Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2026; 22(3):1142-1161. doi:10.7150/ijbs.124499 This issue Cite
Review
Microbiota-Gut-Kidney Axis and Targeted Therapeutic Strategies in Kidney Diseases
1. School of Basic Medical Sciences, Anhui Medical University, Hefei 230032, China.
2. Department of Cardiovascular Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei 230032, China.
3. Inflammation and Immune Mediated Diseases Laboratory of Anhui Province, The Key Laboratory of Anti-Inflammatory of Immune Medicines, Ministry of Education, Anhui Institute of Innovative Drugs, School of Pharmacy, Anhui Medical University, Hefei 230032, China.
4. Department of Nephrology, the Second Affiliated Hospital of Anhui Medical University, 678 Furong Road, Hefei 230601, Anhui, China.
# Zheng-hao Sun, Qian Gong and Zi-lai Wang contributed equally to this work.
Received 2025-8-31; Accepted 2025-12-1; Published 2026-1-1
Abstract
The gut microbiota, as a source of profound genetic and metabolic capacity, affects every aspect of human biology including health, development and aging, as well as disease. Studies have demonstrated the crosstalk between the gut microbiota and the kidney, which is directly referred to as the “microbiota-gut-kidney axis”. Most gut microbiota metabolites are associated with metabolic, immune, and inflammatory pathways. Disruption of gut microbiota homeostasis in patients with kidney disease contributes to further loss of kidney function, forming a vicious cycle. This discovery may provide a potential avenue of treatment for kidney diseases, creating a new therapeutic paradigm. Moreover, new therapeutic strategies seem to be beneficial for kidney health via the modulation of the gut microbiota. Although these strategies show early beneficial results, their long-term effectiveness and safety require further investigation and confirmation. In this review, we discuss the interactions between the gut microbiota and kidney diseases and explore related therapeutic strategies. A comprehensive understanding of the microbiota-gut-kidney axis will facilitate the development of efficient therapeutic measures for kidney diseases.
Keywords: microbiota-gut-kidney axis, kidney diseases, therapeutic strategies, gut microbiota dysbiosis
1. Introduction
Kidney diseases, including acute kidney injury (AKI), chronic kidney disease (CKD), renal cell carcinoma (RCC) and various renal dysfunctions have emerged as crucial public health issues globally [1]. They are highly prevalent, with incidence rates continuing to increase, and are strongly linked to metabolic disorders and immune dysfunction [1-3]. The kidneys, one of the organs with the highest metabolic rates in the human body, play an irreplaceable role in maintaining internal body fluid homeostasis by filtering approximately 180 liters of plasma (primary urine) each day [2]. In addition to waste excretion, the kidneys are crucial for regulating blood pressure, electrolyte balance, and certain endocrine functions [2]. Recently, an increasing number of researchers have pointed out the crosstalk between the kidneys and other organs [4-6], suggesting that kidney diseases may affect multiple organs through complex pathways.
In recent years, the microbiota-gut-kidney axis has received increased attention. Trillions of bacteria inhabit the gut, which is the largest microecosystem, and alterations in gut bacteria influence host health [7]. Given the function of the gut microbiota in immune homeostasis modulation, intestinal epithelial barrier integrity maintenance, and metabolite generation, the gut microbiota displays potent systemic roles in other non-intestinal organs, including the kidneys [8-10]. Diseases such as diabetes, obesity, and inflammatory bowel disease are strongly associated with gut microbiota dysbiosis [11-13]. The relationship between gut microbiota and kidney diseases has received considerable attention. In patients with kidney disease, gut microbiota dysbiosis manifests as relatively low diversity and a higher abundance of pathogenic bacteria, which might lead to systemic inflammation and kidney injury [14]. Furthermore, kidney disease is associated with alterations in microbiota-derived metabolites that are thought to be critical for disease development. For example, the accumulation of uremic toxins in patients with CKD and patients undergoing dialysis can lead to kidney injury [15, 16].
Given the close relationship between gut microbiota and kidney diseases, strategies targeting the gut microbiota have become a focus of research. For example, Modified Huangfeng Decoction has been shown to ameliorate diabetic kidney disease (DKD) by modulating gut microbiota and its metabolites [17]. Lactobacillus reuteri can influence the gut microbiota, leading to reduced glycated hemoglobin and serum cholesterol in type 2 diabetes (T2D) patients [18]. Administration of the probiotic Lactobacillus casei Zhang to CKD patients can slow the decline in kidney function [19]. As research progresses, these strategies are increasingly demonstrating clinical translational potential. In this review, we provide a comprehensive overview of the microbiota-gut-kidney axis and its potential therapeutic targets and modulations, such as probiotics, synbiotics, fecal microbiota transplantation (FMT), and dietary intake, as well as the potential use of this new burgeoning field for the treatment of kidney diseases. This will improve understanding of the pathways of molecular mechanisms of kidney diseases and the development of new drugs targeting the microbiota-gut-kidney axis.
2. Microbiota-gut-kidney axis in kidney diseases
2.1 Microbiota-gut-kidney axis
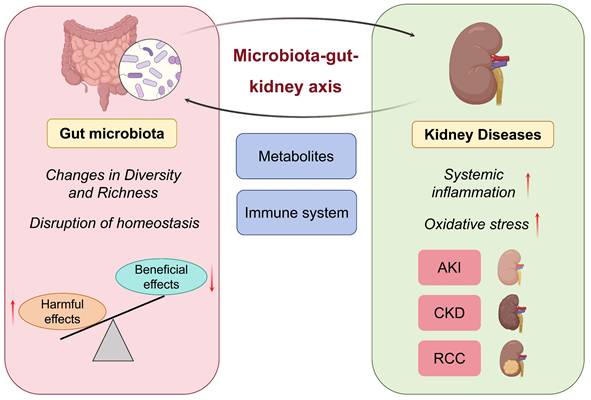
The microbiota-gut-kidney axis is a new research field that has attracted more and more attention from scholars. The relationship between gut microbiota and kidney diseases has emerged as a novel concept in nephrology research. This may overthrow the traditional concept of kidney disease and lay the foundation for new theories and clinical guidelines for the prevention and treatment of kidney diseases. The gut microbiota affects kidney function via metabolic and immunological pathways, and kidney disease can further shape the gut microbiota by disturbing systemic metabolism and immune responses [19, 20]. One study demonstrated that patients with kidney disease exhibited reduced gut microbiota richness and an increased proportion of pathogenic species. Harmful bacteria generate metabolites that induce harmful pathways of inflammation and oxidative stress, ultimately leading to the worsening of kidney function [21]. When kidney function declines to levels at which metabolic waste is no longer adequately filtered from the bloodstream, gut microbiota dysbiosis is promoted, creating a vicious cycle (Figure 1). To combat this, researchers have scrambled to develop strategies to escape this spiral. Evidence has shown that rehabilitation of the gut microbial environment mitigates the progression of kidney diseases. It has been suggested that probiotics, which have the potential to reduce the production of uremic toxins by restoring gut microbiota balance, may improve clinical outcomes in CKD patients [22]. Another study suggested that FMT could restore gut microbiota homeostasis and intestinal integrity to reduce the burden on the kidneys [23]. These findings demonstrate the therapeutic potential of regulating the gut microbiota in kidney disease management.
2.2 Gut microbiota-derived metabolites in kidney diseases
Metabolites are directly influenced by compositional changes in the gut microbiota and are closely associated with the progression of kidney disease [24]. Gut microbiota dysbiosis promotes the production of harmful metabolites and impairs the intestinal barrier, leading to an increased absorption of toxic substances. This process can exacerbate kidney inflammation and injury [25]. During the course of kidney disease, various dangerous metabolites, such as indoxyl sulfate (IS), p-cresol sulfate (PCS), trimethylamine N-oxide (TMAO), and Advanced Glycation End Products (AGEs), are produced.
With decreased kidney function, IS and PCS, which are toxic metabolites produced by the gut microbiota, freely accumulate in the plasma, leading to deleterious health consequences. IS is a tryptophan metabolite that is naturally excreted in urine by the kidneys. IS accumulates in the circulation due to kidney dysfunction, contributing to oxidative stress and inflammation, which promotes the progression of kidney fibrosis [26]. It has been reported that higher IS levels not only inhibit the growth of beneficial bacteria including Bifidobacterium and Lactobacillus, but also increase the ratio of pathogenic bacteria, and therefore aggravate systemic inflammation [27]. PCS induces the production of reactive oxygen species (ROS), leading to oxidative stress and promoting the progression of kidney disease [28]. Furthermore, PCS can induce insulin resistance in CKD, and blocking PCS may be a potential therapeutic approach for the treatment of CKD [29]. TMAO is another common harmful metabolite. The gut microbiota generates TMA during the metabolism of choline and L-carnitine, and TMA is oxidized by the liver to TMAO. An imbalance in the gut microbiota is closely associated with the development and prognosis of CKD, and increased TMAO level is an important factor in its prognosis. These findings suggest that interventions to prevent TMAO elevation may be beneficial in the prevention and prognosis of kidney disease. For example, inhibiting TMAO generation significantly alleviated kidney injury in adenine-induced CKD mice [30, 31]. AGEs are noxious and permanent products of non-enzymatic reactions between carbonyl compounds and proteins or lipids. AGEs may disrupt the structure and function of the kidney through accumulation and induce downstream signaling of the AGE-receptor for AGEs pathway, thereby initiating oxidative stress and a pronounced inflammatory process, leading to further kidney injury [32]. In summary, gut microbiota-derived metabolites are essential mediators of the microbiome-gut-kidney axis, and their homeostasis may be clinically relevant in the prevention and treatment of kidney diseases.
Microbiota-gut-kidney axis in kidney diseases. Gut microbiota exhibits bidirectional interplay with various kidney diseases.
2.3 Interaction between gut microbiota and AKI
2.3.1 Impact of AKI on gut microbiota
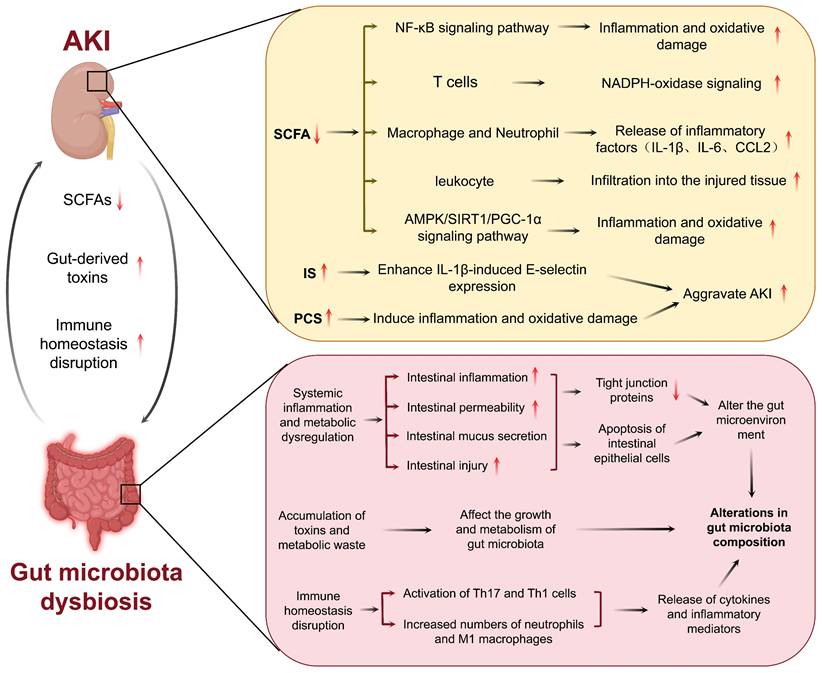
AKI is a clinical syndrome characterized by a rapid decline in kidney function and diverse pathogenic mechanisms. The gut microbiota confers several beneficial effects on the host by preserving intestinal barrier function, modulating the immune system, and producing vital metabolites such as short chain fatty acids (SCFAs). Nevertheless, in AKI, kidney injury may perturb this balance resulting in gut microbiota dysbiosis. In a cisplatin-induced AKI model, a reduction in the beneficial bacterium Lactobacillus was observed in feces [33]. In a rat renal ischemia/reperfusion (I/R) AKI model, the levels of Staphylococcus spp. and Rothia spp. were increased and were positively associated with increased serum levels of creatinine and urea [34]. AKI disrupts gut microbiota homeostasis via various mechanisms. First, AKI alters the anatomy and physiology of the gut mucosa. AKI-induced systemic inflammation and metabolic abnormalities affect the intestinal microenvironment, which is characterized by intestinal inflammation, damage, increased permeability, and altered mucus secretion. Such alterations alter the living environment of the gut microbiota, further altering its composition and structure [35, 36]. Moreover, impaired kidney excretory function for uremic toxins and metabolic waste products in AKI can cause the retention of these substances, some of which can translocate to the intestinal tract via systemic circulation, thereby affecting the growth and metabolic activity of the gut microbiota. These alterations manifest as a decrease in beneficial bacteria and an increase in pathogenic bacteria [36-38]. In addition, since AKI causes immune dysregulation, homeostasis of the gut microbiota could be disturbed. The pro-inflammatory cytokines and mediators released by the activated immune system can affect gut microbiota composition [38]. For example, AKI has been found to disturb intestinal immune homeostasis, which is characterized by increased Th1 and Th17 responses, higher infiltration of neutrophils and pro-inflammatory M1 macrophages, as well as upregulated levels of IFN-γ [38]. These changes not only affect the function of the intestinal barrier, resulting in a greater inflammatory response, but also enable the entry of gut-derived toxins into the blood circulation, aggravating kidney injury.
