects [64]. MetS, along with overweight, hyperglycemia, etc. is characterized by a proinflammatory state which may result in ED [65]. These abnormal inflammatory factors such as tumor necrosis factor-α, interleukin-1β and interleukin-6 may also inhibit the production of testosterone through influencing the steroidogenesis in Leydig cells [66].
3.6 Programmed cell death
Programmed cell death such as apoptosis, autophagy, pyroptosis, and ferroptosis plays a significant role in the pathogenesis of vascular ED. Excessive apoptosis reduces endothelial NOS activity as well as interacts with oxidative stress to produce ROS to damage vascular endothelial cells [67]. Autophagy maintains vascular endothelial cell homeostasis in the CC by removing damaged organelles, reducing ROS accumulation, stabilizing endothelial NOS, and regulating apoptosis. However, autophagy can also induce excessive apoptosis under pathological conditions [68]. Ferroptosis may contribute to the dysfunction of penile vascular endothelial cells through oxidative damage and iron metabolism disorders, and it is likely to occur during the initiation and progression of atherosclerosis [69]. Pyroptosis forms pores in the cell membrane via Gasdermin D, releasing pro-inflammatory factors and triggering local inflammation, which impairs endothelial function. Chronic inflammation disrupts endothelial integrity and reduces NO bioavailability, while inflammasomes like NOD-like receptor thermal protein domain associated protein 3 may activate oxidative stress, and finally aggravate vascular damage [70]. Although different types of programmed cell death exhibit distinct features, they are interconnected through mutual enhancement, conversion, and suppression. In the state of MetS, oxidized low-density lipoprotein can inhibit autophagic flux by suppressing the sirtuin 1/forkhead box protein O1 pathway to promote apoptosis and adhesion molecule expression in endothelial cells [71]. And low androgen levels can induce ferroptosis of endothelial cells in rat penile tissue [72], as well as inhibit erectile function of rats by reducing NOS through pyroptosis of endothelial cells in the CC [73].
4. Impacts of MetS-Associated Pathological Conditions on Penile Vascular Endothelium
MetS encompasses pathological conditions mainly including glucose metabolism disorder, hypertension, dyslipidemia and obesity [8,21,22]. Each pathological state may damage the vascular endothelium of the CC through multiple potential mechanisms.
4.1 Glucose metabolism disorder
There is a clear pathophysiological connection between glucose metabolism disorders (such as DM and insulin resistance) and ED. A meta-analysis found that ED affects more than half of men with the glucose metabolism disorder have a prevalence odds of approximately 3.5 times more than healthy controls [74]. ED is considered a key factor connecting sexual dysfunction and cardiovascular issues in DM patients, as endothelial cells are more vulnerable to damage compared to other blood vessel cells [75]. Previous research showed reduced expression of genes (e.g., VEGF-A, VEGF-B, VEGF-C) involved in angiogenesis and vascular endothelial function in DM-associated ED compared to healthy men [76]. Kurt et al found that DM-associated ED patients have lower flow-mediated dilation, higher ET-1 levels, and elevated ischemia-modified albumin (an early ischemic marker) compared to non-DM-associated ED subjects [77]. In addition, reduced serum testosterone levels in men with DM are an independent factor of endothelial dysfunction, contributing to vascular disease and ED [78], and evidence also showed a decrease in circulating EPC correlates with penile endothelial dysfunction and testosterone levels in DM-associated ED [79].
To further investigate how abnormal glucose metabolism induces vascular endothelial damage in the penis, Felten et al showed that endothelium-dependent vasodilation in the rat penis can be reversed by high levels of L-arginine [80]. Also, elevated binding of ligands to the potent vasoconstrictor, ET-1 and endothelin A and endothelin B receptors have been noted in rat and rabbit penile tissues [81]. In diabetic rodent penile tissue, altered expression of angiogenic mediators like VEGF and Ang-1, which disrupt intracellular signaling pathways [82,83]. It is well acknowledged, VEGF expression is reduced in diabetic CC, affecting VEGF-mediated signaling, decreasing endothelial NOS activation, and reducing endothelial cell viability [84]. Similarly, Ang-1 therapy administered intracorporeally boosted endothelial NOS activation in DM rat, enhancing endothelial content and ameliorating ED [83]. In addition to the endothelium-derived relaxing factors and endothelium-derived contracting factor, inflammatory factors also play a significant role in influencing the vascular endothelium of the penis. From the evidence of Mao et al, interleukin-6, tumor necrosis factor-α, and interleukin-1β in serum levels of DM-associated ED rats were increased, while interleukin-10 and interleukin-4 were decreased [85]. In addition, the change of inflammatory factors often followed with oxidative stress and programmed cell death [85].
Limited literature exists on oxidative stress marker changes in penile tissue from DM patients. In DM rats, these changes mirror those in other vascular areas, including high malondialdehyde levels and low glutathione concentrations [86]. Confocal microscopy imaging of DM penile tissue showed elevated superoxide production in the vascular endothelium, and ROS may compromise NO bioavailability, which contributes to endothelial dysfunction and results in long-term vascular impairment in DM penile tissues. Studies have also demonstrated that the use of ROS scavengers can significantly improve penile vascular endothelial defects in streptozotocin-induced DM rats [87]. Meanwhile, oxidative stress can trigger programmed cell death. Increased oxidative stress elevates cell apoptosis while reducing endothelial content, ultimately impairing penile hemodynamics and erectile function in animal models [88]. According to Li et al, the proportion of endothelial cell pyroptosis (24.4 ± 3.69%), endothelial cell apoptosis (22.13 ± 2.43%), total cell pyroptosis (14.75 ± 0.93%), and total apoptosis (14.82 ± 1.08%) in the CC of the DM rats were significantly greater than those in the normoglycemic rats (P < 0.01) [89]. In fact, apoptosis occurring in the endothelial cells of penile blood vessels has also been confirmed by the clinical evidence [90]. However, there is few evidence regarding autophagy, ferroptosis and pyroptosis occurring in the vascular endothelial cells of the CC in patients with DM-associated ED. Li et al observed that endothelial cell pyroptosis is the dominant form of cell death in the initial phases of DMED development [89], and suppressing NOD-like receptor thermal protein domain associated protein 3 expression can significantly reduce pyroptotic activity in diabetic penile tissue of rats, enhancing the vascular endothelial function [91]. Xin et al found that the nuclear factor erythroid 2-related factor 2-heme oxygenase-1/glutathione peroxidase 4 axis in DM-associated ED rats was inhibited, leading to ferroptosis and oxidative stress, which impaired endothelial/smooth muscle cell function and further aggravated cavernous fibrosis [92]. Another study demonstrated that the NO/cGMP pathway was inhibited in the DM-associated ED rats, accompanied by the occurrence of autophagy and apoptosis, as well as the high expression of mammalian target of rapamycin in the penis [93].
RhoA/ROCK activity is elevated in CC of DM-associated ED rats [94]. RhoA/ROCK can promote advanced glycation end products (AGEs) in high glucose environment [95]. AGEs are irreversible compounds formed when sugars react with proteins, lipids, or nucleic acids without enzyme control (glycation) [96]. Research indicated that chronic hyperglycemia stimulates AGEs production, which disrupt NO generation in endothelial cells by blocking endothelial NOS phosphorylation [97]. AGEs also contribute to the generation of ROS, and affect the expression of endothelial growth factors to damage endothelial function in penis [98].
4.2 Hypertension
Epidemiological data showed that ED affects more than 30% of individuals with hypertension, compared to a prevalence rate of about 9.6% in the overall population [99]. Jensen et al observed that the main reason for ED among hypertensive patients were penile impairment (found in 89%), probably due to atherosclerosis [100]. Hypertension may compromise penile blood flow through vascular damage affecting the pudendal arteries as well as penile blood vessels [101]. Elevated blood pressure causes morphological changes in the penile vasculature and ED, and hypertension-induced remodeling, which is characterised by fibre derangement and mild cytoplasmic inflammatory may also substantially impair arterial inflow to the penis [102]. Hypertension promotes the release of vasoactive compounds, stimulates endothelial cell proliferation, and causes damage to smooth muscle cells, Schwann cells, as well as increased vascular intimal permeability [103]. Specifically, common effects of hypertension on the penile vascular endothelium involve in disorders of NO/cGMP pathway, RhoA/ROCK pathway, endothelium-derived vasoactive factors, oxidative stress, and programmed cell death.
The role of NO and other possible mediators in endothelial dysfunction of hypertensive rats [104,105]. In spontaneously hypertensive rats, endothelial-mediated relaxation of CC strips in response to acetylcholine was significantly impaired, suggesting a defect in endothelium-dependent reactivity and a corresponding reduction in NO [106]. Clinical evidence showed that endothelial function of hypertensive patients has an inverse relationship with ADMA (a competitive inhibitor of endothelial NOS) [107]. Endothelial NOS was also significantly decreased in the hypertensive animal model [108]. Therefore, impairment of NO/cGMP pathway in hypertensive rats may explain reduced efficacy of phosphodiesterase-5 inhibitor (PDE5I) as observed in the previous experiments [109]. In addition, other relevant pathologic mechanisms may also influence hypertension-associated endothelial injury in penile vasculature by acting directly or indirectly on the NO/cGMP pathway.
ED may arise from an impaired response to vasoconstrictive stimuli like Ang II and ET-1. In fact, elevated Ang II and ET-1 levels in systemic and cavernosal blood have been observed in hypertensive men with ED [110,111]. As the strongest vasoconstrictor, ET-1 may also constrict the internal pudendal artery (the main blood vessel supplying the penis), exacerbating localized blood flow disturbances in the penis. Hypertension is closely linked to Ang II, a potent vasoconstrictor that influences its onset and chronic persistence. Ang II regulates aldosterone production in the adrenal gland [112]. It also influences cardiovascular homeostasis by activating the Ang II type 1 receptor, mainly found in cardiovascular cells [113]. Excessive activation of the Ang II type 1 receptor induces NADPH oxidase, which is a major generator of ROS in vascular and CC, and elevated ROS level may diminish NO bioavailability. Clinical evidence demonstrated that increased ROS production and decreased NO bioavailability were in hypertensive patients [114]. Elevated levels of spongy lipid peroxidation in the CC of hypertensive rats [115]. High levels of ROS may diminish the availability of NO, either via peroxynitrite production or through other mechanisms. In addition, Ang II and aldosterone can interact and jointly contribute to the progression of target organ damage by activating the RhoA/ROCK pathway [116]. Similarly, the overactivation of ET-1 has also been identified as a significant pathway for the activation of the RhoA/ROCK signaling [117]. The protein expression of ROCK1 and ROCK2 were significantly higher in hypertension-associated ED rats than that in the normal control rats, with a reduction of endothelial NOS, cGMP, and an elevation of norepinephrine-induced hyper-contractions, and acetylcholine-induced hypo-relaxations in the penile tissue [118].
