10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2026; 22(5):2265-2284. doi:10.7150/ijbs.126977 This issue Cite
Review
Gut-Lung Microbiota Axis Shapes the Immune Microenvironment and Immunotherapeutic Response in Lung Cancer
1. Department of Respiratory and Critical Care Medicine, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
2. Department of Respiratory and Critical Care Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 430000.
*These authors contributed equally to this work.
Received 2025-10-29; Accepted 2026-1-12; Published 2026-2-1
Abstract
The gut-lung axis microbiota plays a pivotal role in shaping the tumor immune microenvironment (TIME) and regulating immunotherapeutic responses in lung cancer. This review highlights that pulmonary and gut microbial dysbiosis drives lung cancer development through inducing chronic inflammation, remodeling the immune microenvironment, and reprogramming metabolism. Lung cancer patients exhibit distinct microbial signatures characterized by altered microbiotal diversity and enrichment of specific taxa like Streptococcus, Veillonella, and Bacteroidetes in the airways, along with gut microbial shifts involving decreased Firmicutes/Bacteroidetes ratio. These microbial alterations promote tumor progression via activation of pro-inflammatory pathways (e.g., interleukin-17 (IL-17)/interleukin-23 (IL-23) axis) and suppression of antitumor immunity.Notably, the gut-lung microbiome exerts a profound impact on immunotherapeutic efficacy: responders are enriched with beneficial microbes like Akkermansia muciniphila and Bifidobacterium that enhance CD8⁺ T cell responses, while non-responders show elevated levels of Gammaproteobacteria and Fusobacterium associated with immunosuppression. Regulatory mechanisms include systemic immune modulation by microbial metabolites such as short-chain fatty acids, as well as activation of key signaling pathways including cGAS-STING and CD40L-CD40/NF-κB. Emerging translational applications encompass lung cancer diagnosis and immunotherapeutic response prediction via microbial biomarkers, as well as therapeutic interventions including fecal microbiota transplantation (FMT) and probiotic supplementation. Future studies should clarify microbe-host interaction mechanisms and develop personalized microbiota-based strategies to overcome immunotherapy resistance, offering the potential to revolutionize precision oncology through integrating microbiota modulation with conventional therapies.
Keywords: lung cancer, gut-lung axis, immunotherapy, microbiota
Introduction
Emerging epidemiological data reveal that in 2023, lung cancer accounted for approximately 2.30 million new cases and 2.04 million deaths globally, ranking first among all malignant neoplasms [1]. Projections indicate that in 2025, the United States will record 226,650 incident cases of lung cancer, representing approximately 11.1% of all new cancer diagnoses, with 124,730 attributable deaths constituting about 20.2% of total cancer mortality [2]. This highlights an urgent need for revolutionary breakthroughs in current diagnostic and therapeutic approaches. Immune checkpoint inhibitors (ICIs) have revolutionized the therapeutic landscape for lung cancer. Compared to conventional chemotherapy and targeted therapies, ICIs restore T-cell function by blocking immune checkpoint proteins including programmed death 1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), thereby significantly prolonging survival in subsets of advanced non-small cell lung cancer (NSCLC) patients [3]. Nevertheless, immunotherapy remains hindered by significant constraints, with less than 50% of patients attaining sustained clinical responses from ICIs monotherapy while the majority confront primary or acquired resistance [4, 5]. Furthermore, the accurate quantification of PD-L1 expression persists as a challenging task owing to the difficulties in sample acquisition and intratumoral heterogeneity [6, 7]. Meanwhile, emerging biomarkers such as tumor mutational burden (TMB) are unable to completely surmount the challenge of precise patient stratification [8]. The microbiota-cancer interplay has emerged as a promising therapeutic avenue (Figure 1). Landmark studies first demonstrated colonic commensals promote colorectal tumorigenesis [9], subsequently establishing microbial regulation of chemotherapy/immunotherapy responses [10, 11] and CTLA-4 blockade efficacy [12]. Microbiota now constitute key immunotherapy modulators, with their roles in lung cancer progressively elucidated. Research findings suggest that the lungs are not in a sterile state, and airway microbiome dysbiosis can increase the risk of chronic respiratory diseases [13, 14]. Particularly in lung cancer, dysbiotic microbiota regulates tumorigenesis through host interactions and remodels the tumor immune microenvironment (TIME) via mechanisms such as pro-inflammatory factor release, induction of immune tolerance, and disruption of barrier function [15]. Notably, this comprehension has extended from the pulmonary realm to the domain of the gut-lung axis, indicating that the distal gut microbiota also assumes a crucial regulatory function in pulmonary diseases [16, 17]. The lungs and the gut share a common embryogenic origin, exhibit similarities in structure and function, and possess comparable mucosal immune characteristics. The bidirectional regulatory mechanism between the lungs and the gut is mainly accomplished through the bidirectional migration of immune cells, mediators, and microbiota [18, 19]. Furthermore, the microbiome has the potential to function as a novel biomarker for prognosticating the efficacy of ICIs and as a target for enhancement. Interventions such as fecal microbiota transplantation and probiotic supplementation can significantly enhance antitumor immunotherapy responsiveness [20, 21]. These findings provide new strategies for overcoming resistance to tumor immunotherapy.