Fortunately, researchers have identified several therapies that can alleviate AKI dysbiosis of the gut microbiota. For example, modulation of gut microbiota through probiotics, prebiotics, or supplementation with SCFAs may help attenuate inflammation and kidney injury in AKI [19, 39]. The second case involves Qiongyu paste, a traditional Chinese medicine product that modulates the gut microbiota and increases the production of SCFAs to attenuate kidney fibrosis and inflammation in AKI [40]. In summary, AKI induces an imbalance in the gut microbiota, which exacerbates kidney injury through a vicious cycle.
2.3.2 Involvement of gut microbiota in AKI
In contrast, disrupted gut microbiota has emerged as a crucial contributor to the development of AKI. Transplantation of the gut microbiota from AKI mice into germ-free mice results in exacerbated kidney injury [41]. Consequently, researchers have proposed that modulation of gut microbiota dysbiosis could serve as an innovative therapeutic approach for the treatment of AKI. For instance, the administration of antibiotics to mice to deplete the gut microbiota attenuated the recruitment of local renal macrophages and bone marrow monocytes, impaired inflammatory responses, and protected against tubular injury of the kidneys [42]. In mouse kidneys, depletion of gut microbiota with a cocktail of antibiotics before renal I/R injury could significantly reduce the concentrations of TNF-α, IL-6, and MCP-1 [42].
Alterations in the composition of the gut microbiota have been linked to significant modifications in metabolite profiles, with recent research highlighting the significance of gut microbiota-derived metabolites in AKI (Table 1). Notably, SCFAs and amino acid metabolites play pivotal roles in immune regulation and kidney injury processes. These metabolites can impact immune responses and metabolic functions through diverse host pathways, resulting in intricate effects on the development of AKI. Notably, SCFAs such as acetate, propionate, and butyrate, which are derived from the fermentation of dietary fibers by the gut microbiota, exhibit potent renoprotective properties [38, 43]. For example, sodium butyrate has been observed to mitigate the activation of the nuclear factor-kappa B (NF-κB) signaling pathway, thereby suppressing inflammation and oxidative damage in contrast-induced AKI [44]. SCFAs also modulate immune cells. For example, acetate treatment ameliorates sepsis-induced AKI by inhibiting NADPH-oxidase signaling in T cells [45]. SCFAs ameliorate AKI by regulating macrophage and neutrophil activation, thereby reducing the production of inflammatory cytokines such as IL-1β, IL-6, and CCL2 [19]. In I/R-induced AKI, SCFAs have been demonstrated to inhibit leukocyte migration into injured tissues and reduce inflammation [46]. Additionally, SCFAs participate in metabolic regulation, as evidenced in sepsis-induced AKI where acetate alleviates kidney oxidative stress and inflammation by activating the AMPK/SIRT1/PGC-1α pathway, consequently contributing to the preservation of kidney function [47]. In the gut, SCFAs contribute to the maintenance of gut barrier integrity [48]. In summary, changes in SCFA levels caused by gut microbiota dysbiosis may lead to the onset of new AKIs or the exacerbation of existing AKIs.
The role and mechanisms of gut microbiota-derived metabolites in AKI.
| Model | Metabolite | Function | Mechanism | Reference |
|---|---|---|---|---|
| Contrast-induced AKI | SCFAs | Protective | Sodium butyrate reduces inflammation and oxidative stress through suppression of NF-κB pathway activation. | [44] |
| Sepsis-induced AKI | SCFAs | Protective | Acetate ameliorates AKI by suppressing NADPH oxidase signaling in T cells. | [45] |
| I/R-induced AKI | SCFAs | Protective | SCFAs inhibit the release of inflammatory cytokines by modulating macrophage and neutrophil activity, ultimately ameliorating AKI. | [19] |
| I/R-induced AKI | SCFAs | Protective | SCFAs reduce leukocyte infiltration into injured tissues and attenuate kidney inflammation. | [46] |
| Sepsis-induced AKI | SCFAs | Protective | Acetate ameliorates oxidative stress and inflammation by activating the AMPK/SIRT1/PGC-1α axis. | [47] |
| I/R-induced AKI | IS | Pathogenic | IS exacerbates the progression of AKI through the ROS/MAPKs/NFκB/AP-1 pathway. | [51] |
| I/R-induced AKI | PCS | Pathogenic | PCS promotes kidney injury by inducing inflammation and oxidative stress. | [52, 53] |
AKI, acute kidney injury; IS, indoxyl sulfate; PCS, p-cresol sulfate; SCFAs, short-chain fatty acids.
Interaction between gut microbiota and AKI. AKI induces alterations in gut microbiota homeostasis, compromises intestinal barrier function, leading to gut damage. Gut microbiota dysbiosis subsequently exacerbates progression of AKI.
In addition to SCFAs, amino acid metabolites have been investigated as critical regulators of AKI pathogenesis. Uremic toxins, such as IS and PCS, are classic examples of highly toxic compounds that accumulate during AKI [49]. For example, increased IS levels in the serum of patients with AKI correlate with higher mortality and poor prognosis [50]. IS enhances IL-1β-induced E-selectin expression through the ROS/MAPKs/NF-κB/AP-1 pathway, thereby exacerbating the progression of AKI [51]. Similarly, high PCS levels are associated with kidney function damage in patients with AKI. During AKI, PCS exacerbates kidney injury by inducing inflammation and oxidative stress, thereby manifesting cytotoxic effects [52, 53]. However, not all amino acid metabolites are harmful to human health. Another tryptophan metabolite, indole-3-propionic acid (IPA) is known to exhibit protective effects on the kidney. Studies have indicated that IPA protects proximal tubular cells by inhibiting the expression of inflammatory genes [54].
In summary, gut microbiota-derived metabolites are critical for kidney function in patients with AKI. SCFAs can be used to treat AKI by preventing inflammation and oxidative stress-induced kidney injury. In contrast, uremic toxins exert nephrotoxic effects by augmenting inflammation and ROS generation. Thus, the modulation of the gut microbiota and its metabolites may represent a novel treatment strategy for AKI (Figure 2).
2.4 Interaction between gut microbiota and CKD
2.4.1 Impact of CKD on gut microbiota
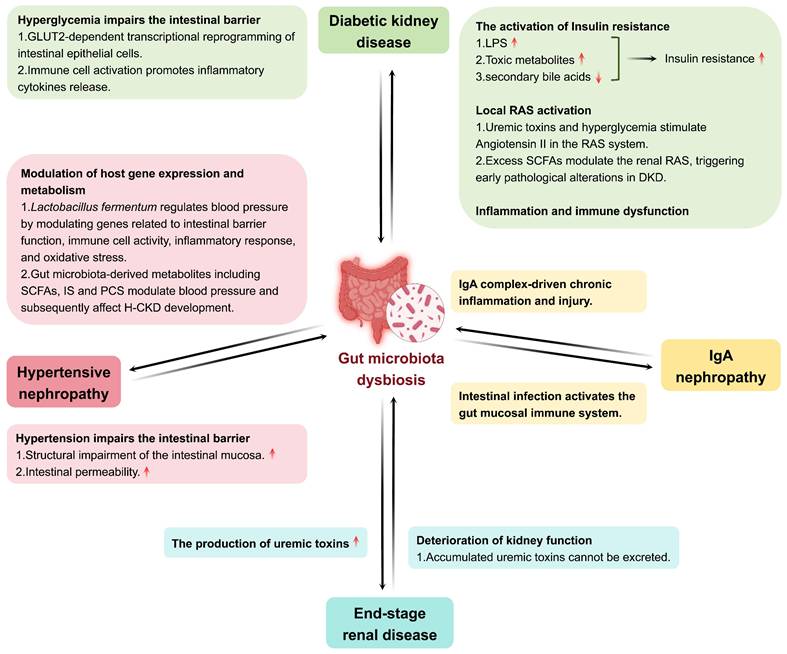
CKD has emerged as a major global public health challenge, creating a significant societal burden. It is characterized by the presence of kidney injury markers or a reduced glomerular filtration rate (GFR) lasting more than three months [55, 56]. Over the past three decades, the prevalence and mortality rates of CKD have increased substantially, with forecasts predicting further increases through at least 2029 [57]. CKD develops progressively, and gut microbiota dysbiosis is increasingly recognized to be involved in the pathophysiology of CKD. The degree of gut microbiota dysbiosis differs in various CKD forms, including DKD, IgA nephropathy (lgAN), hypertensive nephropathy (H-CKD), and end-stage renal disease (ESRD) (Figure 3).
Interaction between gut microbiota and CKD. Gut microbiota dysbiosis engages in a bidirectional pathogenic relationship with multiple forms of CKD, including DKD, lgAN, H-CKD, and ESRD.\
Diabetic kidney disease
DKD is the most common cause of CKD, and many studies have shown that the gut microbiota is closely related to DKD [58]. Hyperglycemia is a typical symptom of DKD. Multiple studies have indicated that hyperglycemia has an undeniable impact on the intestinal barrier and gut microbiota. First, hyperglycemia drives intestinal barrier permeability through Glut2-dependent transcriptional reprogramming of intestinal epithelial cells, thereby disrupting the integrity of tight and adherens junctions [59]. Second, hyperglycemia may promote immune cell hyperactivation in the gut [60]. The activated immune cells promote the release of inflammatory cytokines and damage the intestinal barrier. Damage to the intestinal barrier allows for the effects of extraintestinal noxious substances on the gut microbiota. For example, dysbiosis of the gut microbiota occurs in both type 1 diabetes (T1D) and T2D. The gut microbiota composition of patients with T1D has been shown to be different than healthy subjects and the Firmicutes/Bacteroidetes ratio is shifted to a lower one compared to healthy individuals [61]. Another study reported that an increased abundance of Bacteroides in patients with T1D was correlated with poor glycemic control [62]. Compared to healthy controls, patients with T2D also exhibited lower gut microbiota diversity and richness, similar to that observed in patients with T1D [63, 64]. The abundance of butyrate-producing bacteria (Bifidobacterium, Akkermansia, Faecalibacterium) was also lower in patients with T2D [61]. Furthermore, a reduction in Blautia genus was observed in patients with T2D, which was negatively correlated with glycated hemoglobin and glucose [65]. Dysbiosis leads to elevated systemic levels of harmful metabolites, which further promote kidney injury by inducing oxidative stress and activating inflammatory pathways. This not only worsens the inflammatory state of DKD, but may also promote the development of complications. As mentioned previously, hyperglycemia directly affects the gut microbiota and causes a vicious cycle, resulting in the production of more uremic toxins that contribute to the progression of DKD [66].
IgA nephropathy
lgAN is an immune-mediated CKD in which IgA immune complexes are deposited in the renal tubular interstitium and mediate chronic inflammation and kidney injury [67]. Subsequently, chronic kidney inflammation further aggravates gut microbiota dysbiosis by affecting the intestinal environment through the blood circulation. Patients with lgAN have been observed to experience gut microbiota dysbiosis, known to include decreased SCFA-producing bacteria and increased pro-inflammatory bacteria. SCFAs are required for the preservation of the intestinal barrier function and protection against inflammation. In lgAN, deficits in SCFA-producing bacteria and their fermentation products disrupt this barrier, permitting systemic inflammatory mediators to transcend the circulation, and induce greater kidney inflammation and immune activation [68]. Furthermore, the severity of gut microbiota dysbiosis directly correlates with lgAN progression [69, 70]. These findings suggest a potential impact of IgAN on the gut microbiota.
Hypertensive nephropathy
H-CKD, a type of CKD, is kidney injury caused by long-term hypertension. Kidney injury can cause sodium retention and increase blood volume, which in turn increases blood pressure. Some authors have indicated that alterations in the structure of the gut microbiota are directly related to hypertension. For example, studies show that hypertensive rats display intestinal mucosal damage and detachment of epithelial cells from the mucosal surface [71]. Additionally, it has been suggested that hypertension can increase the permeability of the intestinal epithelial barrier [72]. This breakdown of intestinal barrier function may be a critical mechanism by which hypertension influences the gut microbiota. The impact of hypertension on the gut microbiota is specifically characterized by a significant reduction in microbial abundance and diversity, with notable enrichment of Prevotella and Klebsiella accompanied by decreased levels of beneficial bacteria [71]. Furthermore, there was no difference in the diversity of gut microbiota between hypertensive patients with and without CKD. However, there are differences between specific bacterial species. In H-CKD, the signature bacteria are Veillonella parvula and Oxalobacter formigenes and their relative abundance is significantly higher in the H-CKD as compared with hypertension patients. Besides, Veillonella parvula and Oxalobacter formigenes are potential biomarkers for distinguishing H-CKD patients from those with pure hypertension [71]. In metabolism, the level of tauorsodeoxycholic acid was significantly increased in H-CKD patients compared to hypertensive patients without CKD, whereas the levels of other bile acid metabolites did not significantly differ between the two groups [71]. These results indicated that H-CKD may affect the gut microbiota.