Penile vascular endothelial injury in hypertension-associated ED rats is also related to the programmed cell death. Luan et al found endothelial dysfunction in hypertension-associated ED rats, with the increased expression of the NOD-like receptor thermal protein domain associated protein 3 inflammasome, caspase-1, gasdermin-D, and the pro-inflammatory cytokines interleukin-1β and interleukin-18 in the CC, supporting the involvement of pyroptosis in vascular endothelial injury related to hypertension-associated ED [119]. In addition to pyroptosis, when hypertensive state exists alone in rats, caspase 3-mediated apoptosis, beclin-1/LC3-II-mediated autophagy, may also decrease the expression of NO and endothelial NOS in the penis, and finally affect the penile vascular endothelium through multiple pathways [120].
4.3 Dyslipidemia
There is a close association between dyslipidemia and penile vascular endothelial dysfunction. In a study of 154 men with ED, 74% had a low-density lipoprotein cholesterol level greater than 120 mg/dl (3.1 mmol/l) [121]. From the results of Wei et al, every mmol/liter of increase in total cholesterol was associated with 1.32 times the risk of ED (95% confidence interval 1.04-1.68), while every mmol/liter of increase in high density lipoprotein cholesterol was associated with 0.38 times the risk (95% confidence interval 0.18-0.80) [122]. Similarly, an elevated triglyceride/high-density lipoprotein ratio emerged as an independent risk factor for ED, with vascular endothelial dysfunction serving as the probable underlying cause [123]. Dyslipidemia and ED were significantly related in older men, possibly because atherosclerotic damage takes longer to develop [124]. Due to the limited clinical data, some published animal experiments have shown that hyperlipidemia-associated ED rats may exhibit reduced endothelial cell numbers, downregulated cell-to-cell junctions, increased CC fibrosis, and a lower smooth muscle-to-collagen ratio [125]. Gholami et al also found a decrease in the content of endothelial cells in the CC of the high-cholesterol rat model [126]. Meanwhile, electron microscopy in cholesterol plus phosphate buffered saline treated rats showed denuded endothelial lining of the sinusoids covered by numerous platelets in the CC [126]. From the previous evidence, in dyslipidemia-related ED, angiogenesis is disrupted due to (1) endothelial NOS downregulation and NO depletion, (2) superoxide-mediated NO degradation, and (3) oxidized low-density lipoprotein/lysophosphatidylcholine-induced inhibition of endothelial migration—key processes driven by arterial wall atherogenesis [127]. Moreover, the pathological mechanisms of these endothelial injuries seem to be attributed to disorders of the NO/cGMP pathway, abnormal endothelium-derived vasoactive factors, oxidative stress, etc.
In apolipoprotein E knockout mice aged 30-35 weeks, researchers observed significant impairment of NO-dependent endothelial relaxation in aortic tissue [128,129]. Interestingly, although NOS enzymatic activity remained normal during initial disease progression [130], the bioavailability of NO was already reduced [129]. Another study by Xie et al showed that the high-cholesterol diet induced time-dependent alterations in endothelial-dependent and -independent vascular responses, endothelial cell density, smooth muscle-to-collagen ratio, selective phospho-endothelial NOS-Ser1177 expression, and cGMP levels in the penis of mouse model [131]. Among them, the endothelium-dependent relaxation function of mice on a high-cholesterol diet was impaired at the 8th and 12th weeks. Additionally, the ratio of phosphorylation of endothelial NOS at Ser1177/total endothelial NOS decreased to 46% at the 12th week, while the cGMP level significantly dropped at the 12th week. This further indicated that a high-cholesterol diet may damage vascular endothelium by affecting the NO/cGMP pathway, thereby reducing erectile function [131].
In rabbits fed a 0.5% high-cholesterol diet, VEGF receptor-2 decreased in the CC, while VEGF increased initially but declined later [132], another study by Ryu et al also showed that in rats on a 4% high-cholesterol diet for 3 months, VEGF and VEGF receptor-2 were downregulated in the CC [133]. VEGF is an angiogenic growth factor that participates in promoting angiogenesis and vascular permeability in both physiological and pathological processes. In vascular ED, it mainly promotes angiogenesis, improves endothelial function, and enhances penile blood perfusion [134]. Similarly, intracavernous injection of VEGF seems to be able to improve the endothelial injury in the CC caused by hyperlipidemia in the rabbit model [135].
Oxidative stress is also involved in the endothelial injury of the CC vessels related to dyslipidemia. The CC of animals subjected to a high-cholesterol diet demonstrated increased ROS [136]. NADPH oxidase activation acts as a key contributor to oxidative stress, resulting in endothelial NOS disorder and exacerbating endothelial impairment in hypercholesterolemia-associated ED [136]. In the rat model of hyperlipidemia-associated ED, oxidative stress responses occur in the endothelial cells of the CC, accompanied by an increase in the apoptotic index [137]. In addition, oxidized low-density lipoprotein plays a significant role in hyperlipidemia-associated ED, as elevated levels have been detected in the human penile tissue [138]. Within the vascular system, oxidized low-density lipoprotein enhances superoxide generation through multiple pathways, including the induction of uncoupled endothelial NOS, activation of NADPH oxidase and xanthine oxidase enzymes, and disruption of mitochondrial electron transport chain function [139]. However, the role of oxidized low-density lipoprotein in penile vessels and how it affects endothelial cells remains to be further elucidated.
4.4 Obesity
Obesity is a major global health concern and often associates with ED. Men with abdominal obesity may have an elevated ED risk, and the severity of obesity is also directly proportional to the severity of ED [140]. In a cross-sectional study based on National Health and Nutrition Examination Surveys, higher visceral adiposity index is independently related to ED risk [141], The visceral adiposity index can clearly reveal the influence of waist circumference, body mass index, triglyceride, etc., and is considered more practical in assessing the impact of obesity on ED patients [142]. However, for metabolically healthy patients who meet obesity criteria, the impact on erectile function may be smaller due to the absence of organic damage. As Moura et al have verified, metabolically unhealthy obese individuals had lower mean peak systolic velocity (28.1 cm/s vs. 36.9 cm/s) and IIEF-5 than metabolically healthy obese individuals, while there were no significant differences in IIEF-5 scores, mean peak systolic velocity, or hypogonadism prevalence found between metabolically healthy obese and non-obese patients [143]. Similarly, in the experiment by Odom et al, mice fed a high-fat diet for 12 weeks developed obesity characteristics, and interestingly, endothelium-dependent and nondependent diastolic function was unchanged in both systemic and penile vasculature, and the 12-week high-fat diet did not affect penile neurotransmitter-mediated diastolic function [144]. We speculate that these normal obese subjects may not have insulin resistance or hypogonadism or that this healthy metabolic state of obesity does not cause significant organic damage in a short period of time. In fact, metabolically healthy obese individuals are likely to become metabolically unhealthy in subsequent years with a high probability [145]. A clinical trial reported obesity without insulin resistance preserved endothelial function while insulin-resistant obese individuals had endothelial dysfunction [146]. Indeed, insulin resistance is an independent risk factor for ED in young men as clinical evidence confirmed [147]. Also, obesity disrupts the hypothalamic-pituitary-gonadal axis, causing hypogonadism and testosterone deficiency, while adipose-mediated aromatization of testosterone to estrogen exacerbates hormonal dysregulation. Corona et al showed that among 2435 ED patients, obesity-related comorbidities with low testosterone levels correlated more strongly with impaired penile blood flow than obesity alone [148]. Although there is debate about whether obesity causes ED, but evidence showed that obesity-induced metabolic abnormalities do lead to ED. And these obesity-associated ED studies also emphasized the importance of penile vascular endothelial function [146,149], which were related to NO/cGMP pathway, low testosterone levels, adipokine dysregulation, oxidative stress, etc.
In obese animals, ED is associated with decreased NO bioavailability in erectile tissue. On the one hand, the low testosterone level associated with obesity affects the function of penile vascular endothelium and reduces the activity of endothelial NOS through multiple pathways. On the other hand, insulin resistance also mediates the connection between obesity and NO deficiency [150]. The insulin induces vasodilation by increasing the expression of endothelial NOS and NO production through the activation of phosphoinositide 3-kinase/protein kinase B pathways [151]. Elevated levels of ROS can lead to a decrease in NO levels and impaired endothelial cell function. Current evidence suggest that a significant portion of ROS in the penile vascular endothelium of obese rats with ED is derived from adipokine dysregulation [149]. Adipokines, which are cytokines secreted by adipose tissue, play crucial roles in regulating metabolic functions, including energy metabolism, inflammatory responses, and vascular homeostasis. Dysregulated adipokines disrupts normal adipokine signaling, promoting oxidative stress through ROS generation and contributing to endothelial dysfunction [152]. In the state of obesity, pro-inflammatory adipokines (such as leptin, resistin, etc.) directly promote inflammatory responses, and anti-inflammatory adipokines (such as adiponectin) are inhibited. A decrease in adiponectin levels observed in obese patients is thought to contribute to insulin resistance [153]. Adiponectin also improves endothelial function, reduces endothelial cell apoptosis, and decreases the risk of atherosclerosis by reducing the expression of adhesion molecules in blood vessels, the formation of foam cells, and the proliferation of vascular smooth muscle cells [154]. This is also the reason why obese patients with low levels of adiponectin are more prone to vascular endothelial dysfunction [155]. Meanwhile, hypoxia and cell death caused by the expansion due to adipose tissue accumulation further recruit macrophages and promote the release of tumor necrosis factor-α, interleukin-6, and interleukin-1β, which induce inflammatory gene transcript in endothelial cells and suppresses endothelial NOS expression [156]. Clinical evidence showed that inflammatory cytokines in the plasma of obese individuals are related to body mass index [157]. These highly expressed pro-inflammatory factors may cause inflammatory responses and insulin resistance, and high levels of tumor necrosis factor-α are associated with the occurrence of ED [158]. In animal models, tumor necrosis factor-α perfusion of the CC showed weakened non-adrenergic non-cholinergic nerve-mediated relaxation, and the expression of endothelial NOS was inhibited [159].