End-stage renal disease
ESRD is a late phase of CKD and is described as a severe and irreversible kidney injury. In patients with ESRD, the retention of uremic toxins damages the intestinal barrier and directly changes the gut microbiota. Gut microbiota dysbiosis is more pronounced in patients with ESRD and mainly reflects the composition and metabolism of the gut microbiota. Some studies have provided evidence that patients with ESRD have low gut microbiota diversity, fewer beneficial bacterial species, and a high prevalence of potential pathogens [73]. Dysbiosis of the gut microbiota disrupts immune regulation in various types of cells in the intestine, leading to impaired barrier function and an increase in systemic inflammation, as well as harmful translocation of uremic toxins into the blood circulation [73, 74]. Furthermore, kidney dysfunction in patients with ESRD affects gut microbiota metabolism. For example, levels of uremic toxins and secondary bile acids are dramatically increased in the serum metabolome. Uremic toxin precursors such as indole are elevated and SCFAs are significantly lower in the fecal metabolome [75]. Interestingly, gut microbiota dysbiosis improves following kidney transplantation, as reflected by a return to normal microbial abundance and diversity, and significant blood microbial uremic toxin reduction [76, 77]. This suggests that improving kidney function in patients with ESRD may alleviate gut microbiota dysbiosis.
The role and mechanisms of gut microbiota-derived metabolites in CKD.
| Model | Metabolite | Function | Mechanism | Reference |
|---|---|---|---|---|
| DKD | LPS | Pathogenic | LPS promotes systemic inflammation in DKD patients and facilitates pancreatic islet cell apoptosis and insulin resistance. | [85, 86] |
| DKD | IS and PCS | Pathogenic | IS and PCS induce insulin resistance. | [87] |
| DKD | Secondary bile acids | Protective | Secondary bile acids alleviate inflammation and insulin resistance through FXR/TGR5 activation. | [88, 89] |
| DKD | IS and PCS | Pathogenic | Uremic toxins activate Angiotensin II, leading to kidney vasoconstriction, glomerular hyperfiltration, secretion of inflammatory and profibrotic cytokines, extracellular matrix deposition, and podocyte morphological changes, thereby promoting the progression of DKD. | [87, 91, 93] |
| DKD | SCFAs | Protective | SCFAs reduce the levels of inflammatory cytokines, chemokines, and fibrosis-promoting proteins in DKD. | [86, 94] |
| DKD | Excessive SCFAs | Pathogenic | Excessive SCFAs modulate the intrarenal RAS, triggering early pathological changes in DKD. | [91, 93, 95] |
| DKD | TMAO, IS, PCS, and PS | Pathogenic | Activates the immune system, resulting in the overproduction of inflammatory cytokines. | [99] |
| IgAN | IS, PCS, TMAO, and phenylacetylglutamine | Pathogenic | Stimulates the intestinal mucosal immune system. | [69] |
| H-CKD | SCFAs | Protective | SCFAs decrease blood pressure by activating GPR41. | [72] |
| H-CKD | SCFAs | Pathogenic | SCFAs increase blood pressure by activating Olfr78. | [72] |
| H-CKD | IS and PCS | Pathogenic | IS and PCS increase inflammation and oxidative stress. | [107] |
| ESRD | IS, PCS, and TMAO | Pathogenic | Exacerbates kidney fibrosis and oxidative stress. | [75] |
lgAN, IgA nephropathy; H-CKD, hypertensive nephropathy; DKD, diabetic kidney disease; ESRD, end-stage renal disease; IS, indoxyl sulfate, PCS, p-cresol sulfate; SCFAs, short chain fatty acids; TMAO, trimethylamine N-oxide.
2.4.2 Involvement of gut microbiota in CKD
Gut microbiota dysbiosis is a crucial factor in CKD progression [78, 79]. Metabolic alterations driven by dysbiosis of the gut microbiome are also important in CKD (Table 2). The number of beneficial bacteria decreases, whereas that of pathogenic bacteria increases in patients with CKD [80]. Additionally, gut microbiota dysbiosis is known to be linked to the modification of gut barrier function and an increase in intestinal permeability, both of which would contribute to metabolite transport into the systemic circulation, leading to systemic chronic inflammation [81]. Persistent chronic inflammation is a secondary injury in the kidney that further accelerates CKD [82]. Owing to the diminished kidney function, the elimination of uremic toxins is reduced. These toxins accumulate in the body and trigger the aryl hydrocarbon receptor (AHR) signaling pathway for subsequent injury by promoting oxidative stress and inflammation [83]. The following section discusses the function of the gut microbiota and their metabolites in CKD, which is critical for creating gut microbiota-targeted therapy.
Diabetic kidney disease
Excessive accumulation of LPS, IS, and PCS in the body may cause insulin resistance. LPS receptors are directly or indirectly involved in inducing insulin resistance [84]. It has been shown that gut microbiota dysbiosis can result in an excess of LPS-producing microbiota in the lumen of the gut, promoting systemic low-grade inflammation and enhancing apoptosis of islet cells and insulin resistance in patients with DKD [85, 86]. The elevation of toxic metabolites such as PCS and IS can also contribute to insulin resistance [87]. In addition, bile acids have also been considered gut microbiota metabolites associated with insulin resistance. The gut microbiota transforms primary bile acids into secondary bile acids, which can modulate glucose metabolism, ameliorate insulin resistance, and improve DKD by binding to their receptors, nuclear farnesoid X receptor (FXR) and membrane-bound Takeda G protein-coupled receptor 5 (TGR5) [87]. Gut microbiota dysbiosis leads to the loss of secondary bile acids and suppresses the activation of bile acid receptors FXR and TGR5, contributing to inflammation and insulin resistance [88, 89].
The renin-angiotensin system (RAS) plays an important role in the progression of DKD. Local RAS activation, not circulating RAS, is one of the major triggers of DKD [90]. A growing body of evidence has indicated that there is an interaction between gut microbiota and RAS activation [91, 92]. The key component of the RAS, Angiotensin II, can be stimulated by uremic toxins and hyperglycemia, leading to renal vasoconstriction, glomerular hyperfiltration, secretion of inflammatory and profibrotic factors, extracellular matrix deposition, and morphological changes in podocytes, thereby promoting the progression of DKD [87, 91, 93]. Furthermore, although there is evidence that SCFAs have protective effects in DKD [86, 94], researchers have also pointed out that gut microbiota dysbiosis can lead to excessive SCFA production, particularly of acetate, which may bind to receptors in the kidney and modulate the intrarenal RAS, inducing pathological changes in the early stages of DKD [91, 93, 95].
Although DKD is generally regarded as a non-immune disease, accumulating evidence has suggested that inflammatory responses and the immune system are important for its pathogenesis. The development and progression of DKD are accompanied by inflammation. Chemokines, inflammatory cytokines, and adhesion molecules are involved in several inflammatory pathways that contribute to the complex molecular networks and processes in DKD [96]. Several anti-inflammatory treatments can relieve kidney injury in patients with DKD. SCFAs may participate in modulating anti-inflammatory responses by inhibiting histone deacetylase (HDAC) and binding G protein-coupled receptors (GPRs) [97, 98]. SCFAs caused reduced levels of inflammatory cytokines, chemokines, and fibrosis-promoting proteins in DKD, leading to improvements in albuminuria, glomerular hypertrophy, podocyte injury, and interstitial fibrosis [86, 94]. From the perspective of immunity, dysbiosis of the gut microbiota activates immune cells, resulting in immune dysregulation [91]. In addition, the accumulation of gut microbiota-derived metabolites, such as phenyl sulfate (PS), TMAO, IS, and PCS, can continuously stimulate the immune system, potentially leading to the excessive production of inflammatory factors, thereby exacerbating kidney injury in DKD [99].
In summary, gut microbiota dysbiosis is associated with insulin sensitivity, RAS activation, inflammation, and immune system disorders. These factors may contribute separately to the progression toward DKD but may also work synergistically. To better target these mechanisms and prevent DKD progression, future research should expand on the basis of the implicated molecular pathways that regulate gut dysbiosis in DKD, especially those related to metabolism, to derive individualized results.
IgA nephropathy
In recent years, it has been emphasized that manipulating the gut microbiota and kidney crosstalk is a novel potential treatment for IgAN [100, 101]. IgAN is thought to be initiated by intestinal infections that stimulate the immune system in the intestinal mucosa. Abnormally glycosylated IgA antibodies secreted by the immune cells of the intestinal mucosa are deposited in the kidney [102]. The gut microbiota and their metabolites participate in the induction of mucosal immunity in IgAN. These metabolites included IS, PCS, TMAO, and phenylacetylglutamine [69]. In an IgAN model, depletion of the gut microbiota using antibiotics resulted in decreased IgA1 mesangial deposition, urinary protein levels, and glomerular inflammation [103, 104]. Fresh FMT from healthy donors also significantly reduced proteinuria in two patients with refractory IgAN [105]. These findings suggest that the targeted modification of the gut microbiota or its metabolites could be a novel therapeutic option for the treatment of IgAN. Although the modulation of the gut microbiota and its metabolites may constitute a promising therapeutic approach for IgAN, owing to the complexity of the human gut microbiota, it is challenging to identify the gut microbiota or metabolites that are causally associated with IgAN.
Hypertensive nephropathy Hypertension is characterized by H-CKD and regulated by the gut microbiota. Gut microbiota has also been reported to be involved in the regulation of hypertension, mainly by acting on host gene expression and metabolite profiles [106]. For instance, Lactobacillus fermentum alters blood pressure by regulating the genes associated with intestinal barrier function, immune function, inflammation, and oxidative stress [106]. Additionally, metabolites from the gut microbiota can control blood pressure, which may influence the progression of H-CKD. SCFAs decrease blood pressure by activating GPR41 and increase it by stimulating Olfr78 [72]. In addition, higher IS and PCS levels have been associated with increased inflammation and oxidative stress, both of which could be involved in the progression of hypertension and kidney injury [107]. However, whether gut microbiota plays a regulatory role in H-CKD requires further investigation.
End-stage renal disease Gut microbiota is also involved in the development of ESRD. Gut microbiota is involved in the morbidity and treatment of ESRD. Germ-free mice receiving the gut microbiota of ESRD patients exhibited increases in serum uremic toxins and enhanced kidney fibrosis and oxidative stress, which in turn resulted in further aggravation of kidney injury. The gut microbiota of patients with ESRD is enriched with specific bacterial species, Eggerthella lenta and Fusobacterium nucleatum, which produce toxins by metabolizing aromatic amino acids [75]. Supplementation with probiotics diminishes the abundance of these bacterial groups, decreases toxicity, and enhances kidney function [75]. In addition, the gut microbiota can modulate metabolic and immune homeostasis, which can reduce complications in ESRD patients, such as constipation and cardiovascular diseases [73, 108].
Moreover, altered gut microbiota in hemodialysis patients has also been recognized as a potential risk factor for mortality [109], indicating the necessity of recognizing gut microbiota as a novel therapeutic target for the management of ESRD. Dialysis is the treatment modality of choice for patients with ESRD. Emerging evidence has highlighted the association between gut microbiota and dialysis. For instance, the gut microbiota profile of patients undergoing hemodialysis has been reported to be characterized mainly by a dramatic decrease in Bacteroidetes and an increase in Pseudomonas [74]. Various gut microbiota are also found in patients treated with different dialysis therapies such as hemodialysis and peritoneal dialysis [110]. In addition, dialysis induces alterations in gut microbiota metabolism, consequently affecting the efficiency of dialysis [111]. Together, these findings highlight the critical function of the gut microbiota in the disease progression and therapeutic control of ESRD.
2.5 Interaction between gut microbiota and RCC
2.5.1 Impact of RCC on gut microbiota
RCC is a malignant transformation of tubular epithelial cells, and is the most common and fatal urological neoplasm [112]. Recently, alterations in the gut microbiota of patients with RCC have been emphasized. Compared to controls, the abundance of Bacteroides and Akkermansia was significantly increased, and the abundance of Blautia, Bifidobacterium, and Megamonas was significantly decreased in patients with RCC [113]. This suggests that RCC may affect the gut microbiota.
2.5.2 Involvement of gut microbiota in RCC
The occurrence and development of RCC are considered to be a multifactorial process, and the gut microbiota may serve as a risk factor for RCC. Previous studies have indicated that the gut microbiota can induce tumorigenesis via several major mechanisms. First, pathogenic microorganisms can directly invade host cells, resulting in cell damage and affecting genome integrity, cell death, and proliferation signaling. These modifications can facilitate the conversion of normal cells into tumor cells [114]. Second, pathogenic microorganisms can induce local tissue inflammation by releasing inflammatory molecules such as ROS, reactive nitrogen species (RNS), cytokines, and chemokines secreted by immune cells, which promote tumor growth and metastasis [115]. Third, specific microorganisms can prevent the activation of immune cells, resulting in immune dysfunction, allowing the tumor to escape destruction by the host immune system [115]. Fourth, pathogenic microorganisms can produce biologically active products or secretions that change the living environment of host cells and destroy the normal biological barrier of host cells, which can also cause tumor development through the host circulatory system away from the microbial growth site [116-118]. These findings provide important information concerning the role of gut microbiota in the development and progression of RCC.