5. Management and Potential Drug Targets for Penile Vascular Endothelial Dysfunction in Patients with Metabolic Syndrome
Currently, a healthy lifestyle is the preferred treatment option for organic ED according to sexual medicine experts [160]. Firstly, evidence from systematic reviews has shown that aerobic training can significantly enhance male erectile function [161], which may be related to mechanisms such as exercise reducing oxidative stress, increasing the production of lactic acid, NO, and cortisol, and raising total testosterone levels [162]. Preclinical studies in a rabbit model of MetS-associated hyperglycemia have demonstrated that endurance exercise alone can restore the normal function of the hypothalamic-pituitary-gonadal axis [163]. Secondly, ED caused by smoking is mainly related to endothelial damage, reduced NO, and oxidative stress. Therefore, quitting smoking helps restore erectile function [164]. Moreover, a healthy diet, including one rich in fruits and vegetables and low in fat, has been shown to be associated with less ED in the general population [165], and the potential of plant-based diets in MetS-associated ED was also highlighted [166]. Finally, limiting alcohol intake, controlling weight, maintaining regular sleep, and avoiding emotional stress are all crucial for MetS-associated ED.
MetS-associated ED involves the management of multiple diseases. For individuals with insulin resistance and DM, the viewpoint of Corona et al indicates that metformin, glucagon-like peptide-1 agonist, and sodium-glucose cotransporter-2 inhibitor are more effective in improving ED [167]. Metformin is recommended as the first-line treatment for type 2 DM, as it can improve endothelial dysfunction by enhancing endothelium-dependent vasodilation and reducing sympathetic overactivity, ultimately restoring erectile responses in the CC of DM animal models through the NO/cGMP pathway [168]. Vignozzi et al also showed that long-term use of metformin can restore erectile function in MetS animal models by increasing adenosine and NO signaling [169]. For individuals with a higher body mass index, factors such as lipid metabolism disorders and decreased testosterone levels have a more significant impact on erectile function. Glucagon-like peptide-1 receptor agonists improve erectile function by reducing weight and regulating lipid metabolism, and show more significant improvement in erectile function compared to metformin [170]. Other antidiabetic drugs, such as thiazolidinediones and α-glucosidase inhibitors, show promising mechanisms but lack strong clinical validation for their effects on ED [170]. The impact of antihypertensive drugs represented by β-blockers on ED has been confirmed. β-blockers may cause ED by reducing perfusion pressure, directly acting on penile and vascular smooth muscle cells, or reducing testosterone levels, which also decreases patients' treatment compliance [171]. However, evidence from meta-analyses indicated that nebivolol can significantly reduce the incidence and progression risk of ED [172]. As a third-generation β-blocker, nebivolol blocks α-adrenergic receptors and induces the release of NO, showing significant advantages in reversing ED caused by first- and second-generation β-blockers. Additionally, inhibiting Ang II has a positive impact on endothelial function in patients with hypertension and MetS [173], and patients with hypertension-associated ED can benefit from the treatment with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers [174]. In animal experiments, angiotensin receptor blockers can reduce ROS generation in penile tissues of aged rats and hypercholesterolemic mice and enhance NO production [175,176]. For patients with dyslipidemia-associated ED, statins not only lower cholesterol but also show the effect of enhancing the activity of endothelial NOS [177], and have potential in improving MetS-associated ED. Park et al reported that the target of statins in improving ED lies in antioxidation and inhibition of the RhoA/ROCK signaling pathway [178]. However, these evidences seem insufficient and further in-depth research is still needed.
MetS pathological manifestations and their main relevant mechanisms
| MetS pathological manifestations | Main mechanisms in endothelial dysfunction of ED | Ref. |
|---|---|---|
| glucose metabolism disorder | NO/cGMP pathway RhoA/ROCK pathway endothelium-derived vasoactive factors sex hormone levels oxidative stress programmed cell death | [80-82,87,90,94] |
| hypertension | NO/cGMP pathway RhoA/ROCK pathway endothelium-derived vasoactive factors oxidative stress programmed cell death | [106,110,114,116,118,119] |
| dyslipidemia | NO/cGMP pathway endothelium-derived vasoactive factors oxidative stress | [131,133,136,137] |
| obesity | NO/cGMP pathway sex hormone levels oxidative stress | [148-150] |
Abbreviation: ED, erectile dysfunction; MetS, metabolic syndrome; NO, nitric oxide; cGMP, cyclic guanosine monophosphate; RhoA, ras homolog family member A; ROCK, rho-associated coiled-coil containing protein kinase
Vascular endothelial dysfunction in MetS-associated ED involves multiple mechanisms discussed in our review (Table 1). Due to individual differences and underlying diseases, individualized therapy should be implemented while providing necessary treatment for the underlying diseases, focusing on endothelial-related therapeutic targets. It is well known that PDE5I is the first-line treatment recommended by guidelines and is effective for various organic ED, including MetS-associated ED. However, the main target of PDE5I is cGMP, which is hydrolyzed to inactive 5'-guanylic acid. Although PDE5I may have positive effects on improving NO and endothelial NOS and inhibiting ROCK [179], but more clinical evidence is still needed to support it. For MetS-associated ED patients, PDE5I, which is often well-tolerated and safe, should also take into account the high failure rate of PDE5I monotherapy for MetS-associated ED [180]. Also, all the different formulations of organic nitrates are considered to increase NO levels, which in turn increases cGMP levels, and the combined application with PDE5I often leads to hypotension and is not recommended in clinical practice. Testosterone plays a significant role in MetS-associated ED. Testosterone replacement therapy including the use of oral formulations of testosterone undecanoate, has significantly increased testosterone levels and erectile function in patients with low androgen levels, and it has long-term safety [181]. However, patients may still face the problem of hypogonadism after discontinuation of the drug. To overcome this issue, many researchers have attempted to combine daily injections of testosterone undecanoate with PDE5I, which has further improved the sustained therapeutic effect [182] and is recommended for ED patients with obesity and type 2 DM [183]. At the same time, for patients with low testosterone levels, TRT also shows potential in improving total cholesterol levels [184]. Nevertheless, the current situation of androgen abuse should also be taken seriously. For instance, TRT is not recommended in case of ED with normal testosterone level, as well as in men seeking for reproductive potential, due to inhibition of the spermatogenesis [185]. The extensive research on RhoA/ROCK pathway in endothelial dysfunction related to MetS-associated ED has made it a reasonable drug target. Sopko et al reviewed the physiological and pathological mechanisms of the RhoA/ROCK pathway in penile vascular endothelial dysfunction and summarized the possible treatment directions [186]. Although Lasker et al found that selective ROCK inhibitor can improve erectile function in rats with nerve injury and independently of the NO/cGMP pathway [187]. There is still a long way to go for the application of the ROCK inhibitor in patients troubled by MetS-associated ED, including the complexity of MetS etiology and the adverse reactions such as systemic blood pressure reduction after use of ROCK inhibitors [186].
Regenerative therapy in penile vascular endothelium may cure MetS-associated ED by enhancing vascular endothelial growth factor, regulating the NO/cGMP pathway, and controlling programmed cell death. Evidence indicated that low-intensity extracorporeal shock wave therapy can cause minor damage to vascular endothelial cells, stimulating the release of VEGF, promoting new blood vessel formation, and restoring penile blood flow [188], and has been widely applied in clinical practice [189]. The potential of stem cell therapy in improving MetS-associated endothelial dysfunction should not be overlooked. Liu et al's research demonstrated that erectile function recovery in DM rats was observed following the administration of VEGF-secreting adipose derived stem cells, which enhanced endothelial function and increased smooth muscle and pericyte levels through VEGF action [190]. Human urine-derived stem cells isolated from the urine of healthy adult males, when injected into the CC of DM-associated ED rats, could increase autophagic activity in CC endothelial cells, thereby improving vascular endothelial function [191]. Additionally, extracellular vesicles from human urine-derived stem cells can promote endothelial cell proliferation and angiogenesis, increase endothelial NOS content in the CC. RNA sequencing analysis results also indicate their potential in enhancing vascular endothelial integrity [192]. Due to the high cost, the lack of long-term risk assessment, individual differences in therapeutic effects, the absence of standardized dosing regimens, and certain ethical controversies, the stem cell therapy has not yet been included in the routine recommended treatment options for ED.
In clinical practice, the efficacy of using antioxidants alone to treat ED is limited, and they are often used as complementary and alternative therapies in ED patients. By combining with PDE5I, they can exert therapeutic advantages for vascular ED [193]. The impact of antioxidants on ED has been studied in various animal models, such as vitamin E, melatonin, and α-lipoic acid, which have been confirmed to improve penile vascular lesions related to MetS [194]. Our team has summarized the mechanism of action of medicinal plants on DM-associated ED in previous review [195]. Many medicinal plants have the advantage of multi-target regulation of DM-associated ED. They can not only regulate oxidative stress, inhibit malondialdehyde and ROS in CC, and activate the NO/cGMP pathway, but also play an important role in endothelial dysfunction caused by programmed cell death [195]. Therefore, in some East Asian countries, many researchers have mixed several medicinal plants to make traditional Chinese medicine prescriptions, which also have potential in the treatment of MetS-associated ED as well as other male reproductive diseases [196,197]. In addition, as we mentioned, plant-based diets are receiving increasing attention. By evaluating the impact of dietary antioxidants and plant extracts on penile vascular endothelial damage related to MetS, researchers can develop effective antioxidant-based treatment strategies that are easier to implement in daily life and facilitate patient management.
6. Perspectives and Future Directions
We reviewed the multiple mechanisms of penile vascular endothelial injury related to ED in MetS and briefly analyzed the current treatment progress (Figure 2). Combining the existing evidence, the treatment of CC vascular endothelial injury based on the RhoA/ROCK pathway still faces numerous challenges. Therefore, the NO/cGMP pathway remains a key focus for future research, serves as the core target for treating CC vascular endothelial injury in MetS-associated ED. Although modulating endothelium-derived vasoactive factors, antioxidation and regulating programmed cell death are beneficial for improving CC endothelial dysfunction related to MetS, limited literature evidence, low bioavailability, lack of cell specificity and low clinical efficacy as well as other factors have restricted the drug development based on these targets. The existing MetS-associated animal models induced by high-fat diets are mainly rodent models, including (diabetic ED rats, hyperlipidemic ED rats, etc.), which may only lead to obesity or insulin resistance, but lack key manifestations such as hypertension or inflammation. In addition, animals with simple metabolic abnormalities are difficult to show significant ED in a short time and may require additional interventions (such as vascular injury, drug induction), which further increases the difficulty and cost of modeling. Due to ethical limitations on extracting endothelial cells from human CC, human umbilical vein endothelial cells are commonly used because of their easy accessibility and ease of culture. However, they differ from microvascular endothelial cells in the CC in terms of gene expression, functional characteristics, and response to androgens, making it difficult to fully simulate the local pathological and physiological processes of the penis. The extraction of endothelial cells from animal models reduces ethical risks and enables controlled mechanism exploration. This also means that on the basis of optimizing the MetS animal model, the extraction technology should be continuously improved to ensure the activity and purity of penile vascular cells. In addition, by screening differentially expressed genes (such as endothelial NOS and inflammatory factors) through transcriptomics, validating key proteins (such as VEGF and ICAM-1) through proteomics, and analyzing small molecule metabolites (such as NO and oxidative stress markers) through metabolomics, a molecular network of endothelial injury can be jointly constructed [33]. This will facilitate the discovery of biomarkers and therapeutic targets for endothelial injury related to MetS-associated ED, and provide personalized treatment strategies.
Common therapies for penile vascular endothelial injury associated with MetS-associated ED and future research directions. Abbreviations: NO, nitric oxide; cGMP, cyclic guanosine monophosphate; RhoA, ras homolog family member A; ROCK, rho-associated coiled-coil containing protein kinase; PDE5I, phosphodiesterase-5 inhibitor; TRT, testosterone replacement therapy. Created in BioRender. Wang, H. (2025) https://BioRender.com/fbi4hq5.
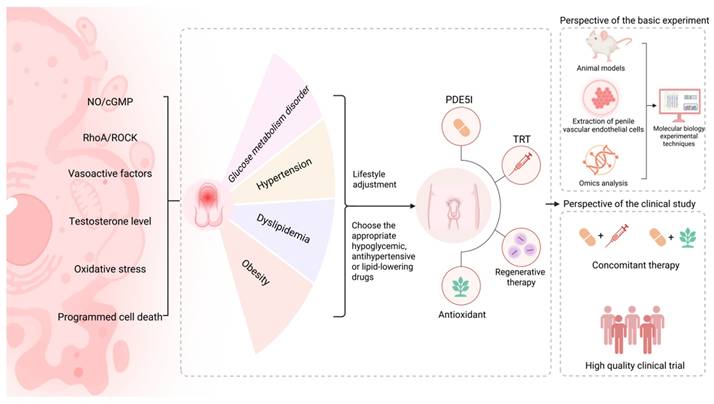
With the in-depth research on combined therapy for MetS-associated ED, simultaneous treatment of metabolic control and ED is recommended. Clinical physicians should monitor the metabolic-related indicators and the patients' own conditions in a timely manner, and guide the basic medication with necessary lifestyle guidance. This also means the participation of multiple disciplines, including the department of sexual medicine, urology, nutrition, cardiology, and endocrinology, to provide effective and comprehensive guidance for personalized management. In terms of improving the quality of sexual life, concomitant therapy (including the application of PDE5I combined with TRT or PDE5I combined with antioxidant therapy, etc.) is still necessary [193,196], which involves multi-target regulation of ED related to MetS. We expect more high-level clinical evidence to confirm the efficacy and safety of concomitant therapy for MetS-associated ED patients, and also expect the clinical application value of regenerative therapy, which may benefit those patients who are ineffective with PDE5I and unwilling to receive the penile prosthesis implantation.
7. Conclusion
MetS-associated ED is a refractory ED. Due to the multiple pathological features included in MetS, it involves various different mechanisms. Existing studies have clarified that MetS causes a decline in erectile quality and sexual life satisfaction in patients by affecting penile vascular endothelial function. NO/cGMP pathway, RhoA/ROCK pathway, endothelium-derived vasoactive factors, testosterone level, oxidative stress, programmed cell death, etc. may be involved in the penile vascular endothelial dysfunction of MetS-associated ED, and are reflected in the main pathological features of MetS such as glucose metabolism disorder, hypertension, dyslipidemia, and obesity. Currently, the drug targets for treating penile vascular endothelial injury in MetS-associated ED mainly focus on the NO/cGMP pathway. Patients can benefit from lifestyle adjustments and the rational application of hypoglycemic drugs, antihypertensive drugs, or lipid-lowering drugs. At the same time, the application of combined therapy also has potential in the management of MetS-associated ED.
Acknowledgements
Funding
This work was financially supported by the High-Level Key Discipline Construction Project of Traditional Chinese Medicine by the National Administration of Traditional Chinese Medicine (zyyzdxk-2023238).
Author contributions
All authors revised and approved the manuscript. Hao Wang prepared the figures and manuscript. Jun Guo participated in the manuscript writing. Jun Guo and Eric Chung designed the review, supervised all figures, and cowrote the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC. et al. European Association of Urology Guidelines on Sexual and Reproductive Health-2021 Update: Male Sexual Dysfunction. Eur Urol. 2021;80:333-57
2. Kessler A, Sollie S, Challacombe B, Briggs K, Van Hemelrijck M. The global prevalence of erectile dysfunction: a review. BJU Int. 2019;124:587-99
3. Mulhall J, King R, Glina S, Hvidsten K. Importance of and satisfaction with sex among men and women worldwide: results of the global better sex survey. J Sex Med. 2008;5:788-95
4. Uddin SMI, Mirbolouk M, Dardari Z, Feldman DI, Cainzos-Achirica M, DeFilippis AP. et al. Erectile Dysfunction as an Independent Predictor of Future Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. Circulation. 2018;138:540-2
5. An J, Xiang B, Peng J, Li D. Understanding the erectile dysfunction-cardiovascular disease connection: clinical and pathophysiological insights. Sex Med Rev. 2025;13:406-22
6. Jalali S, Zareshahi N, Behnoush AH, Azarboo A, Shirinezhad A, Hosseini SY. et al. Association of insulin resistance surrogate indices and erectile dysfunction: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2024;22:148
7. Pellegrino F, Sjoberg DD, Tin AL, Benfante NE, Briganti A, Montorsi F. et al. Relationship Between Age, Comorbidity, and the Prevalence of Erectile Dysfunction. Eur Urol Focus. 2023;9:162-7
8. Neeland IJ, Lim S, Tchernof A, Gastaldelli A, Rangaswami J, Ndumele CE. et al. Metabolic syndrome. Nat Rev Dis Primers. 2024;10:77
9. Golden SH, Robinson KA, Saldanha I, Anton B, Ladenson PW. Clinical review: Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94:1853-78
10. Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, Nkeck JR, Nyaga UF, Ngouo AT. et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res Clin Pract. 2022;188:109924
11. Wang W, Zhao S, Zhou R, Yu PZ, Pan SY, Huan PF. et al. Associations between metabolic syndrome and erectile dysfunction: evidence from the NHANES 2001-2004. Front Public Health. 2025;13:1543668
12. Kupelian V, Shabsigh R, Araujo AB, O'Donnell AB, McKinlay JB. Erectile dysfunction as a predictor of the metabolic syndrome in aging men: results from the Massachusetts Male Aging Study. J Urol. 2006;176:222-6
13. Karoli R, Fatima J, Verma P, Bhat S, Siddiqi Z, Beg MS. et al. Study of Association of Erectile Dysfunction with Metabolic Syndrome and Its Correlation with Endothelial Dysfunction in an Indian Population. J Assoc Physicians India. 2024;72:17-20
14. Esposito K, Giugliano F, Martedì E, Feola G, Marfella R, D'Armiento M. et al. High proportions of erectile dysfunction in men with the metabolic syndrome. Diabetes Care. 2005;28:1201-3
15. Müller A, Mulhall JP. Cardiovascular disease, metabolic syndrome and erectile dysfunction. Curr Opin Urol. 2006;16:435-43
16. Matfin G, Jawa A, Fonseca VA. Erectile dysfunction: interrelationship with the metabolic syndrome. Curr Diab Rep. 2005;5:64-9
17. Castela Â, Costa C. Molecular mechanisms associated with diabetic endothelial-erectile dysfunction. Nat Rev Urol. 2016;13:266-74
18. Sanchez E, Pastuszak AW, Khera M. Erectile dysfunction, metabolic syndrome, and cardiovascular risks: facts and controversies. Transl Androl Urol. 2017;6:28-36