In the treatment of RCC, the association of gut microbiota with the immune microenvironment and immunotherapies has been reported [119]. For instance, Akkermansia muciniphila and Bacteroides salyersiae have been shown to activate CD4+ and CD8+ T cells, reshape the immune microenvironment, and elicit systemic immune responses, thereby enhancing the antitumor efficacy of immunotherapy [120]. Furthermore, Bifidobacterium spp may enhance the efficacy of PD-1/PD-L1 inhibitors by activating antigen-presenting cells and promoting Th1 immune responses [121]. The gut microbiota modulates the immune microenvironment of RCC and the response to immune checkpoint inhibitors (ICIs) largely via its metabolites. SCFAs, common gut microbiota metabolites, influence T cell function, suppress inflammation, and induce tumor cell apoptosis [122]. For example, the abundance of Akkermansia muciniphila is increased in RCC patients who experience clinical benefit from ICIs therapy [119]. This bacterium produces SCFAs, which recruit CD4+ T cells and dendritic cells via an IL-12-mediated mechanism, thereby enhancing the efficacy of ICIs in RCC [123]. Firmicutes also produce SCFAs, which significantly improve treatment responses and thereby enhance clinical outcomes in RCC patients [119, 124]. Furthermore, Bacteroides can modulate the response to ICIs in RCC patients by inducing a T cell-mediated adaptive immune response through the secretion of capsular polysaccharides [124]. Gut microbiota dysbiosis leads to an increase in the tryptophan metabolite kynurenine. Kynurenine binds to and activates the AHR. On one hand, this activation drives epithelial-mesenchymal transition in renal cancer cells, enhancing their migration, invasion, and suppressing apoptosis. On the other hand, sustained AHR activation suppresses anti-tumor immune functions, such as T cell function, thereby promoting tumor immune evasion and metastasis, as well as facilitating the formation of an immunosuppressive microenvironment in RCC [113]. In summary, the gut microbiota influences both the immune microenvironment of RCC and the response to ICIs through immune and metabolic mechanisms.
3. Strategies targeting gut microbiota
3.1 Probiotics, prebiotics and synbiotics
Probiotics have attracted increasing attention as therapeutic agents for gut microbiota dysbiosis and kidney diseases. As described above, the gut barrier deteriorates in AKI, leading to the translocation of metabolites into the systemic circulation, leading to systemic inflammation and kidney dysfunction. Probiotics can modulate gut microbiota, which is helpful in decreasing systemic inflammation and toxin translocation in patients with AKI. Some probiotics may mitigate gut microbiota dysbiosis and inhibit proinflammatory cytokines [19]. For instance, the probiotic Bifidobacterium has been described as a potential method to mitigate AKI by modulating the gut microbiota, lowering gut inflammation, and improving the intestinal barrier [125]. In addition, the association between dysbiosis of the gut microbiota and CKD is complex. The inefficient clearance and excessive accumulation of uremic toxins produced by gut microbiota dysbiosis are pathogenic factors in CKD progression [126, 127]. Patients with CKD have been reported to have gut microbiota dysbiosis, which is manifested by a low level of beneficial bacteria and a high level of pathogenic bacteria. Probiotic bacteria can manipulate gut microbiota and suppress the generation of uremic toxins. Probiotics, such as Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium longum, have been found to improve kidney function, reduce the concentration of uremic toxins, increasing the quality of life in patients with CKD [128]. In addition, probiotics may reduce gut inflammation and regulate other complications associated with CKD [129]. Although these studies demonstrated the potential benefits of probiotics, more clinical trials are required to confirm long-term protection against kidney disease and to determine active therapeutic protocols.
Indigestible fibers, which belong to the category of prebiotics, have gained considerable interest with regard to the management of kidney diseases. It has been demonstrated that oligofructose-enriched inulin is a prebiotic that elevates the production of SCFA in the circulation and modulates the level of serum inflammation associated with the modification of the composition of the gut microbiota in CKD rats [130]. They may also reduce the concentration of uremic toxins and the activity of proinflammatory cytokines, leading to significant enhancement of kidney function and retardation of CKD progression [131]. In addition, clinical trials have reported that inulin-type prebiotics reduce serum uric acid levels in patients with ESRD via the extrarenal pathway, including elevated uric acid degradation and increased purine-degrading species in the gut [132]. Other studies have shown increased abundance of beneficial gut bacteria and a decreased abundance of pathogenic bacteria following oligofructose-enriched inulin supplementation, likely resulting in reduced gut-derived toxin levels and improved metabolic profiles in patients with CKD [133]. These studies emphasize the therapeutic effect of prebiotics against kidney diseases, which is partly mediated by modifying the gut microenvironment for the proliferation of beneficial bacteria.
Furthermore, synbiotics have great potential for modification of gut microbiota and affect host health. Synbiotics have the advantages of both probiotics and prebiotics to better colonize and function as beneficial bacteria. They are effective against metabolic diseases and chronic inflammation and enhance general gastrointestinal health. For instance, synbiotic therapy, combining high-molecular-weight inulin, fructo-oligosaccharides, and galacto-oligosaccharides as prebiotics with nine probiotic strains from Lactobacillus, Bifidobacteria, and Streptococcus genera, effectively reduces serum levels of IS and PCS in patients with CKD by restoring the gut microbiota composition [134]. Other reports have also suggested that long-term use of synbiotics increases the abundance of beneficial bacteria, prevents kidney injury, and reduces serum inflammatory markers [135]. Nonetheless, synbiotics face many hurdles in their clinical application. This is an intricate process underlying the matching of probiotics with prebiotics, as using different strains tends to produce diverse effects based on their individual attributes and disease states. For example, although synbiotics have been shown to improve gut microbiota diversity and stability in several clinical trials, they may have negative effects on body weight and metabolism [136]. Moreover, the viability of probiotics, the efficacy of prebiotics, and their stability within the gut are critical factors determining the effectiveness of synbiotics. Clinical trials have shown that probiotics are highly susceptible to gastric acid and bile as they migrate through the gastrointestinal tract. Thus, there is an urgent need to design stable carriers for the formulation of synbiotic products [137].
3.2 Fecal microbiota transplantation
3.2.1 Application of FMT in kidney disease
FMT, an innovative therapeutic approach, aims to rebalance the gut microbiota by transferring healthy donor microbes and has been demonstrated to have remarkable potential for the treatment of a wide range of diseases [138-140]. With the development of research related to the application of FMT in kidney diseases, especially AKI, CKD, and ESRD, several positive findings have emerged. The reconstitution of healthy gut microbiota may be an attractive therapeutic opportunity to either mitigate the progression of microbiota-related diseases or restore kidney health [141]. FMT has been shown to help decrease intestinal permeability, repair gut barrier function, and reduce systemic inflammation in AKI [142]. Furthermore, FMT alleviates kidney inflammation in CKD by enhancing the production of SCFAs [23]. In another CKD model study, FMT enriched the gut microbiota diversity, reduced uremic toxin levels, and improved kidney function [143]. Clinical trials in patients with CKD have also shown that the levels of uremic toxins are reduced after [77]. In patients with ESRD, immunosuppressive treatments and antibiotics disrupt the gut microbiota, thereby increasing the risk of infection and transplant rejection [144]. FMT may reduce the risk of transplant rejection in patients with ESRD by restoring gut microbial balance and attenuating systemic inflammation [145]. FMT is a promising new therapy for establishing normal gut microbiota in patients with kidney disease. It has potential uses in AKI, CKD, and ESRD. However, current studies primarily use animal models and there have been few clinical trials exploring its applications. Therefore, further confirmation through large-scale clinical studies is required.
3.2.2 Feasibility and ethical challenges of FMT
The use of FMT for the treatment of kidney diseases is highly feasible. FMT not only plays a role in ameliorating kidney function by modulating the gut microbiota but is also beneficial for the regulation of body metabolism and immunity. However, the safety and efficacy of FMT must be confirmed using standardized donor screening and transplantation procedures. Under strict selection of healthy donors and standardized procedures, FMT may provide a novel therapy for kidney diseases [23].
Despite the therapeutic potential of FMT, its implementation remains fraught with multiple ethical issues. FMT requires the extensive and careful screening of donors to avoid passing on infection and diseases. Nevertheless, there may still be defects in the current screening protocols, and infections may not be completely prevented even after screening [141]. Donor screening is also associated with personal privacy issues, for which a high level of protection of donor information is critical. Furthermore, the safety of FMT has not been extensively confirmed in patients with kidney disease. The affected immune function in patients on immunosuppressive therapy might make such patients more vulnerable to infections post-FMT, resulting instead in an aggravation of the original disease. This risk is particularly pronounced in patients with ESRD on immunosuppressive regimens, who may face a substantially higher risk of FMT-related infections compared with other patient populations. ESRD is characterized by chronic uremia, which itself causes profound immune dysregulation [146, 147]. Additionally, many ESRD patients are treated with corticosteroids, calcineurin inhibitors, or other immunosuppressive agents, further compromising their immune defenses [148]. These individuals often present with multiple comorbidities such as diabetes and cardiovascular disease, which independently increase the risk of infection [149, 150]. In ESRD patients, impaired intestinal barrier integrity [146] and immunosuppression create a condition that favors bacterial translocation [151, 152]. In this context, even commensal or low-pathogenicity microorganisms from FMT could breach the compromised barrier, causing bacteremia or invasive sepsis. Thus, in patients undergoing immunosuppression, such as those with ESRD, extra caution is required when administering FMT. Further studies are needed before the clinical application of FMT. Finally, another ethical concern arising from the use of FMT is informed consent. Effective FMT is still under investigation; thus, patients must be counseled on potential risks [23]. Such challenges may eventually hinder the application of FMT as a potential treatment for kidney disease, unless mitigation strategies are established at the outset. These strategies should range from rigorous research on long-term safety and efficacy to stringent regulatory oversight.
3.3 Antibiotics and adsorbents
Gut-targeted drugs, such as antibiotics and adsorbents, are gut-modulating agents and potential drugs for kidney function. For example, in pediatric patients with sepsis-induced AKI, the concomitant use of metronidazole and sulbactam decreased AKI biomarker levels [153]. However, they target healthy gut microbiota, not only facilitating the emergence of antibiotic resistance but also inducing immune homeostatic disruption. Additionally, the therapeutic effect of drugs is influenced by the modulation of the gut microbiota by antibiotics. In patients with kidney cancer, a history of antibiotic use is associated with reduced benefit of immunotherapy [121]. Furthermore, the adsorbent may interact with the gut microbiota metabolites and prevent their absorption into the circulatory system. Some adsorbents, such as AST-120, may contribute to the protection of the gut barrier, suppression of intestinal permeability to toxins, and reduction of kidney burden through manipulation of the gut microbiota in CKD [154]. In summary, gut-directed therapies constitute new avenues for the treatment of kidney disease, particularly those aimed at decreasing gut-derived uremic toxins and remodeling the gut microbiota.
Therapeutic strategies targeting gut microbiota.
| Strategies | Diseases | Application examples | Reference |
|---|---|---|---|
| Probiotics | AKI | Bifidobacterium mitigates AKI through regulation of the gut microbiota, inhibition of intestinal inflammation, and restoration of the intestinal barrier. | [125] |
| Probiotics | CKD | Probiotic supplementation with Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium longum improves kidney function and reduces serum uremic toxin levels in CKD patients. | [128] |
| Prebiotics | CKD | Oligofructose-enriched inulin can increase circulating SCFA levels, modulate serum inflammation by altering the composition of the gut microbiota. | [130] |
| Prebiotics | ESRD | Inulin-type prebiotics reduced serum uric acid levels in ESRD patients by modulating the gut microbiota. | [132] |
| Prebiotics | CKD | Oligofructose-enriched inulin improves the gut microbiota dysbiosis and its metabolic profile in patients with CKD. | [133] |
| Synbiotics | CKD | Synbiotic therapy reduces serum IS and PCS levels and inflammation in patients with CKD by restoring the gut microbiota composition. | [134] |
| FMT | AKI | FMT decreases intestinal permeability, repairs gut barrier function, and reduces systemic inflammation in AKI. | [142] |
| FMT | CKD | FMT improves kidney function in CKD patients by modulating the gut microbiota, enhancing the production of SCFAs, and reducing uremic toxin levels. | [23, 143] |
| FMT | ESRD | FMT restores the balance of gut microbiota and reduces systemic inflammation, potentially lowering the risk of transplant rejection in patients with ESRD. | [145] |
| Antibiotics | AKI | The combination of metronidazole and sulbactam can reduce the levels of AKI markers. | [153] |
| Adsorbents | CKD | AST-120 is an oral adsorbent that reduces the progression of CKD by adsorbing uremic toxins and their precursors in the gut. | [154] |
| Low-protein diets | CKD | A low-protein diet promotes the proliferation of beneficial bacteria, such as Lactobacillaceae and Bacteroidaceae. | [155] |
| Low-protein diets | CKD | A low-protein diet reduces the production of IS and PCS. | [156] |
| Dietary fiber | DKD | SCFAs produced by dietary fiber fermentation slow DKD progression. | [157] |
| Dietary fiber | CKD | A diet rich in dietary fiber enriches SCFA-producing bacteria, and the SCFAs generated by these bacteria can alleviate kidney inflammation and fibrosis. | [158, 159] |
AKI, acute kidney injury; CKD, chronic kidney disease; DKD, diabetic kidney disease; ESRD, end-stage renal disease; IS, indoxyl sulfate; PCS, p-cresol sulfate; SCFAs, short chain fatty acids.
3.4 Dietary interventions
Feeding a specialized low-protein and high-fiber diet can beneficially influence gut microbiota profiles and contribute to kidney health. Rebalancing the gut microbiota with low-protein diets has been reported to stimulate the proliferation of beneficial bacteria, such as Lactobacillaceae and Bacteroidaceae [155]. Furthermore, research has shown that low-protein diets decrease the intake of proteins and may decrease the production of IS and PCS [156]. SCFAs are produced by the fermentation of dietary fiber in the colon and are crucial for slowing the progression of DKD [157]. A diet abundant in dietary fiber can theoretically enhance the production of SCFA-producing bacteria. Subsequently, the SCFAs generated by these fermentative bacteria interact with their receptors, thereby mitigating kidney inflammation and fibrosis in CKD [158, 159]. Moreover, SCFAs can promote Treg cell differentiation, which may modulate kidney inflammation [160].
In summary, uremic toxin levels could be reduced using low-protein and high-fiber diets, potentially mediated by the modulation of gut microbiota metabolic pathways, which could ultimately benefit the metabolic and anti-inflammatory functions of the kidney. Furthermore, apart from their potential kidney protective effects, these diets offer an effective non-pharmacological therapeutic modality for patients (Table 3 and Figure 4).
3.5 The dual role of SCFAs in kidney disease
Targeting the gut microbiota to modulate their metabolites represents a promising therapeutic strategy. Although multiple studies have confirmed that increasing SCFA levels through microbiota modulation can ameliorate kidney disease, SCFAs do not always exert therapeutic effects. On the contrary, under specific concentrations and pathological conditions, they may exhibit detrimental effects. In immune regulation, high concentrations of butyrate not only fail to suppress TNF-α but also promote the expression of IL-1β while inhibiting the anti-inflammatory cytokine IL-10, ultimately demonstrating a pro-inflammatory effect. It can also induce macrophage death, which exacerbates the inflammation [161]. Furthermore, low concentrations of butyrate help enhance intestinal barrier integrity, whereas high concentrations may disrupt the barrier structure by inducing epithelial cell apoptosis, promoting immune cell migration, and triggering local inflammation [162]. These findings suggest that SCFAs may have an "unfriendly" aspect in the context of kidney diseases. Some studies have pointed out that long-term exposure to SCFA concentrations above physiological levels can impair kidney structure and function. The underlying mechanism involves SCFAs-induced mTOR activation, which promotes the infiltration of inflammatory T cells (Th1/Th17) into kidney tissues. This initiates a cascade of chronic inflammation, fibrosis, and cellular proliferation, culminating in obstructive uropathy and kidney injury [163]. In DKD, elevated acetate activates the RAS, inducing glomerular hypertension, proteinuria and tubulointerstitial injury, which accelerates CKD progression. SCFAs may also synergize with other uremic toxins to collectively promote kidney inflammation and fibrosis [91, 93]. Furthermore, acetate can promote the expression of proteins involved in cholesterol synthesis and uptake in renal tubular epithelial cells, leading to lipid accumulation and interstitial fibrosis [95].
Targeting gut microbiota as a therapeutic strategy for kidney diseases. Multiple gut microbiota-targeted therapeutic approaches can be employed to treat kidney diseases, including probiotics, prebiotics, synbiotics, FMT, antibiotics, adsorbents, and dietary interventions.
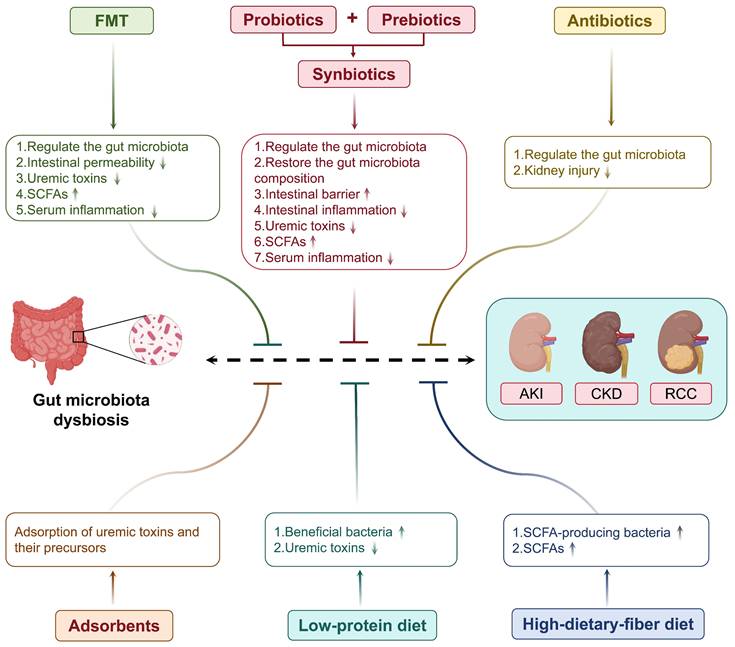
In summary, SCFAs exert a complex dual role in kidney disease, with outcomes dictated by concentration and pathological context. At appropriate levels, they help maintain the intestinal barrier and modulate immune function. Conversely, elevated concentrations or specific disease states can shift their function toward promoting kidney injury via pro-inflammatory, pro-fibrotic and lipid-dysregulating pathways. Future interventions targeting SCFAs must carefully consider their dose-response relationships and systemic physiological context to avoid potential adverse effects.
4. Personalized medicine, challenges and future research
4.1 Personalized medicine
Personalized gut microbiota detection and regulation have attracted considerable attention. According to previous studies, variations in the composition and functions of the gut microbiota exist among individuals due to differences in genetics, diet, and age, consequently causing different therapeutic responses [164]. Accordingly, regulation of the gut microbiota can provide personalized treatment for patients. For example, differences in the gut microbiota among cancer patients can determine drug activity and adverse effects of targeted therapy [165], and modulation of the microbiota may enhance therapeutic efficacy and reduce side effects [166]. Similarly, kidney disease also has considerable potential for individualized detection and regulation of the gut microbiota. Some gut microbes have been identified as major contributors to kidney disease. For example, enhanced Enterobacteriaceae and Bacteroides are significantly associated with a decline in kidney function, whereas a reduction in beneficial bacteria such as Lactobacillus is correlated with the loss of kidney-protective actions [77, 167]. Furthermore, the gut microbiota is associated with rejection responses in kidney transplant patients [168]. Thus, tailored gut microbiota composition analysis may not only be used as a promising early diagnostic biomarker but also as a foundation for defining personalized therapeutic approaches.
4.2 Challenges in clinical application
However, the clinical application of the gut microbiota in kidney disease still faces some challenges. Gut microbiota shows differences between individuals, which makes development of personalized treatment strategies challenging, which is the first problem. In addition, differences in the strains and dosages of probiotics used in studies make the results of clinical trials irreproducible, which complicates the creation of standardized treatment guidelines [169]. More importantly, the long-term safety of gut microbiota regulatory strategies remains to be fully established. This issue is of paramount concern for patients with severe disease or immunosuppression. Individuals with uremia or on immunosuppressive therapy often have impaired immunity, increasing their vulnerability to infections linked to FMT. First, despite rigorous donor screening, the risk of transmitting undetectable pathogens cannot be entirely eliminated. Such pathogens, while potentially harmless to healthy individuals, can cause severe and persistent infections in these patients. Secondly, the FMT procedure itself may induce bacterial translocation, increasing the risk of bacteremia and sepsis, especially in patients who already have intestinal wall edema and compromised barrier function. More insidiously, there is a risk of colonization by multidrug-resistant organisms. If undetected resistant bacteria from a donor establish a long-term reservoir in the recipient's gut, it could compromise future antibiotic treatments for subsequent infections, creating a persistent latent health risk. Finally, for immunocompromised patients, FMT poses a unique long-term immune risk by introducing donor immune cells that may launch a delayed, potentially fatal attack on host. To mitigate these risks, rigorous donor selection, proactive surveillance, and long-term follow-up are essential. In conclusion, despite its promise for treating kidney disease, gut microbiota modulation requires solutions to key challenges in standardization and safety. The development of standardized guidelines and rigorous clinical trials to confirm efficacy and safety is essential next steps before this therapy can be widely adopted.
4.3 Future research
Emerging evidence suggests that gut microbiota characteristics can predict treatment response in kidney disease patients, going beyond their role as diagnostic biomarkers. For instance, gut microbiota dysbiosis in CKD patients is correlated with disease severity, and gut microbiota characteristics show potential as prognostic biomarkers [66]. Research on IgAN provides strong supporting evidence. An analysis of fecal microbiota from 55 IgAN patients revealed a significant enrichment of Pseudomonas in non-responders, which was closely associated with poor treatment outcomes [170]. Moreover, in a recent prospective study of 69 patients with metastatic RCC treated with nivolumab, a higher response rate was linked to increased levels of Bacteroides salyersiae, Akkermansia muciniphila, and Eubacterium siraeum, along with a lower abundance of Clostridium clostridioforme and C. hathewayi in the fecal microbiota [171]. In summary, the characteristics of the gut microbiota show promise in predicting responses to specific therapies in patients with kidney diseases.
Beyond bacteria, fungi significantly contribute to the microbiota-gut-kidney axis. Despite constituting a minor fraction of the gut microbiota, they regulate gut microecology through antagonistic or synergistic interactions with bacteria, which is essential for maintaining gut microbiota homeostasis [172]. A key mechanism of their impact on host health is through immune modulation [173, 174]. Fungi such as Candida, Saccharomyces, and Aspergillus can impair the intestinal barrier, allowing components like β-glucan to enter circulation. This promotes systemic inflammation and aggravates kidney injury in CKD [175]. Conversely, Paecilomyces cicadae can improve DKD in mice by modulating the gut microbiota via the fermentation of astragalus [176, 177]. Collectively, fungi play multiple roles in the regulation of kidney health. As another common gut microbe, viruses can infect bacteria to regulate the composition and metabolism of the gut microbiota. This process indirectly influences uremic toxin production and immune homeostasis, which is particularly relevant in CKD patients [178]. Furthermore, some viruses carry auxiliary metabolic genes that alter gut microbiota metabolism, including bile acid production, thereby modulating host immunity and triggering systemic inflammation in CKD [178]. Enterovirus infection significantly increases the risk of nephrotic syndrome in children. Notably, Coxsackievirus may induce the disease by either modulating host immunity or directly damaging glomerular cells [179]. Although research on fungi and viruses in the microbiota-gut-kidney axis is still nascent, they may emerge as novel targets for the diagnosis and intervention of kidney diseases in the future.
5. Conclusion
The function of the microbiota-gut-kidney axis has attracted increasing attention in basic research and clinical practice. Kidney health is related to the gut microbiota, which can affect kidney physiology through the immune and inflammatory pathways. Gut microbiota dysbiosis is a manifestation of kidney disease and is one of the key regulators of its development. Modulation of gut microbiota may provide promising therapeutic opportunities for kidney diseases. Gut microbiota-targeted therapies such as probiotics, prebiotics, synbiotics, and FMT can restore the gut microbiota and ameliorate both inflammation and gut microbiota-derived uremic toxins. Research on individualized treatment strategies is ongoing. Precise assessment of the gut microbiota of each patient allows the development of tailored approaches to modulate it in order to enhance therapeutic efficacy. Although these results are encouraging, more clinical evidence is required to validate the efficacy and safety of these strategies, which are widely accepted as standard treatments. In summary, the modulation of the gut microbiota is a promising therapeutic strategy for treating kidney diseases. However, before these techniques can be safely and effectively introduced into the clinic, issues regarding standardization and ethics must be addressed in future studies.
Abbreviations
AKI: acute kidney injury; CKD: chronic kidney disease; RCC: renal cell carcinoma; FMT: fecal microbiota transplantation; IS: indoxyl sulfate; PCS: p-cresol sulfate; TMAO: trimethylamine N-oxide; AGEs: Advanced Glycation End Products; AHR: aryl hydrocarbon receptor; ROS: reactive oxygen species; SCFAs: short chain fatty acids; I/R: ischemia/reperfusion; NF-κB: nuclear factor-kappa B; IPA: indole-3-propionic acid; GFR: glomerular filtration rate; DKD: diabetic kidney disease; H-CKD: hypertensive nephropathy; ESRD: end-stage renal disease; T1D: type 1 diabetes; T2D: type 2 diabetes; FXR: farnesoid X receptor; TGR5: Takeda G protein-coupled receptor 5; RAS: renin-angiotensin system; HDAC: histone deacetylase; GPRs: G protein-coupled receptors; PS: phenyl sulfate; RNS: reactive nitrogen species; ICIs: immune checkpoint inhibitors.
Acknowledgements
We would like to thank Biorender Platform (https://www.biorender.com) for drawing assistance.
Funding
This work was supported by the National Natural Science Foundation of China (No.82270738), the National Key R&D Program (2022YFC2502503).
Author contributions
SZH, GQ and WZL read a large number of relevant literatures and drafted the manuscript; LC, WJN, YJT and JML participated in the review and revision of the manuscript; ZDF, WJ, XSS, ZFJ, LXY, SXG, QZ and JJ eventually edited the manuscript; SW, PP and MXM supervised and advanced the process. All authors contributed to manuscript revision, read, and approved the submitted version.
Competing interests
The authors have declared that no competing interest exists.