19. Corona G, Mannucci E, Schulman C, Petrone L, Mansani R, Cilotti A. et al. Psychobiologic correlates of the metabolic syndrome and associated sexual dysfunction. Eur Urol. 2006;50:595-604
20. Zhu D, Pham QM, Wang C, Colonnello E, Yannas D, Nguyen BH. et al. Erectile Dysfunction and Oxidative Stress: A Narrative Review. Int J Mol Sci. 2025;26:3073
21. Ma D, Xi J. Correlation study and full management of metabolic syndrome and sperm DNA fragmentation index, a regional multicentre prospective long term follow-up study from Anhui, China. Front Cell Dev Biol. 2025;13:1586069
22. Rahali D, Dallagi Y, Hupkens E, Veegh G, Mc Entee K, Asmi ME. et al. Spermatogenesis and steroidogenesis disruption in a model of metabolic syndrome rats. Arch Physiol Biochem. 2023;129:222-32
23. Bolat MS, Bolat IA, Dündar C, Asci R. Which is better to predict erectile dysfunction and male sexual function in the context of metabolic syndrome: triglyceride-glucose index or visceral adiposity index?: a retrospective cross-sectional study. Int Urol Nephrol. 2024;56:2869-76
24. Demir T, Demir O, Kefi A, Comlekci A, Yesil S, Esen A. Prevalence of erectile dysfunction in patients with metabolic syndrome. Int J Urol. 2006;13:385-8
25. Salama MN, Eid AA, Hatem A, Swidan AK. Prevalence of erectile dysfunction in Egyptian males with metabolic syndrome. Aging Male. 2020;23:257-63
26. Gamidov SI, Mamedov MN, Sotnikova EM, Guseĭnov MM. Metabolic syndrome and erectile dysfunction. Ter Arkh. 2007;79:21-5
27. Corona DG, Vena W, Pizzocaro A, Rastrelli G, Sparano C, Sforza A. et al. Metabolic syndrome and erectile dysfunction: a systematic review and meta-analysis study. J Endocrinol Invest. 2023;46:2195-211
28. Tomada N, Tomada I, Botelho F, Cruz F, Vendeira P. Are all metabolic syndrome components responsible for penile hemodynamics impairment in patients with erectile dysfunction? The role of body fat mass assessment. J Sex Med. 2011;8:831-9
29. Koca O, Calışkan S, Oztürk M, Güneş M, Kılıçoğlu G, Karaman MI. Vasculogenic erectile dysfunction and metabolic syndrome. J Sex Med. 2010;7:3997-4002
30. Akorede BA, Hassan SA, Akhigbe RE. Penile erection and cardiovascular function: effects and pathophysiology. Aging Male. 2024;27:2336627
31. Besiroglu H, Otunctemur A, Ozbek E. The relationship between metabolic syndrome, its components, and erectile dysfunction: a systematic review and a meta-analysis of observational studies. J Sex Med. 2015;12:1309-18
32. Chung E, Rhee H. Impact of Physical Exercise Program Interventions on Erectile Function and Cardiovascular Health in Males with Prostate Cancer. World J Mens Health. 2022;40:361-7
33. Wang H, Chung E. Revisiting experimental models of erectile dysfunction and their value in drug discovery and development. Expert Opin Drug Discov. 2025;20:499-516
34. Sánchez A, Contreras C, Martínez MP, Climent B, Benedito S, García-Sacristán A. et al. Role of neural NO synthase (nNOS) uncoupling in the dysfunctional nitrergic vasorelaxation of penile arteries from insulin-resistant obese Zucker rats. PLoS One. 2012;7:e36027
35. Mollace R, Scarano F, Bava I, Carresi C, Maiuolo J, Tavernese A. et al. Modulation of the nitric oxide/cGMP pathway in cardiac contraction and relaxation: Potential role in heart failure treatment. Pharmacol Res. 2023;196:106931
36. Yi LY, Hsieh HH, Lin ZQ, Hung KF, Sun YC. Exploring the Role of ROCK Inhibition in Corneal Edema Through Crosstalk Between Epithelial and Endothelial Cells. J Ophthalmol. 2024;2024:9381303
37. Hossen F, Sun GY, Lee JC. Oligomeric Tau-induced oxidative damage and functional alterations in cerebral endothelial cells: Role of RhoA/ROCK signaling pathway. Free Radic Biol Med. 2024;221:261-72
38. Ni XQ, Zhu JH, Yao NH, Qian J, Yang XJ. Statins suppress glucose-induced plasminogen activator inhibitor-1 expression by regulating RhoA and nuclear factor-κB activities in cardiac microvascular endothelial cells. Exp Biol Med (Maywood). 2013;238:37-46
39. Lee SH, Huang H, Choi K, Lee DH, Shi J, Liu T. et al. ROCK1 isoform-specific deletion reveals a role for diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 2014;306:332-43
40. Feng ZH, Zhang XH, Zhao JQ, Ma JZ. Involvement of Rho-associated coiled-coil kinase signaling inhibition in TGF-β1/Smad2, 3 signal transduction in vitro. Int J Ophthalmol. 2017;10:1805-11
41. Zhang H, Feng CH, He S, Deng MX, Meng H, Chen M. et al. Leech-Centipede Granules Suppress EndMT to Improve Erectile Dysfunction in Rats with Diabetes Mellitus via TGF-β/Smad Pathway. Chin J Integr Med. 2023;29:28-36
42. Bivalacqua TJ, Usta MF, Champion HC, Kadowitz PJ, Hellstrom WJ. Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J Androl. 2003;24(Suppl 6):S17-S37
43. Bełtowski J, Wójcicka G, Jamroz-Wiśniewska A, Marciniak A. Resistance to acute NO-mimetic and EDHF-mimetic effects of leptin in the metabolic syndrome. Life Sci. 2009;85:557-67
44. El Melegy NT, Ali ME, Awad EM. Plasma levels of endothelin-1, angiotensin II, nitric oxide and prostaglandin E in the venous and cavernosal blood of patients with erectile dysfunction. BJU Int. 2005;96:1079-86
45. Francavilla S, Properzi G, Bellini C, Marino G, Ferri C, Santucci A. Endothelin-1 in diabetic and nondiabetic men with erectile dysfunction. J Urol. 1997;158:1770-4
46. Rakhmat II, Nugraha GI Ariyanto EF, Pratiwi YS Linasari D, Fatimah SN et al. Strong Association of Metabolic Parameters with ADMA and VCAM-1 in Normo-Weight Subjects with Metabolic Syndrome. Diabetes Metab Syndr Obes. 2024;17:833-9
47. Tan HW, Liu X, Bi XP, Xing SS, Li L, Gong HP. et al. IL-18 overexpression promotes vascular inflammation and remodeling in a rat model of metabolic syndrome. Atherosclerosis. 2010;208:350-7
48. Wang J, Wu P, Liu Q, Ben L, Chen G, Han Z. et al. Effect of a three-piece inflatable penile prosthesis combined with a phosphodiesterase-5 inhibitor on erectile dysfunction. J Int Med Res. 2021;49:300060520985365
49. Bocchio M, Desideri G, Scarpelli P, Necozione S, Properzi G, Spartera C. et al. Endothelial cell activation in men with erectile dysfunction without cardiovascular risk factors and overt vascular damage. J Urol. 2004;171:1601-4
50. Chen Z, Jiang J, Jiang R. A low testosterone level impairs erectile function by increasing endocan expression in rat penile corpus cavernosum. J Sex Med. 2024;21:663-70
51. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA. et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593-600
52. Foresta C, Caretta N, Lana A, De Toni L, Biagioli A, Ferlin A. et al. Reduced number of circulating endothelial progenitor cells in hypogonadal men. J Clin Endocrinol Metab. 2006;91:4599-602
53. Foresta C, Zuccarello D, De Toni L, Garolla A, Caretta N, Ferlin A. Androgens stimulate endothelial progenitor cells through an androgen receptor-mediated pathway. Clin Endocrinol (Oxf). 2008;68:284-9
54. Cai J, Hong Y, Weng C, Tan C, Imperato-McGinley J, Zhu YS. Androgen stimulates endothelial cell proliferation via an androgen receptor/VEGF/cyclin A-mediated mechanism. Am J Physiol Heart Circ Physiol. 2011;300:1210-21
55. Wang Y, Jiang R. Androgens and erectile dysfunction: from androgen deficiency to treatment. Sex Med Rev. 2024;12:458-68
56. Cakir E, Ozcan O, Yaman H, Akgul EO, Bilgi C, Erbil MK. et al. Elevated plasma concentration of asymmetric dimethylarginine that is reduced by single dose testosterone administration in idiopathic hypogonadotropic hypogonadism patients. J Clin Endocrinol Metab. 2005;90:1651-4
57. Babcock MC, DuBose LE, Hildreth KL, Stauffer BL, Kohrt WM, Wenner MM. et al. Endothelial dysfunction in middle-aged and older men with low testosterone is associated with elevated circulating endothelin-1. Am J Physiol Regul Integr Comp Physiol. 2025;328:253-61
58. Mills TM, Chitaley K, Wingard CJ, Lewis RW, Webb RC. Effect of Rho-kinase inhibition on vasoconstriction in the penile circulation. J Appl Physiol (1985). 2001;91:1269-73
59. Blaya R, Blaya P, Rhoden L, Rhoden EL. Low Testosterone Levels and Metabolic Syndrome in Aging Male. Curr Pharm Des. 2017;23:4470-4
60. Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217:47-71
61. Saad F, Gooren L, Haider A, Yassin A. Effects of testosterone gel followed by parenteral testosterone undecanoate on sexual dysfunction and on features of the metabolic syndrome. Andrologia. 2008;40:44-8
62. Kaltsas A, Zikopoulos A, Dimitriadis F, Sheshi D, Politis M, Moustakli E. et al. Oxidative Stress and Erectile Dysfunction: Pathophysiology, Impacts, and Potential Treatments. Curr Issues Mol Biol. 2024;46:8807-34
63. Touyz RM, Rios FJ, Alves-Lopes R, Neves KB, Camargo LL, Montezano AC. Oxidative Stress: A Unifying Paradigm in Hypertension. Can J Cardiol. 2020;36:659-70
64. Bekkouche L, Bouchenak M, Malaisse WJ, Yahia DA. The Mediterranean diet adoption improves metabolic, oxidative, and inflammatory abnormalities in Algerian metabolic syndrome patients. Horm Metab Res. 2014;46:274-82
65. Oteri V, Galeano F, Panebianco S, Piticchio T, Le Moli R, Frittitta L. et al. Influence of Mediterranean Diet on Sexual Function in People with Metabolic Syndrome: A Narrative Review. Nutrients. 2024;16:3397
66. Lee RK, Chughtai B, Te AE, Kaplan SA. Sexual function in men with metabolic syndrome. Urol Clin North Am. 2012;39:53-62
67. Zhang J, Xin S, Mao J, Liu X, Wang T, Liu J. et al. The role of programmed cell death in diabetes mellitus-induced erectile dysfunction: from mechanisms to targeted therapy. Reprod Biol Endocrinol. 2025;23:32
68. Luo PY, Zou JR, Chen T, Zou J, Li W, Chen Q. et al. Autophagy in erectile dysfunction: focusing on apoptosis and fibrosis. Asian J Androl. 2025;27:166-76
69. Jia B, Li Z, Zhao D, Fu Q. Research Progress on Ferroptosis in Organic Erectile Dysfunction. Arch Esp Urol. 2023;76:746-54
70. Zhu B, Niu Y, Guo H, Jin X, Liu F. Pyroptosis and inflammation-mediated endothelial dysfunction may act as key factors in the development of erectile dysfunction (Review). Mol Med Rep. 2023 28
71. Wu Z, Huang C, Xu C, Xie L, Liang JJ, Liu L. et al. Caveolin-1 regulates human trabecular meshwork cell adhesion, endocytosis, and autophagy. J Cell Biochem. 2019;120:13382-91
72. Shi HX, Zhao X, Yang H, Cheng Y, Jiang J, Jiang R. Low androgen levels induce ferroptosis of rat penile cavernous endothelial cells. Sex Med. 2023;11:qfad043
73. Chen ZB, Li G, Lin H, Jiang J, Jiang R. Low androgen status inhibits erectile function by increasing pyroptosis in rat corpus cavernosum. Andrology. 2021;9:1264-74
74. Kouidrat Y, Pizzol D, Cosco T, Thompson T, Carnaghi M, Bertoldo A. et al. High prevalence of erectile dysfunction in diabetes: a systematic review and meta-analysis of 145 studies. Diabet Med. 2017;34:1185-92
75. Terentes-Printzios D, Ioakeimidis N, Rokkas K, Vlachopoulos C. Interactions between erectile dysfunction, cardiovascular disease and cardiovascular drugs. Nat Rev Cardiol. 2022;19:59-74
76. Begum M, Choubey M, Tirumalasetty MB, Arbee S, Sadik S, Mohib MM. et al. Exploring the Molecular Link Between Diabetes and Erectile Dysfunction Through Single-Cell Transcriptome Analysis. Genes (Basel). 2024;15:1596
77. Kurt HA, Demirci E, Alan C. Endothelial Dysfunction and Ischemia-Modified Albumin Levels in Males with Diabetic and Nondiabetic Erectile Dysfunction. Dis Markers. 2022;2022:3661822
78. Akishita M, Hashimoto M, Ohike Y, Ogawa S, Iijima K, Eto M. et al. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res. 2007;30:1029-34
79. Maiorino MI, Bellastella G, Petrizzo M, Della Volpe E, Orlando R, Giugliano D. et al. Circulating endothelial progenitor cells in type 1 diabetic patients with erectile dysfunction. Endocrine. 2015;49:415-21
80. Felten DL, Felten SY, Melman A. Noradrenergic innervation of the penis in control and streptozotocin-diabetic rats: evidence of autonomic neuropathy. Anat Rec. 1983;206:49-59
81. Sullivan ME, Dashwood MR, Thompson CS, Muddle JR, Mikhailidis DP, Morgan RJ. Alterations in endothelin B receptor sites in cavernosal tissue of diabetic rabbits: potential relevance to the pathogenesis of erectile dysfunction. J Urol. 1997;158:1966-72
82. Jesmin S, Sakuma I, Salah-Eldin A, Nonomura K, Hattori Y, Kitabatake A. Diminished penile expression of vascular endothelial growth factor and its receptors at the insulin-resistant stage of a type II diabetic rat model: a possible cause for erectile dysfunction in diabetes. J Mol Endocrinol. 2003;31:401-18
83. Jin HR, Kim WJ, Song JS, Piao S, Tumurbaatar M, Shin SH. et al. Intracavernous delivery of synthetic angiopoietin-1 protein as a novel therapeutic strategy for erectile dysfunction in the type II diabetic db/db mouse. J Sex Med. 2010;7:3635-46
84. Lund M, Valsgaard Vammen D, Hanna M, Høyer S, Lund L. Placebo-Controlled Study of Effects of Low-Energy Shockwave Therapy (LE-ESWT) on Erectile Tissue in a Diabetic Animal Model. Res Rep Urol. 2023;15:123-9
85. Mao Y, Zha Y, Zang Y, Gao Y, Sun J, Liu Y. et al. Isorhamnetin improves diabetes-induced erectile dysfunction in rats through activation of the PI3K/AKT/eNOS signaling pathway. Biomed Pharmacother. 2024;177:116987
86. Ryu JK, Kim DJ, Lee T, Kang YS, Yoon SM, Suh JK. The role of free radical in the pathogenesis of impotence in streptozotocin-induced diabetic rats. Yonsei Med J. 2003;44:236-41
87. Yang H, Xiong W, Jiang J, Jiang R. Icariin inhibits hyperglycemia-induced cell death in penile cavernous tissue and improves erectile function in type 1 diabetic rats. Sex Med. 2025;13:qfaf017
88. Liu T, Peng YF, Jia C, Yang BH, Tao X, Li J. et al. Ginsenoside Rg3 improves erectile function in streptozotocin-induced diabetic rats. J Sex Med. 2015;12:611-20
89. Li J, Jiang Q, Jiang J, Jiang R. Mode of cell death in the penile cavernous tissue of type 1 diabetes mellitus rats. J Sex Med. 2024;21:652-62
90. Costa C, Soares R, Castela A, Adães S, Hastert V, Vendeira P. et al. Increased endothelial apoptotic cell density in human diabetic erectile tissue-comparison with clinical data. J Sex Med. 2009;6:826-35
91. Luo C, Peng Y, Zhou X, Fan J, Chen W, Zhang H. et al. NLRP3 downregulation enhances engraftment and functionality of adipose-derived stem cells to alleviate erectile dysfunction in diabetic rats. Front Endocrinol (Lausanne). 2022;13:913296
92. Xin S, Song W, Mao J, Hu P, Chen Z, Liu J. et al. Therapeutic potential of hesperidin in diabetes mellitus-induced erectile dysfunction through Nrf2-mediated ferroptosis and oxidative stress. Andrology. 2024 [Epub ahead of print]
93. Lin H, Wang T, Ruan Y, Liu K, Li H, Wang S. et al. Rapamycin Supplementation May Ameliorate Erectile Function in Rats With Streptozotocin-Induced Type 1 Diabetes by Inducing Autophagy and Inhibiting Apoptosis, Endothelial Dysfunction, and Corporal Fibrosis. J Sex Med. 2018;15:1246-59
94. Liu K, Cui K, Feng H, Li R, Lin H, Chen Y. et al. JTE-013 supplementation improves erectile dysfunction in rats with streptozotocin-induced type Ⅰ diabetes through the inhibition of the rho-kinase pathway, fibrosis, and apoptosis. Andrology. 2020;8:497-508
95. Shu A, Du Q, Chen J, Gao Y, Zhu Y, Lv G. et al. Catalpol ameliorates endothelial dysfunction and inflammation in diabetic nephropathy via suppression of RAGE/RhoA/ROCK signaling pathway. Chem Biol Interact. 2021;348:109625
96. Zhang Y, Zhang Z, Tu C, Chen X, He R. Advanced Glycation End Products in Disease Development and Potential Interventions. Antioxidants (Basel). 2025;14:492
97. Jing C, Zhang G, Liu Z, Xu Q, Li C, Cheng G. et al. Peroxidasin promotes diabetic vascular endothelial dysfunction induced by advanced glycation end products via NOX2/HOCl/Akt/eNOS pathway. Redox Biol. 2021;45:102031
98. Musicki B, Kramer MF, Becker RE, Burnett AL. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2005;102:11870-5
99. Rosen RC, Fisher WA, Eardley I, Niederberger C, Nadel A, Sand M. The multinational Men's Attitudes to Life Events and Sexuality (MALES) study: I. Prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin. 2004;20:607-17
100. Jensen J, Lendorf A, Stimpel H, Frost J, Ibsen H, Rosenkilde P. The prevalence and etiology of impotence in 101 male hypertensive outpatients. Am J Hypertens. 1999;12:271-5
101. Hale TM, Hannan JL, Carrier S, deBlois D, Adams MA. Targeting vascular structure for the treatment of sexual dysfunction. J Sex Med. 2009;6(Suppl 3):S210-S220
102. Ajeigbe OF, Oboh G, Ademosun AO, Umar HI. Fig (Ficus exasperata and Ficus asperifolia)-Supplemented diet improves sexual function, endothelial nitric oxide synthase and suppresses tumour necrosis factor-alpha genes in hypertensive rats. Andrologia. 2022;54:e14289
103. Jiang R, Chen JH, Jin J, Shen W, Li QM. Ultrastructural comparison of penile cavernous tissue between hypertensive and normotensive rats. Int J Impot Res. 2005;17:417-23
104. Olabiyi AA, Tope-Eniola OS, Oluwatuyi AO, Alabi O, Ademola OG, Oguntimehin OM. et al. Quercetin boosts nitric oxide levels and modulates the activities of arginase, acetylcholinesterase and adenosine deaminase in the corpus cavernosum of cyclosporine-treated rats. Andrologia. 2022;54:e14404
105. Gonzaga NA, do Vale GT, da Silva CBP, Pinheiro LC, Leite LN, Carneiro FS. et al. Treatment with nitrite prevents reactive oxygen species generation in the corpora cavernosa and restores intracavernosal pressure in hypertensive rats. Nitric Oxide. 2020;94:19-26
106. Behr-Roussel D, Bernabe J, Compagnie S, Rupin A, Verbeuren TJ, Alexandre L. et al. Distinct mechanisms implicated in atherosclerosis-induced erectile dysfunction in rabbits. Atherosclerosis. 2002;162:355-62
107. Ono Y, Nakaya Y, Bando S, Soeki T, Ito S, Sata M. Telmisartan decreases plasma levels of asymmetrical dimethyl-L-arginine and improves lipid and glucose metabolism and vascular function. Int Heart J. 2009;50:73-83
108. Yilmaz E, Kaya-Sezginer E, Yilmaz-Oral D, Cengiz T, Bayatli N, Gur S. Effects of hydrogen sulphide donor, sodium hydrosulphide treatment on the erectile dysfunction in L-NAME-induced hypertensive rats. Andrologia. 2019;51:e13240
109. Wang TD, Lee CK, Chia YC, Tsoi K, Buranakitjaroen P, Chen CH. et al. Hypertension and erectile dysfunction: The role of endovascular therapy in Asia. J Clin Hypertens (Greenwich). 2021;23:481-8
110. Becker AJ, Uckert S, Stief CG, Scheller F, Knapp WH, Hartmann U. et al. Plasma levels of angiotensin II during different penile conditions in the cavernous and systemic blood of healthy men and patients with erectile dysfunction. Urology. 2001;58:805-10
111. Elkamshoushi AM, Badae NM, Kabary MG, Omar SI. Evaluation of daily avanafil efficacy in improving the endothelial function in Egyptian males with erectile dysfunction. Andrologia. 2021;53:e13833