References
1. Zhu Z, Hu J, Chen Z, Feng J, Yang X, Liang W. et al. Transition of acute kidney injury to chronic kidney disease: role of metabolic reprogramming. Metabolism. 2022;131:155194
2. Miguel V, Shaw IW, Kramann R. Metabolism at the crossroads of inflammation and fibrosis in chronic kidney disease. Nat Rev Nephrol. 2025;21:39-56
3. Qu L, Jiao B. The Interplay between Immune and Metabolic Pathways in Kidney Disease. Cells. 2023 12
4. Matsuura R, Doi K, Rabb H. Acute kidney injury and distant organ dysfunction-network system analysis. Kidney Int. 2023;103:1041-55
5. Zoccali C, Tripepi G, Dounousi E, Mallamaci F. Chronic kidney disease (CKD) as a systemic disease: whole body autoregulation and inter-organ cross-talk. Kidney Blood Press Res. 2014;39:134-41
6. Nigam SK, Bush KT. Uraemic syndrome of chronic kidney disease: altered remote sensing and signalling. Nat Rev Nephrol. 2019;15:301-16
7. Martin AJM, Serebrinsky-Duek K, Riquelme E, Saa PA, Garrido D. Microbial interactions and the homeostasis of the gut microbiome: the role of Bifidobacterium. Microbiome Res Rep. 2023;2:17
8. Minaya DM, Kim JS, Kirkland R, Allen J, Cullinan S, Maclang N. et al. Transfer of microbiota from lean donors in combination with prebiotics prevents excessive weight gain and improves gut-brain vagal signaling in obese rats. Gut Microbes. 2024;16:2421581
9. Lv J, Lang G, Wang Q, Zhao W, Shi D, Zhou Z. et al. Lactobacillus helveticus attenuates alcoholic liver injury via regulation of gut microecology in mice. Microb Biotechnol. 2024;17:e70016
10. Knauf F, Brewer JR, Flavell RA. Immunity, microbiota and kidney disease. Nat Rev Nephrol. 2019;15:263-74
11. Crudele L, Gadaleta RM, Cariello M, Moschetta A. Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. EBioMedicine. 2023;97:104821
12. Liu BN, Liu XT, Liang ZH, Wang JH. Gut microbiota in obesity. World J Gastroenterol. 2021;27:3837-50
13. Qiu P, Ishimoto T, Fu L, Zhang J, Zhang Z, Liu Y. The Gut Microbiota in Inflammatory Bowel Disease. Front Cell Infect Microbiol. 2022;12:733992
14. Pluznick JL. The gut microbiota in kidney disease. Science. 2020;369:1426-7
15. Wang H, Ainiwaer A, Song Y, Qin L, Peng A, Bao H. et al. Perturbed gut microbiome and fecal and serum metabolomes are associated with chronic kidney disease severity. Microbiome. 2023;11:3
16. Beker BM, Colombo I, Gonzalez-Torres H, Musso CG. Decreasing microbiota-derived uremic toxins to improve CKD outcomes. Clin Kidney J. 2022;15:2214-9
17. Ni Y, Yang W, Wang S, Pan Y, Du H, Zheng L. et al. Modified huangfeng decoction alleviates diabetic nephropathy by activating autophagy and regulating the gut microbiota. Phytomedicine. 2025;141:156677
18. Hsieh MC, Tsai WH, Jheng YP, Su SL, Wang SY, Lin CC. et al. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: a randomized, double-blinded, placebo-controlled trial. Sci Rep. 2018;8:16791
19. Zhu H, Cao C, Wu Z, Zhang H, Sun Z, Wang M. et al. The probiotic L. casei Zhang slows the progression of acute and chronic kidney disease. Cell Metab. 2021;33:1926-42 e8
20. He M, Wei W, Zhang Y, Xiang Z, Peng D, Kasimumali A. et al. Gut microbial metabolites SCFAs and chronic kidney disease. J Transl Med. 2024;22:172
21. Li F, Wang M, Wang J, Li R, Zhang Y. Alterations to the Gut Microbiota and Their Correlation With Inflammatory Factors in Chronic Kidney Disease. Front Cell Infect Microbiol. 2019;9:206
22. Cooper TE, Khalid R, Chan S, Craig JC, Hawley CM, Howell M. et al. Synbiotics, prebiotics and probiotics for people with chronic kidney disease. Cochrane Database Syst Rev. 2023;10:CD013631
23. Bian J, Liebert A, Bicknell B, Chen XM, Huang C, Pollock CA. Faecal Microbiota Transplantation and Chronic Kidney Disease. Nutrients. 2022 14
24. Yu M, Li L, Ren Q, Feng H, Tao S, Cheng L. et al. Understanding the Gut-Kidney Axis in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: An Analysis of Gut Microbiota Composition. Front Pharmacol. 2022;13:783679
25. Yang Y, Wu C. Traditional Chinese Medicine in Ameliorating Diabetic Kidney Disease Via Modulating Gut Microbiota. 2021; 8: 8.
26. Hou H, Horikawa M, Narita Y, Jono H, Kakizoe Y, Izumi Y. et al. Suppression of Indoxyl Sulfate Accumulation Reduces Renal Fibrosis in Sulfotransferase 1a1-Deficient Mice. Int J Mol Sci. 2023 24
27. Di Iorio BR, Rocchetti MT, De Angelis M, Cosola C, Marzocco S, Di Micco L. et al. Nutritional Therapy Modulates Intestinal Microbiota and Reduces Serum Levels of Total and Free Indoxyl Sulfate and P-Cresyl Sulfate in Chronic Kidney Disease (Medika Study). J Clin Med. 2019 8
28. Wei CW, Wu TK, Wu SC, Chen YL, Pan YR, Chien YC. et al. Curcumin enhances p-cresyl sulfate-induced cytotoxic effects on renal tubular cells. Int J Med Sci. 2022;19:1138-46
29. Koppe L, Pillon NJ, Vella RE, Croze ML, Pelletier CC, Chambert S. et al. p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol. 2013;24:88-99
30. Zixin Y, Lulu C, Xiangchang Z, Qing F, Binjie Z, Chunyang L. et al. TMAO as a potential biomarker and therapeutic target for chronic kidney disease: A review. Front Pharmacol. 2022;13:929262
31. Zhang W, Miikeda A, Zuckerman J, Jia X, Charugundla S, Zhou Z. et al. Inhibition of microbiota-dependent TMAO production attenuates chronic kidney disease in mice. Sci Rep. 2021;11:518
32. Ma Y, Wang X, Lin S, King L, Liu L. The Potential Role of Advanced Glycation End Products in the Development of Kidney Disease. Nutrients. 2025 17
33. Lee TH, Park D, Kim YJ, Lee I, Kim S, Oh CT. et al. Lactobacillus salivarius BP121 prevents cisplatin-induced acute kidney injury by inhibition of uremic toxins such as indoxyl sulfate and p-cresol sulfate via alleviating dysbiosis. Int J Mol Med. 2020;45:1130-40
34. Saranya GR, Viswanathan P. Gut microbiota dysbiosis in AKI to CKD transition. Biomed Pharmacother. 2023;161:114447
35. Xie RC, Zhang JC, Lin XM, Huang T, Wang YT, Zhang LF. et al. Inhibition of colon C5a/C5a receptor signalling pathway confers protection against LPS-induced acute kidney injury via gut microbiota-kidney axis. Eur J Pharmacol. 2024;969:176425
36. Zhang J, Ankawi G, Sun J, Digvijay K, Yin Y, Rosner MH. et al. Gut-kidney crosstalk in septic acute kidney injury. Crit Care. 2018;22:117
37. Rabb H, Pluznick J, Noel S. The Microbiome and Acute Kidney Injury. Nephron. 2018;140:120-3
38. Yang J, Kim CJ, Go YS, Lee HY, Kim MG, Oh SW. et al. Intestinal microbiota control acute kidney injury severity by immune modulation. Kidney Int. 2020;98:932-46
39. Yang J, Ji GE, Park MS, Seong YJ, Go YS, Lee HY. et al. Probiotics partially attenuate the severity of acute kidney injury through an immunomodulatory effect. Kidney Res Clin Pract. 2021;40:620-33
40. Zou YT, Zhou J, Zhu JH, Wu CY, Shen H, Zhang W. et al. Gut Microbiota Mediates the Protective Effects of Traditional Chinese Medicine Formula Qiong-Yu-Gao against Cisplatin-Induced Acute Kidney Injury. Microbiol Spectr. 2022;10:e0075922
41. Shah N, Rabb H. Intestinal Microbiota in Experimental Acute Kidney Injury. Nephron. 2023;147:25-30
42. Emal D, Rampanelli E, Stroo I, Butter LM, Teske GJ, Claessen N. et al. Depletion of Gut Microbiota Protects against Renal Ischemia-Reperfusion Injury. J Am Soc Nephrol. 2017;28:1450-61
43. Li L, Ma L, Fu P. Gut microbiota-derived short-chain fatty acids and kidney diseases. Drug Des Devel Ther. 2017;11:3531-42
44. Chou YT, Kan WC, Shiao CC. Acute Kidney Injury and Gut Dysbiosis: A Narrative Review Focus on Pathophysiology and Treatment. Int J Mol Sci. 2022 23
45. Al-Harbi NO, Nadeem A, Ahmad SF, Alotaibi MR, AlAsmari AF, Alanazi WA. et al. Short chain fatty acid, acetate ameliorates sepsis-induced acute kidney injury by inhibition of NADPH oxidase signaling in T cells. Int Immunopharmacol. 2018;58:24-31
46. Lei J, Xie Y, Sheng J, Song J. Intestinal microbiota dysbiosis in acute kidney injury: novel insights into mechanisms and promising therapeutic strategies. Ren Fail. 2022;44:571-80
47. Shi X, Xing J, Wang Y, Li J, Chai R, Yu X. [Effect and related mechanism of acetate in alleviating acute kidney injury in septic rats through G-protein coupled receptor 43]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2024;36:1147-52
48. Felizardo RJF, Watanabe IKM, Dardi P, Rossoni LV, Camara NOS. The interplay among gut microbiota, hypertension and kidney diseases: The role of short-chain fatty acids. Pharmacol Res. 2019;141:366-77
49. Rydzewska-Rosolowska A, Sroka N, Kakareko K, Rosolowski M, Zbroch E, Hryszko T. The Links between Microbiome and Uremic Toxins in Acute Kidney Injury: Beyond Gut Feeling-A Systematic Review. Toxins (Basel). 2020 12
50. Wang W, Hao G, Pan Y, Ma S, Yang T, Shi P. et al. Serum indoxyl sulfate is associated with mortality in hospital-acquired acute kidney injury: a prospective cohort study. BMC Nephrol. 2019;20:57
51. Shen WC, Liang CJ, Huang TM, Liu CW, Wang SH, Young GH. et al. Indoxyl sulfate enhances IL-1beta-induced E-selectin expression in endothelial cells in acute kidney injury by the ROS/MAPKs/NFkappaB/AP-1 pathway. Arch Toxicol. 2016;90:2779-92
52. Veldeman L, Vanmassenhove J, Van Biesen W, Massy ZA, Liabeuf S, Glorieux G. et al. Evolution of protein-bound uremic toxins indoxyl sulphate and p-cresyl sulphate in acute kidney injury. Int Urol Nephrol. 2019;51:293-302
53. Falconi CA, Fogaca-Ruiz F, da Silva JV, Neres-Santos RS, Sanz CL, Nakao LS. et al. Renocardiac Effects of p-Cresyl Sulfate Administration in Acute Kidney Injury Induced by Unilateral Ischemia and Reperfusion Injury In Vivo. Toxins (Basel). 2023 15
54. Yisireyili M, Takeshita K, Saito S, Murohara T, Niwa T. Indole-3-propionic acid suppresses indoxyl sulfate-induced expression of fibrotic and inflammatory genes in proximal tubular cells. Nagoya J Med Sci. 2017;79:477-86
55. Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J. et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089-100
56. Hahn D, Hodson EM, Fouque D. Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst Rev. 2020;10:CD001892
57. Zou Y, Guo X, Liu S. Metabolites Mediate the Causal Associations Between Gut Microbiota and Chronic Kidney Disease: A Mendelian Randomization Study and Therapeutical Strategy from Traditional Chinese Medicine Perspective. 2025; 12: e24-00068.