112. Zhou QY, Pan JQ, Liu W, Jiang ZT, Gao FY, Zhao ZW. et al. Angiotensin II: A novel biomarker in vascular diseases. Clin Chim Acta. 2025;568:120154
113. Ajoolabady A, Pratico D, Ren J. Angiotensin II: Role in oxidative stress, endothelial dysfunction, and diseases. Mol Cell Endocrinol. 2024;592:112309
114. Fratta Pasini A, Garbin U, Nava MC, Stranieri C, Davoli A, Sawamura T. et al. Nebivolol decreases oxidative stress in essential hypertensive patients and increases nitric oxide by reducing its oxidative inactivation. J Hypertens. 2005;23:589-96
115. Chiangsaen P, Maneesai P, Kukongviriyapan U, Tong-Un T, Ishida W, Prachaney P. et al. Tangeretin ameliorates erectile and testicular dysfunction in a rat model of hypertension. Reprod Toxicol. 2020;96:1-10
116. Ohtsu H, Mifune M, Frank GD, Saito S, Inagami T, Kim-Mitsuyama S. et al. Signal-crosstalk between Rho/ROCK and c-Jun NH2-terminal kinase mediates migration of vascular smooth muscle cells stimulated by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:1831-6
117. Lima VV, Giachini FR, Carneiro FS, Carvalho MH, Fortes ZB, Webb RC. et al. O-GlcNAcylation contributes to the vascular effects of ET-1 via activation of the RhoA/Rho-kinase pathway. Cardiovasc Res. 2011;89:614-22
118. Li Y, Jiang J, He Y, Jiang R, Liu J, Fan Z. et al. Icariin combined with breviscapine improves the erectile function of spontaneously hypertensive rats. J Sex Med. 2014;11:2143-52
119. Luan J, Yu M, Gu Q, Zhou X, Shao Y, Chen T. et al. Fatty acid synthase inhibition improves hypertension-induced erectile dysfunction by suppressing oxidative stress and NLRP3 inflammasome-dependent pyroptosis through activating the Nrf2/HO-1 pathway. Front Immunol. 2024;15:1532021
120. Yang CC, Liao PH, Cheng YH, Chien CY, Cheng KH, Chien CT. Diabetes associated with hypertension exacerbated oxidative stress-mediated inflammation, apoptosis and autophagy leading to erectile dysfunction in rats. J Chin Med Assoc. 2022;85:346-57
121. Walczak MK, Lokhandwala N, Hodge MB, Guay AT. Prevalence of cardiovascular risk factors in erectile dysfunction. J Gend Specif Med. 2002;5:19-24
122. Wei M, Macera CA, Davis DR, Hornung CA, Nankin HR, Blair SN. Total cholesterol and high density lipoprotein cholesterol as important predictors of erectile dysfunction. Am J Epidemiol. 1994;140:930-7
123. Roumeguère T, Wespes E, Carpentier Y, Hoffmann P, Schulman CC. Erectile dysfunction is associated with a high prevalence of hyperlipidemia and coronary heart disease risk. Eur Urol. 2003;44:355-9
124. Moosavi D, Vuckovic I, Kunz HE, Lanza IR. A Randomized Trial of ω-3 Fatty Acid Supplementation and Circulating Lipoprotein Subclasses in Healthy Older Adults. J Nutr. 2022;152:1675-89
125. Li R, Cui K, Wang T, Wang S, Li X, Qiu J. et al. Hyperlipidemia impairs erectile function in rats by causing cavernosal fibrosis. Andrologia. 2017;49:e12693
126. Gholami SS, Rogers R, Chang J, Ho HC, Grazziottin T, Lin CS. et al. The effect of vascular endothelial growth factor and adeno-associated virus mediated brain derived neurotrophic factor on neurogenic and vasculogenic erectile dysfunction induced by hyperlipidemia. J Urol. 2003;169:1577-81
127. Murugesan G, Fox PL. Role of lysophosphatidylcholine in the inhibition of endothelial cell motility by oxidized low density lipoprotein. J Clin Invest. 1996;97:2736-44
128. Barton M, Haudenschild CC, d'Uscio LV, Shaw S, Münter K, Lüscher TF. Endothelin ETA receptor blockade restores NO-mediated endothelial function and inhibits atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 1998;95:14367-72
129. d'Uscio LV, Baker TA, Mantilla CB, Smith L, Weiler D, Sieck GC. et al. Mechanism of endothelial dysfunction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1017-22
130. Villeneuve N, Fortuno A, Sauvage M, Fournier N, Breugnot C, Jacquemin C. et al. Persistence of the nitric oxide pathway in the aorta of hypercholesterolemic apolipoprotein-E-deficient mice. J Vasc Res. 2003;40:87-96
131. Xie D, Odronic SI, Wu F, Pippen AM, Donatucci CF, Annex BH. A mouse model of hypercholesterolemia-induced erectile dysfunction. J Sex Med. 2007;4:898-907
132. Azadzoi KM. Vasculogenic erectile dysfunction: beyond the haemodynamic changes. BJU Int. 2006;97:11-6
133. Ryu JK, Shin HY, Song SU, Oh SM, Piao S, Han JY. et al. Downregulation of angiogenic factors and their downstream target molecules affects the deterioration of erectile function in a rat model of hypercholesterolemia. Urology. 2006;67:1329-34
134. Fu X, Sheikholeslami A, Zhanbyrbekuly U, Davoodi Asl F, Mussin NM, Fazaeli H. et al. Advances in stem cell therapy for erectile dysfunction: preclinical evidence and emerging therapeutic approaches. Front Med (Lausanne). 2025;12:1519095
135. Henry GD, Byrne R, Hunyh TT, Abraham V, Annex BH, Hagen PO. et al. Intracavernosal injections of vascular endothelial growth factor protects endothelial dependent corpora cavernosal smooth muscle relaxation in the hypercholesterolemic rabbit: a preliminary study. Int J Impot Res. 2000;12:334-9
136. Fraga-Silva RA, Costa-Fraga FP, Savergnini SQ, De Sousa FB, Montecucco F, da Silva D. et al. An oral formulation of angiotensin-(1-7) reverses corpus cavernosum damages induced by hypercholesterolemia. J Sex Med. 2013;10:2430-42
137. Huang YC, Wu CT, Chen MF, Kuo YH, Li JM, Shi CS. Intracavernous Injection of Autologous Platelet-Rich Plasma Ameliorates Hyperlipidemia-Associated Erectile Dysfunction in a Rat Model. Sex Med. 2021;9:100317
138. Zouaoui Boudjeltia K, Roumeguere T, Delree P, Moguilevsky N, Ducobu J, Vanhaeverbeek M. et al. Presence of LDL modified by myeloperoxidase in the penis in patients with vascular erectile dysfunction: a preliminary study. Eur Urol. 2007;51:262-9
139. Mosalmanzadeh N, Pence BD. Oxidized Low-Density Lipoprotein and Its Role in Immunometabolism. Int J Mol Sci. 2024;25:11386
140. Fillo J, Levcikova M, Ondrusova M, Breza J, Labas P. Importance of Different Grades of Abdominal Obesity on Testosterone Level, Erectile Dysfunction, and Clinical Coincidence. Am J Mens Health. 2017;11:240-5
141. Xu M, Zhou H, Zhang R, Pan Y, Liu X. Correlation between visceral adiposity index and erectile dysfunction in American adult males: a cross-sectional study based on NHANES. Front Endocrinol (Lausanne). 2023;14:1301284
142. Akdemir AO, Karabakan M, Aktas BK, Bozkurt A, Ozgur EG, Akdogan N. et al. Visceral adiposity index is useful for evaluating obesity effect on erectile dysfunction. Andrologia. 2019;51:e13282
143. Moura A, Tomada I, Tomada N. The influence of metabolic profile of obese men on the severity of erectile dysfunction: are metabolically healthy obese individuals protected? Turk J Urol. 2018;44:455-61
144. Odom MR, Hunt TC, Pak ES, Hannan JL. High-fat diet induces obesity in adult mice but fails to develop pre-penile and penile vascular dysfunction. Int J Impot Res. 2022;34:308-16
145. Gao M, Lv J, Yu C, Guo Y, Bian Z, Yang R. et al. Metabolically healthy obesity, transition to unhealthy metabolic status, and vascular disease in Chinese adults: A cohort study. PLoS Med. 2020;17:e1003351
146. El Assar M, Ruiz de Adana JC, Angulo J, Pindado Martínez ML, Hernández Matías A, Rodríguez-Mañas L. Preserved endothelial function in human obesity in the absence of insulin resistance. J Transl Med. 2013;11:263
147. Chen S, Wu R, Huang Y, Zheng F, Ou Y, Tu X. et al. Insulin resistance is an independent determinate of ED in young adult men. PLoS One. 2013;8:e83951
148. Corona G, Mannucci E, Fisher AD, Lotti F, Petrone L, Balercia G. et al. Low levels of androgens in men with erectile dysfunction and obesity. J Sex Med. 2008;5:2454-63
149. Moon KH, Park SY, Kim YW. Obesity and Erectile Dysfunction: From Bench to Clinical Implication. World J Mens Health. 2019;37:138-47
150. El Assar M, Angulo J, Santos-Ruiz M, Ruiz de Adana JC, Pindado ML, Sánchez-Ferrer A. et al. Asymmetric dimethylarginine (ADMA) elevation and arginase up-regulation contribute to endothelial dysfunction related to insulin resistance in rats and morbidly obese humans. J Physiol. 2016;594:3045-60
151. Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H. et al. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101:1539-45
152. Varra FN, Varras M, Varra VK, Theodosis-Nobelos P. Molecular and pathophysiological relationship between obesity and chronic inflammation in the manifestation of metabolic dysfunctions and their inflammation-mediating treatment options (Review). Mol Med Rep. 2024;29:95
153. Lee Y, Harnois-Leblanc S, Rifas-Shiman SL, Oken E, Hivert MF. Associations of adiponectin concentrations from birth until late adolescence with insulin resistance. Pediatr Obes. 2025;20:e70015
154. Tilg H, Ianiro G, Gasbarrini A, Adolph TE. Adipokines: masterminds of metabolic inflammation. Nat Rev Immunol. 2025;25:250-65
155. Borel JC, Roux-Lombard P, Tamisier R, Arnaud C, Monneret D, Arnol N. et al. Endothelial dysfunction and specific inflammation in obesity hypoventilation syndrome. PLoS One. 2009;4:e6733
156. Roumeguère T, Van Antwerpen P, Fathi H, Rousseau A, Vanhamme L, Franck T. et al. Relationship between oxidative stress and erectile function. Free Radic Res. 2017;51:924-31