58. Tao S, Li L, Li L, Liu Y, Ren Q, Shi M. et al. Understanding the gut-kidney axis among biopsy-proven diabetic nephropathy, type 2 diabetes mellitus and healthy controls: an analysis of the gut microbiota composition. Acta Diabetol. 2019;56:581-92
59. Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E. et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359:1376-83
60. Mori K, Suzuki T, Igarashi T, Inoue K, Asahara T, Nomoto K. et al. Persistent hyperglycemia modulates gut immune function and microbiota in rats. J Intensive Care. 2015;3:34
61. Zaky A, Glastras SJ, Wong MYW, Pollock CA, Saad S. The Role of the Gut Microbiome in Diabetes and Obesity-Related Kidney Disease. Int J Mol Sci. 2021 22
62. Higuchi BS, Rodrigues N, Gonzaga MI, Paiolo JCC, Stefanutto N, Omori WP. et al. Intestinal Dysbiosis in Autoimmune Diabetes Is Correlated With Poor Glycemic Control and Increased Interleukin-6: A Pilot Study. Front Immunol. 2018;9:1689
63. Li Q, Chang Y, Zhang K, Chen H, Tao S, Zhang Z. Implication of the gut microbiome composition of type 2 diabetic patients from northern China. Sci Rep. 2020;10:5450
64. Chavez-Carbajal A, Pizano-Zarate ML, Hernandez-Quiroz F, Ortiz-Luna GF, Morales-Hernandez RM, De Sales-Millan A. et al. Characterization of the Gut Microbiota of Individuals at Different T2D Stages Reveals a Complex Relationship with the Host. Microorganisms. 2020 8
65. Inoue R, Ohue-Kitano R, Tsukahara T, Tanaka M, Masuda S, Inoue T. et al. Prediction of functional profiles of gut microbiota from 16S rRNA metagenomic data provides a more robust evaluation of gut dysbiosis occurring in Japanese type 2 diabetic patients. J Clin Biochem Nutr. 2017;61:217-21
66. Hu X, Ouyang S, Xie Y, Gong Z, Du J. Characterizing the gut microbiota in patients with chronic kidney disease. Postgrad Med. 2020;132:495-505
67. Ren F, Jin Q, Liu T, Ren X, Zhan Y. Causal effects between gut microbiota and IgA nephropathy: a bidirectional Mendelian randomization study. Front Cell Infect Microbiol. 2023;13:1171517
68. Tan J, Dong L, Jiang Z, Tan L, Luo X, Pei G. et al. Probiotics ameliorate IgA nephropathy by improving gut dysbiosis and blunting NLRP3 signaling. J Transl Med. 2022;20:382
69. Zhao J, Bai M, Ning X, Qin Y, Wang Y, Yu Z. et al. Expansion of Escherichia-Shigella in Gut Is Associated with the Onset and Response to Immunosuppressive Therapy of IgA Nephropathy. J Am Soc Nephrol. 2022;33:2276-92
70. Zhu Y, He H, Sun W, Wu J, Xiao Y, Peng Y. et al. IgA nephropathy: gut microbiome regulates the production of hypoglycosilated IgA1 via the TLR4 signaling pathway. Nephrol Dial Transplant. 2024;39:1624-41
71. Li X, Wang L, Ma S, Lin S, Wang C, Wang H. Combination of Oxalobacter Formigenes and Veillonella Parvula in Gastrointestinal Microbiota Related to Bile-Acid Metabolism as a Biomarker for Hypertensive Nephropathy. Int J Hypertens. 2022;2022:5999530
72. Guan Y, Chen K, Quan D, Kang L, Yang D, Wu H. et al. The Combination of Scutellaria baicalensis Georgi and Sophora japonica L. ameliorate Renal Function by Regulating Gut Microbiota in Spontaneously Hypertensive Rats. Front Pharmacol. 2020;11:575294
73. Zhang P, Wang X, Li S, Cao X, Zou J, Fang Y. et al. Metagenome-wide analysis uncovers gut microbial signatures and implicates taxon-specific functions in end-stage renal disease. Genome Biol. 2023;24:226
74. Luo D, Zhao W, Lin Z, Wu J, Lin H, Li Y. et al. The Effects of Hemodialysis and Peritoneal Dialysis on the Gut Microbiota of End-Stage Renal Disease Patients, and the Relationship Between Gut Microbiota and Patient Prognoses. Front Cell Infect Microbiol. 2021;11:579386
75. Wang X, Yang S, Li S, Zhao L, Hao Y, Qin J. et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut. 2020;69:2131-42
76. Winichakoon P, Chaiwarith R, Chattipakorn N, Chattipakorn SC. Impact of gut microbiota on kidney transplantation. Transplant Rev (Orlando). 2022;36:100668
77. Garcia-Martinez Y, Borriello M, Capolongo G, Ingrosso D, Perna AF. The Gut Microbiota in Kidney Transplantation: A Target for Personalized Therapy? Biology (Basel). 2023 12
78. Tao P, Huo J, Chen L. Bibliometric Analysis of the Relationship between Gut Microbiota and Chronic Kidney Disease from 2001-2022. 2024; 11: e00017.
79. Ren Q, Cheng L, Guo F, Tao S, Zhang C, Ma L. et al. Fisetin Improves Hyperuricemia-Induced Chronic Kidney Disease via Regulating Gut Microbiota-Mediated Tryptophan Metabolism and Aryl Hydrocarbon Receptor Activation. J Agric Food Chem. 2021;69:10932-42
80. Voroneanu L, Burlacu A, Brinza C, Covic A, Balan GG, Nistor I. et al. Gut Microbiota in Chronic Kidney Disease: From Composition to Modulation towards Better Outcomes-A Systematic Review. J Clin Med. 2023 12
81. Bhargava S, Merckelbach E, Noels H, Vohra A, Jankowski J. Homeostasis in the Gut Microbiota in Chronic Kidney Disease. Toxins (Basel). 2022 14
82. Ebrahim Z, Proost S, Tito RY, Raes J, Glorieux G, Moosa MR. et al. The Effect of ss-Glucan Prebiotic on Kidney Function, Uremic Toxins and Gut Microbiome in Stage 3 to 5 Chronic Kidney Disease (CKD) Predialysis Participants: A Randomized Controlled Trial. Nutrients. 2022 14
83. Mo Y, Hu D, Yu W, Ji C, Li Y, Liu X. et al. Astragaloside IV attenuates indoxyl sulfate-induced injury of renal tubular epithelial cells by inhibiting the aryl hydrocarbon receptor pathway. J Ethnopharmacol. 2023;308:116244
84. Yoo JY, Groer M, Dutra SVO, Sarkar A, McSkimming DI. Gut Microbiota and Immune System Interactions. Microorganisms. 2020 8
85. Deng L, Yang Y, Xu G. Empagliflozin ameliorates type 2 diabetes mellitus-related diabetic nephropathy via altering the gut microbiota. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867:159234
86. Ni Y, Zheng L, Nan S, Ke L, Fu Z, Jin J. Enterorenal crosstalks in diabetic nephropathy and novel therapeutics targeting the gut microbiota. Acta Biochim Biophys Sin (Shanghai). 2022;54:1406-20
87. Wu X, Zhao L, Zhang Y, Li K, Yang J. The role and mechanism of the gut microbiota in the development and treatment of diabetic kidney disease. Front Physiol. 2023;14:1166685
88. Duan L, An X, Zhang Y, Jin D, Zhao S, Zhou R. et al. Gut microbiota as the critical correlation of polycystic ovary syndrome and type 2 diabetes mellitus. Biomed Pharmacother. 2021;142:112094
89. Sinha SR, Haileselassie Y, Nguyen LP, Tropini C, Wang M, Becker LS. et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microbe. 2020;27:659-70 e5
90. Mao ZH, Gao ZX, Liu DW, Liu ZS, Wu P. Gut microbiota and its metabolites - molecular mechanisms and management strategies in diabetic kidney disease. Front Immunol. 2023;14:1124704
91. Chi M, Ma K, Wang J, Ding Z, Li Y, Zhu S. et al. The Immunomodulatory Effect of the Gut Microbiota in Kidney Disease. J Immunol Res. 2021;2021:5516035
92. Jaworska K, Koper M, Ufnal M. Gut microbiota and renin-angiotensin system: a complex interplay at local and systemic levels. Am J Physiol Gastrointest Liver Physiol. 2021;321:G355-G66
93. Lu CC, Hu ZB, Wang R, Hong ZH, Lu J, Chen PP. et al. Gut microbiota dysbiosis-induced activation of the intrarenal renin-angiotensin system is involved in kidney injuries in rat diabetic nephropathy. Acta Pharmacol Sin. 2020;41:1111-8
94. Fang Q, Liu N, Zheng B, Guo F, Zeng X, Huang X. et al. Roles of Gut Microbial Metabolites in Diabetic Kidney Disease. Front Endocrinol (Lausanne). 2021;12:636175
95. Hu ZB, Lu J, Chen PP, Lu CC, Zhang JX, Li XQ. et al. Dysbiosis of intestinal microbiota mediates tubulointerstitial injury in diabetic nephropathy via the disruption of cholesterol homeostasis. Theranostics. 2020;10:2803-16
96. Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, Garcia-Perez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327-40
97. Li LZ, Tao SB, Ma L, Fu P. Roles of short-chain fatty acids in kidney diseases. Chin Med J (Engl). 2019;132:1228-32
98. Dupraz L, Magniez A, Rolhion N, Richard ML, Da Costa G, Touch S. et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal gammadelta T cells. Cell Rep. 2021;36:109332
99. Wang P, Wang T, Zheng X, Cui W, Shang J, Zhao Z. Gut microbiota, key to unlocking the door of diabetic kidney disease. Nephrology (Carlton). 2021;26:641-9
100. Cheung CK, Rajasekaran A, Barratt J, Rizk DV. An Update on the Current State of Management and Clinical Trials for IgA Nephropathy. J Clin Med. 2021 10
101. Barratt J, Rovin BH, Cattran D, Floege J, Lafayette R, Tesar V. et al. Why Target the Gut to Treat IgA Nephropathy? Kidney Int Rep. 2020;5:1620-4
102. Wang F, Li N, Ni S, Min Y, Wei K, Sun H. et al. The Effects of Specific Gut Microbiota and Metabolites on IgA Nephropathy-Based on Mendelian Randomization and Clinical Validation. Nutrients. 2023 15
103. Di Leo V, Gleeson PJ, Sallustio F, Bounaix C, Da Silva J, Loreto G. et al. Rifaximin as a Potential Treatment for IgA Nephropathy in a Humanized Mice Model. J Pers Med. 2021 11
104. Chemouny JM, Gleeson PJ, Abbad L, Lauriero G, Boedec E, Le Roux K. et al. Modulation of the microbiota by oral antibiotics treats immunoglobulin A nephropathy in humanized mice. Nephrol Dial Transplant. 2019;34:1135-44
105. Zhao J, Bai M, Yang X, Wang Y, Li R, Sun S. Alleviation of refractory IgA nephropathy by intensive fecal microbiota transplantation: the first case reports. Ren Fail. 2021;43:928-33
106. Robles-Vera I, Toral M, de la Visitacion N, Sanchez M, Romero M, Olivares M. et al. The Probiotic Lactobacillus fermentum Prevents Dysbiosis and Vascular Oxidative Stress in Rats with Hypertension Induced by Chronic Nitric Oxide Blockade. Mol Nutr Food Res. 2018;62:e1800298
107. Antza C, Stabouli S, Kotsis V. Gut microbiota in kidney disease and hypertension. Pharmacol Res. 2018;130:198-203
108. Sumida K, Yamagata K, Kovesdy CP. Constipation in CKD. Kidney Int Rep. 2020;5:121-34
109. Lin TY, Wu PH, Lin YT, Hung SC. Gut dysbiosis and mortality in hemodialysis patients. NPJ Biofilms Microbiomes. 2021;7:20
110. Simoes-Silva L, Araujo R, Pestana M, Soares-Silva I, Sampaio-Maia B. The microbiome in chronic kidney disease patients undergoing hemodialysis and peritoneal dialysis. Pharmacol Res. 2018;130:143-51
111. Bao M, Zhang P, Guo S, Zou J, Ji J, Ding X. et al. Altered gut microbiota and gut-derived p-cresyl sulfate serum levels in peritoneal dialysis patients. Front Cell Infect Microbiol. 2022;12:639624
112. Ninkov M, Schmerk CL, Moradizadeh M, Parvathy SN, Figueredo R, Burton JP. et al. Improved MAIT cell functions following fecal microbiota transplantation for metastatic renal cell carcinoma. Cancer Immunol Immunother. 2023;72:1247-60
113. Dai G, Chen X, He Y. The Gut Microbiota Activates AhR Through the Tryptophan Metabolite Kyn to Mediate Renal Cell Carcinoma Metastasis. Front Nutr. 2021;8:712327
114. Yang JW, Wan S, Li KP, Chen SY, Yang L. Gut and urinary microbiota: the causes and potential treatment measures of renal cell carcinoma. Front Immunol. 2023;14:1188520
115. Garrett WS. Cancer and the microbiota. Science. 2015;348:80-6
116. Wang H, Rong X, Zhao G, Zhou Y, Xiao Y, Ma D. et al. The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer. Cell Metab. 2022;34:581-94 e8
117. Hanus M, Parada-Venegas D, Landskron G, Wielandt AM, Hurtado C, Alvarez K. et al. Immune System, Microbiota, and Microbial Metabolites: The Unresolved Triad in Colorectal Cancer Microenvironment. Front Immunol. 2021;12:612826
118. He Y, Fu L, Li Y, Wang W, Gong M, Zhang J. et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 2021;33:988-1000 e7
119. Salgia NJ, Bergerot PG, Maia MC, Dizman N, Hsu J, Gillece JD. et al. Stool Microbiome Profiling of Patients with Metastatic Renal Cell Carcinoma Receiving Anti-PD-1 Immune Checkpoint Inhibitors. Eur Urol. 2020;78:498-502
120. Derosa L, Routy B, Fidelle M, Iebba V, Alla L, Pasolli E. et al. Gut Bacteria Composition Drives Primary Resistance to Cancer Immunotherapy in Renal Cell Carcinoma Patients. Eur Urol. 2020;78:195-206
121. Lalani AA, Xie W, Braun DA, Kaymakcalan M, Bosse D, Steinharter JA. et al. Effect of Antibiotic Use on Outcomes with Systemic Therapies in Metastatic Renal Cell Carcinoma. Eur Urol Oncol. 2020;3:372-81
122. Meza L, Feng M, Lee K, Sperandio R, Pal SK. The Gut Microbiome and Metastatic Renal Cell Carcinoma. J Clin Med. 2023 12
123. Deluce J, Maleki Vareki S, Fernandes R. The role of gut microbiome in immune modulation in metastatic renal cell carcinoma. Ther Adv Med Oncol. 2022;14:17588359221122714
124. Piao XM, Byun YJ, Zheng CM, Song SJ, Kang HW, Kim WT. et al. A New Treatment Landscape for RCC: Association of the Human Microbiome with Improved Outcomes in RCC. Cancers (Basel). 2023 15
125. Meng Y, Zhao M, Ma Q, Hua Q, Hu J, Zhou Q. et al. Bifidobacterium bifidum alleviates adenine-induced acute kidney injury in mice by improving intestinal barrier function. Food Funct. 2024;15:8030-42
126. Tian N, Li L, Ng JK, Li PK. The Potential Benefits and Controversies of Probiotics Use in Patients at Different Stages of Chronic Kidney Disease. Nutrients. 2022 14
127. Nguyen TTU, Kim HW, Kim W. Effects of Probiotics, Prebiotics, and Synbiotics on Uremic Toxins, Inflammation, and Oxidative Stress in Hemodialysis Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Clin Med. 2021 10
128. Huang HW, Chen MJ. Exploring the Preventive and Therapeutic Mechanisms of Probiotics in Chronic Kidney Disease through the Gut-Kidney Axis. J Agric Food Chem. 2024;72:8347-64
129. Zhou J, Li M, Chen Q, Li X, Chen L, Dong Z. et al. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat Commun. 2022;13:3432
130. Melekoglu E, Cetinkaya MA, Kepekci-Tekkeli SE, Kul O, Samur G. Effects of prebiotic oligofructose-enriched inulin on gut-derived uremic toxins and disease progression in rats with adenine-induced chronic kidney disease. PLoS One. 2021;16:e0258145
131. Anegkamol W, Kamkang P, Hunthai S, Kaewwongse M, Taweevisit M, Chuaypen N. et al. The Usefulness of Resistant Maltodextrin and Chitosan Oligosaccharide in Management of Gut Leakage and Microbiota in Chronic Kidney Disease. Nutrients. 2023 15
132. He S, Xiong Q, Tian C, Li L, Zhao J, Lin X. et al. Inulin-type prebiotics reduce serum uric acid levels via gut microbiota modulation: a randomized, controlled crossover trial in peritoneal dialysis patients. Eur J Nutr. 2022;61:665-77
133. Sohn MB, Gao B, Kendrick C, Srivastava A, Isakova T, Gassman JJ. et al. Targeting Gut Microbiome With Prebiotic in Patients With CKD: The TarGut-CKD Study. Kidney Int Rep. 2024;9:671-85
134. Rossi M, Johnson DW, Morrison M, Pascoe EM, Coombes JS, Forbes JM. et al. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin J Am Soc Nephrol. 2016;11:223-31
135. Zheng HJ, Guo J, Wang Q, Wang L, Wang Y, Zhang F. et al. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2021;61:577-98
136. Sergeev IN, Aljutaily T, Walton G, Huarte E. Effects of Synbiotic Supplement on Human Gut Microbiota, Body Composition and Weight Loss in Obesity. Nutrients. 2020 12
137. Han K, Nam J, Xu J, Sun X, Huang X, Animasahun O. et al. Generation of systemic antitumour immunity via the in situ modulation of the gut microbiome by an orally administered inulin gel. Nat Biomed Eng. 2021;5:1377-88
138. Ooijevaar RE, Terveer EM, Verspaget HW, Kuijper EJ, Keller JJ. Clinical Application and Potential of Fecal Microbiota Transplantation. Annu Rev Med. 2019;70:335-51
139. Scheperjans F, Levo R, Bosch B, Laaperi M, Pereira PAB, Smolander OP. et al. Fecal Microbiota Transplantation for Treatment of Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2024;81:925-38
140. Porcari S, Benech N, Valles-Colomer M, Segata N, Gasbarrini A, Cammarota G. et al. Key determinants of success in fecal microbiota transplantation: From microbiome to clinic. Cell Host Microbe. 2023;31:712-33
141. Caggiano G, Stasi A, Franzin R, Fiorentino M, Cimmarusti MT, Deleonardis A. et al. Fecal Microbiota Transplantation in Reducing Uremic Toxins Accumulation in Kidney Disease: Current Understanding and Future Perspectives. Toxins (Basel). 2023 15
142. Gharaie S, Lee K, Newman-Rivera AM, Xu J, Patel SK, Gooya M. et al. Microbiome modulation after severe acute kidney injury accelerates functional recovery and decreases kidney fibrosis. Kidney Int. 2023;104:470-91
143. Barba C, Soulage CO, Caggiano G, Glorieux G, Fouque D, Koppe L. Effects of Fecal Microbiota Transplantation on Composition in Mice with CKD. Toxins (Basel). 2020 12
144. Lauriero G, Abbad L, Vacca M, Celano G, Chemouny JM, Calasso M. et al. Fecal Microbiota Transplantation Modulates Renal Phenotype in the Humanized Mouse Model of IgA Nephropathy. Front Immunol. 2021;12:694787
145. Ye J, Yao J, He F, Sun J, Zhao Z, Wang Y. Regulation of gut microbiota: a novel pretreatment for complications in patients who have undergone kidney transplantation. Front Cell Infect Microbiol. 2023;13:1169500
146. Lamba JK, Tandon C, Tandon S. Gut Microbiota and Chronic Kidney Disease: A Complex Interplay with Implications for Diagnosis and Treatment. Probiotics Antimicrob Proteins. 2025
147. Marasco G, Cannarile DC, Cremon C, Papalia G, Marangoni A, Zucchelli A. et al. Fecal microbiota transplantation for Clostridioides difficile infection in a peritoneal dialysis patient: A case report. Perit Dial Int. 2025;45:247-50
148. Yang LY, Wang YL, Zuo YG. Pemphigoid diseases in patients with end-stage kidney diseases: pathogenesis and treatment. Front Immunol. 2024;15:1427943
149. Popovic L, Bulum T. New Onset Diabetes After Organ Transplantation: Risk Factors, Treatment, and Consequences. Diagnostics (Basel). 2025 15
150. Hubbard D, Colantonio LD, Rosenson RS, Brown TM, Jackson EA, Huang L. et al. Risk for recurrent cardiovascular disease events among patients with diabetes and chronic kidney disease. Cardiovasc Diabetol. 2021;20:58
151. He R, Qi P, Shu L, Ding Y, Zeng P, Wen G. et al. Dysbiosis and extraintestinal cancers. J Exp Clin Cancer Res. 2025;44:44
152. Oami T, Shimazui T, Yumoto T, Otani S, Hayashi Y, Coopersmith CM. Gut integrity in intensive care: alterations in host permeability and the microbiome as potential therapeutic targets. J Intensive Care. 2025;13:16
153. Pevzner IB, Brezgunova AA, Popkov VA, Sintsov MY, Andrianova NV, Zorova LD. et al. The effects of antibiotic therapy on neonatal sepsis-associated acute kidney injury. Life Sci. 2024;338:122359
154. Chen YC, Wu MY, Hu PJ, Chen TT, Shen WC, Chang WC. et al. Effects and Safety of an Oral Adsorbent on Chronic Kidney Disease Progression: A Systematic Review and Meta-Analysis. J Clin Med. 2019 8
155. Hsu CK, Su SC, Chang LC, Shao SC, Yang KJ, Chen CY. et al. Effects of Low Protein Diet on Modulating Gut Microbiota in Patients with Chronic Kidney Disease: A Systematic Review and Meta-analysis of International Studies. Int J Med Sci. 2021;18:3839-50
156. McFarlane C, Krishnasamy R, Stanton T, Savill E, Snelson M, Mihala G. et al. Diet Quality and Protein-Bound Uraemic Toxins: Investigation of Novel Risk Factors and the Role of Microbiome in Chronic Kidney Disease. J Ren Nutr. 2022;32:542-51
157. Li YJ, Chen X, Kwan TK, Loh YW, Singer J, Liu Y. et al. Dietary Fiber Protects against Diabetic Nephropathy through Short-Chain Fatty Acid-Mediated Activation of G Protein-Coupled Receptors GPR43 and GPR109A. J Am Soc Nephrol. 2020;31:1267-81
158. Biruete A, Chen NX, Metzger CE, Srinivasan S, Oa Neill K, Fallen PB. et al. The Dietary Fermentable Fiber Inulin Alters the Intestinal Microbiome and Improves Chronic Kidney Disease Mineral-Bone Disorder in a Rat Model of CKD. bioRxiv. 2023
159. Serrano M, Srivastava A, Buck G, Zhu B, Edupuganti L, Adegbulugbe E. et al. Dietary Protein and Fiber Affect Gut Microbiome and Treg/Th17 Commitment in Chronic Kidney Disease Mice. Am J Nephrol. 2022;53:646-51
160. Liu J, Gu QH, Cui Z, Zhao MH, Jia XY. Short-chain fatty acids ameliorate experimental anti-glomerular basement membrane disease. Clin Immunol. 2024;259:109903
161. Jiang M, Incarnato D, Modderman R, Lazaro AA, Jonkers IH, Bianchi F. et al. Low butyrate concentrations exert anti-inflammatory and high concentrations exert pro-inflammatory effects on macrophages. J Nutr Biochem. 2025;144:109962
162. Liu P, Wang Y, Yang G, Zhang Q, Meng L, Xin Y. et al. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol Res. 2021;165:105420
163. Park J, Goergen CJ, HogenEsch H, Kim CH. Chronically Elevated Levels of Short-Chain Fatty Acids Induce T Cell-Mediated Ureteritis and Hydronephrosis. J Immunol. 2016;196:2388-400
164. Fassarella M, Blaak EE, Penders J, Nauta A, Smidt H, Zoetendal EG. Gut microbiome stability and resilience: elucidating the response to perturbations in order to modulate gut health. Gut. 2021;70:595-605
165. Cheng WY, Wu CY, Yu J. The role of gut microbiota in cancer treatment: friend or foe? Gut. 2020;69:1867-76
166. Fong W, Li Q, Yu J. Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene. 2020;39:4925-43
167. Li N, Wang Y, Wei P, Min Y, Yu M, Zhou G. et al. Causal Effects of Specific Gut Microbiota on Chronic Kidney Diseases and Renal Function-A Two-Sample Mendelian Randomization Study. Nutrients. 2023 15
168. Chen Z, Chang X, Ye Q, Gao Y, Deng R. Kidney transplantation and gut microbiota. Clin Kidney J. 2024;17:sfae214
169. Qu Z, Tian P, Yang B, Zhao J, Wang G, Chen W. Fecal microbiota transplantation for diseases: Therapeutic potential, methodology, risk management in clinical practice. Life Sci. 2022;304:120719
170. Dong Y, Yan G, Zhang Y, Zhou Y, Shang J. Gut microbiota as a predictive tool for outcomes in IgA nephropathy. Ren Fail. 2025;47:2514184
171. Bibbo S, Porcari S, Del Vecchio LE, Severino A, Mullish BH, Ianiro G. et al. Gut microbiota and immunotherapy of renal cell carcinoma. Hum Vaccin Immunother. 2023;19:2268982
172. Liu HY, Li S, Ogamune KJ, Ahmed AA, Kim IH, Zhang Y. et al. Fungi in the Gut Microbiota: Interactions, Homeostasis, and Host Physiology. Microorganisms. 2025 13
173. Huang H, Wang Q, Yang Y, Zhong W, He F, Li J. The mycobiome as integral part of the gut microbiome: crucial role of symbiotic fungi in health and disease. Gut Microbes. 2024;16:2440111
174. Perez JC. Fungi of the human gut microbiota: Roles and significance. Int J Med Microbiol. 2021;311:151490
175. Wu X, Xia Y, He F, Zhu C, Ren W. Intestinal mycobiota in health and diseases: from a disrupted equilibrium to clinical opportunities. Microbiome. 2021;9:60
176. Yang F, Qu Q, Zhao C, Liu X, Yang P, Li Z. et al. Paecilomyces cicadae-fermented Radix astragali activates podocyte autophagy by attenuating PI3K/AKT/mTOR pathways to protect against diabetic nephropathy in mice. Biomed Pharmacother. 2020;129:110479
177. Zhou Q, Yang F, Li Z, Qu Q, Zhao C, Liu X. et al. Paecilomyces cicadae-fermented Radix astragali ameliorate diabetic nephropathy in mice by modulating the gut microbiota. J Med Microbiol. 2022 71
178. Zhang P, Guo R, Ma S, Jiang H, Yan Q, Li S. et al. A metagenome-wide study of the gut virome in chronic kidney disease. Theranostics. 2025;15:1642-61
179. Lin JN, Lin CL, Yang CH, Lin MC, Lai CH, Lin HH. et al. Risk of Nephrotic Syndrome following Enteroviral Infection in Children: A Nationwide Retrospective Cohort Study. PLoS One. 2016;11:e0161004
Author contact
![]() Corresponding authors: Xiao-ming Meng, E-mail address: mengxiaomingedu.cn. Pei Pan, E-mail address: peipan2022com. Wei Shao, E-mail address: ShaoWeiedu.cn.
Corresponding authors: Xiao-ming Meng, E-mail address: mengxiaomingedu.cn. Pei Pan, E-mail address: peipan2022com. Wei Shao, E-mail address: ShaoWeiedu.cn.

 Global reach, higher impact
Global reach, higher impact