157. Polak-Szczybyło E, Tabarkiewicz J. Influence of dietary and lifestyle factors on levels of inflammatory markers (IL-6, IFN-γ and TNF-α) in obese subjects. Cent Eur J Immunol. 2024;49:19-25
158. Matos G, Hirotsu C, Alvarenga TA, Cintra F, Bittencourt L, Tufik S. et al. The association between TNF-α and erectile dysfunction complaints. Andrology. 2013;1:872-8
159. Carneiro FS, Zemse S, Giachini FR, Carneiro ZN, Lima VV, Webb RC. et al. TNF-alpha infusion impairs corpora cavernosa reactivity. J Sex Med. 2009;6(Suppl 3):S311-S319
160. Hassan A, El-Hadidy M, El-Deeck BS, Mostafa T. Couple satisfaction to different therapeutic modalities for organic erectile dysfunction. J Sex Med. 2008;5:2381-91
161. Chen Z, Wang J, Jia J, Wu C, Song J, Tu J. Effect of different physical activities on erectile dysfunction in adult men not receiving phosphodiesterase-5 inhibitors therapy: A systematic review and meta-analysis. Andrology. 2024;12:1632-41
162. Leitão AE, Vieira MCS, Pelegrini A, da Silva EL, Guimarães ACA. A 6-month, double-blind, placebo-controlled, randomized trial to evaluate the effect of Eurycoma longifolia (Tongkat Ali) and concurrent training on erectile function and testosterone levels in androgen deficiency of aging males (ADAM). Maturitas. 2021;145:78-85
163. Morelli A, Filippi S, Comeglio P, Sarchielli E, Cellai I, Pallecchi M. et al. Physical activity counteracts metabolic syndrome-induced hypogonadotropic hypogonadism and erectile dysfunction in the rabbit. Am J Physiol Endocrinol Metab. 2019;316:519-35
164. Leoni LA, Fukushima AR, Rocha LY, Maifrino LB, Rodrigues B. Physical activity on endothelial and erectile dysfunction: a literature review. Aging Male. 2014;17:125-30
165. Esposito K, Giugliano F, Maiorino MI, Giugliano D. Dietary factors, Mediterranean diet and erectile dysfunction. J Sex Med. 2010;7:2338-45
166. Jiménez M. Plant-Based Diet and Erectile Dysfunction: A Narrative Review. J Nutr. 2025;155:1644-52
167. Corona G, Rastrelli G, Sparano C, Vignozzi L, Sforza A, Maggi M. Pharmacotherapeutic strategies for the management of erectile dysfunction in patients with diabetes and pre-diabetes. Expert Opin Pharmacother. 2024;25:2213-23
168. Patel JP, Lee EH, Mena CI, Walker CN. Effects of metformin on endothelial health and erectile dysfunction. Transl Androl Urol. 2017;6:556-65
169. Vignozzi L, Filippi S, Comeglio P, Cellai I, Morelli A, Rastrelli G. et al. Metformin in vitro and in vivo increases adenosine signaling in rabbit corpora cavernosa. J Sex Med. 2014;11:1694-708
170. Yang B, Cheng H, Hu Y, Chen Y, Xu Y, Huang W. et al. Effects of Anti-Diabetic Drugs on Erectile Dysfunction: A Systematic Review and Meta-Analysis. Diabetes Metab Syndr Obes. 2025;18:467-78
171. Hernández-Cerda J, Bertomeu-González V, Zuazola P, Cordero A. Understanding Erectile Dysfunction in Hypertensive Patients: The Need for Good Patient Management. Vasc Health Risk Manag. 2020;16:231-9
172. Lu Y, Li L, Li Q, Sun G. Effect of nebivolol on erectile function: a systematic review and meta-analysis of randomized controlled trials. J Sex Med. 2025;22:307-16
173. Friedrich EB, Teo KK, Böhm M. ACE inhibition in secondary prevention: are the results controversial? Clin Res Cardiol. 2006;95:61-7
174. Bank AJ, Kelly AS, Kaiser DR, Crawford WW, Waxman B, Schow DA. et al. The effects of quinapril and atorvastatin on the responsiveness to sildenafil in men with erectile dysfunction. Vasc Med. 2006;11:251-7
175. Baumhäkel M, Custodis F, Schlimmer N, Laufs U, Böhm M. Improvement of endothelial function of the corpus cavernosum in apolipoprotein E knockout mice treated with irbesartan. J Pharmacol Exp Ther. 2008;327:692-8
176. Park K, Shin JW, Oh JK, Ryu KS, Kim SW, Paick JS. Restoration of erectile capacity in normotensive aged rats by modulation of angiotensin receptor type 1. J Androl. 2005;26:123-8
177. Nachtigal P, Pospisilova N, Vecerova L, Micuda S, Brcakova E, Pospechova K. et al. Atorvastatin Increases Endoglin, SMAD2, Phosphorylated SMAD2/3 and eNOS Expression in ApoE/LDLR Double Knockout Mice. J Atheroscler Thromb. 2009;16:265-74
178. Park J, Kwon OS, Cho SY, Paick JS, Kim SW. Chronic administration of atorvastatin could partially ameliorate erectile function in streptozotocin-induced diabetic rats. PLoS One. 2017;12:e0172751
179. Azmy Nabeh O, Ahmed El-Batrawy F, Anwar Khorshid O, Farouk Soliman G. The potential effect of ambrisentan as monotherapy and combined with tadalafil on diabetic erectile dysfunction in rats. Urologia. 2024;91:159-69
180. Albarakati M, El-Tholoth HS, Alzahrani A, Alghamdi OS, Alquliti A, Alnuami M. et al. Predictors of Phosphodiesterase Type 5 Inhibitor Treatment Failure in Patients Diagnosed With Erectile Dysfunction. Cureus. 2023;15:e50515
181. Honig S, Gittelman M, Kaminetsky J, Wang C, Amory JK, Rohowsky N. et al. Two-Year Analysis of a New Oral Testosterone Undecanoate (TU) Formulation in Hypogonadal Men: Efficacy, Impact on Psychosexual Function, and Safety. J Sex Med. 2022;19:1750-8
182. Park MG, Yeo JK, Cho DY, Kim JW, Kim JW, Oh MM. et al. The efficacy of combination treatment with injectable testosterone undecanoate and daily tadalafil for erectile dysfunction with testosterone deficiency syndrome. J Sex Med. 2015;12:966-74
183. Corona G, Rastrelli G, Vignozzi L, Maggi M. Androgens and male sexual function. Best Pract Res Clin Endocrinol Metab. 2022;36:101615
184. Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M. et al. THERAPY OF ENDOCRINE DISEASE: Testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol. 2016;174:99-116
185. Desai A, Yassin M, Cayetano A, Tharakan T, Jayasena CN, Minhas S. Understanding and managing the suppression of spermatogenesis caused by testosterone replacement therapy (TRT) and anabolic-androgenic steroids (AAS). Ther Adv Urol. 2022;14:17562872221105017
186. Sopko NA, Hannan JL, Bivalacqua TJ. Understanding and targeting the Rho kinase pathway in erectile dysfunction. Nat Rev Urol. 2014;11:622-8
187. Lasker GF, Pankey EA, Allain AV, Murthy SN, Stasch JP, Kadowitz PJ. The selective Rho-kinase inhibitor azaindole-1 has long-lasting erectile activity in the rat. Urology. 2013;81:465.e7-14
188. Chawla ST, Shahan J, Soutipan N, Sorkhi SR, Choi YS, Bae WJ. et al. Radial Type Low-Intensity Extracorporeal Shockwave Therapy Enhances Penile Microvascular Perfusion in an Aging Rat Model: A Novel Interventional Strategy to Treat Erectile Dysfunction. World J Mens Health. 2025;43:396-406
189. Chung E, Bailey W, Wang J. A Prospective, Randomized, Double-Blinded, Clinical Trial Using a Second-Generation Duolith SD1 Low-Intensity Shockwave Machine in Males with Vascular Erectile Dysfunction. World J Mens Health. 2023;41:94-100
190. Liu G, Sun X, Bian J, Wu R, Guan X, Ouyang B. et al. Correction of diabetic erectile dysfunction with adipose derived stem cells modified with the vascular endothelial growth factor gene in a rodent diabetic model. PLoS One. 2013;8:e72790
191. Zhang C, Luo D, Li T, Yang Q, Xie Y, Chen H. et al. Transplantation of Human Urine-Derived Stem Cells Ameliorates Erectile Function and Cavernosal Endothelial Function by Promoting Autophagy of Corpus Cavernosal Endothelial Cells in Diabetic Erectile Dysfunction Rats. Stem Cells Int. 2019;2019:2168709
192. Ouyang B, Xie Y, Zhang C, Deng C, Lv L, Yao J. et al. Extracellular Vesicles From Human Urine-Derived Stem Cells Ameliorate Erectile Dysfunction in a Diabetic Rat Model by Delivering Proangiogenic MicroRNA. Sex Med. 2019;7:241-50
193. Su L, Yang ZT, Qu H, Luo CL, Yuan GX, Wu J. et al. Effect of Antioxidants Supplementation on Erectile Dysfunction: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sex Med Rev. 2022;10:754-63
194. Tang Z, Song J, Yu Z, Cui K, Ruan Y, Wang T. et al. Melatonin Treatment Ameliorates Hyperhomocysteinemia-Induced Impairment of Erectile Function in a Rat Model. J Sex Med. 2019;16:1506-17
195. Wang H, Chang H, Sun D, Wang A, Yan B, Chung E. Therapeutic Challenges of Diabetes Mellitus-Related Erectile Dysfunction and The Potential Therapeutic Role of Medicinal Plants: A Narrative Review. Drug Des Devel Ther. 2025;19:3209-23
196. Feng Y, Shi T, Fu Y, Lv B. Traditional chinese medicine to prevent and treat diabetic erectile dysfunction. Front Pharmacol. 2022;13:956173
197. Wang H, Zhao M, Zhang J, Yan B, Gao Q, Guo J. Traditional Chinese medicine regulates inflammatory factors in chronic prostatitis/chronic pelvic pain syndrome: A review. Integr Med Nephrol Androl. 2023;10:e00001
Author contact
![]() Corresponding authors: Jun Guo, Department of Andrology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, 100091, Beijing, China; E-mail: guojun1126com. Eric Chung, Department of Urology, Princess Alexandra Hospital, University of Queensland, 4000, Brisbane, Australia; E-mail: ericchgcom.
Corresponding authors: Jun Guo, Department of Andrology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, 100091, Beijing, China; E-mail: guojun1126com. Eric Chung, Department of Urology, Princess Alexandra Hospital, University of Queensland, 4000, Brisbane, Australia; E-mail: ericchgcom.

 Global reach, higher impact
Global reach, higher impact