The Gut-Lung Microbiota Axis Dysbiosis Regulates Lung Cancer Progression and Immunotherapeutic Response. Emerging evidence positions gut-lung microbiota axis perturbation as an overarching regulator spanning from lung cancer initiation to immunotherapy outcomes, wherein compartmentalized dysbiosis rewires host-tumor microenvironments via multi-organ crosstalk.
Building on these insights, this study systematically investigates how lung-gut microbiota dysbiosis affects lung carcinogenesis and immunotherapy response. By characterizing microbiota lineage changes and their immune-metabolic networks in tumor microenvironment (TME) regulation, this study proposes novel strategies for microbiota-assisted precision diagnostics and immunotherapy enhancement.
Clinical Evidence Implicating Gut-Lung Microbiota Dysbiosis in Lung Cancer Pathogenesis
Characterization of Microbial Diversity Alterations in Lung Cancer
In health, the dynamic equilibrium of the lung microbiome relies predominantly on the immigration-elimination balance, exhibiting a progressive decline in community richness as distance from the upper respiratory tract source community increases. Impaired airway clearance, increased microbial colonization, and enhanced local proliferation collectively alter lung microbiota diversity in lung cancer patients [13]. Several studies based on 16S rRNA sequencing, next-generation sequencing (NGS), whole metagenomic sequencing (WMS), etc., have indicated a decrease in α-diversity among lung cancer patients (Table 1, Table S1). These findings show reduced microbial richness across various sample types, including tumor tissues, airway samples such as bronchoalveolar lavage fluid (BALF), bronchial brushings, and sputum, when compared to adjacent non-tumor tissue, non-cancer controls, or healthy populations [22-27]. Notably, tumor tissues from patients with recurrence exhibited significantly lower α-diversity compared to non-recurrent cases, and this metric may serve as an independent predictor for recurrence-free survival following pulmonary resection [28]. In addition, advanced-stage (III-IV) patients exhibited decreased α-diversity in sputum samples compared to early-stage (I-II) cases [29], while oral swab samples with higher α-diversity were associated with reduced cancer risk [30]. Conversely, some studies have reported increased α-diversity in tumor tissues [31-33], BALF [34, 35], and even saliva samples [36] from lung cancer patients compared with control groups, which predominantly comprised patients with specific respiratory diseases such as emphysema-only cases, tuberculosis patients, or pneumonia patients. It is worth noting that several studies have not detected any significant differences in microbial α-diversity between the two groups [37-40]. Unlike findings in the lungs, most studies indicate no significant difference in gut microbial α-diversity between lung cancer patients and non-cancer controls, while some reports observe decreased microbial α-diversity [41], alongside increased fungal diversity in patients [42] (Table 2, Table S2). Furthermore, impaired pulmonary ventilation was found to be positively associated with the α-diversity of gut microbiota in patients with lung cancer, indicating that a decline in pulmonary function correlates with an increased microbial diversity [43]. The microbial composition significantly differs between lung cancer patients and healthy controls. Significant β-diversity distinctions have been consistently observed between groups across multiple sampling sites, particularly in lung (BALF, tumor tissue) and gut (stool samples) [23, 26, 27, 31, 32, 36, 37, 42, 44-54], with variations further associated with tumor histology, TNM stages [29, 38], and recurrence outcomes [28]. However, some studies failed to replicate these findings in BALF or stool samples [25, 30, 34, 35, 39, 40, 55-57]. Evidence suggests significant associations between microbial diversity (e.g., α-/ β-diversity) and lung cancer, although inconsistencies remain across studies. Overall, compared to non-cancer controls, lung cancer patients exhibit characteristic alterations in the gut-lung microbiota, typically showing decreased α-diversity and significant β-diversity variations. The concept of "global restructuring of microbial community composition and function" may provide a unifying framework to explain the heterogeneity observed across different studies. Future research should employ larger sample sizes and more comprehensive analytical approaches to systematically characterize the structural and functional features of pulmonary and intestinal microbiota in lung cancer patients.
Lung Cancer-Associated Alterations in Microbial Compositional Profiles
Multiple studies have demonstrated significant differences in lung microbiota composition between lung cancer patients and non-cancer controls (Table S3). Analyses of oral (saliva), lower respiratory tract (BALF/bronchial washing fluid/bronchial brushes), and tumor tissue samples consistently revealed enrichment of Firmicutes in LC patients, accompanied by elevated Bacteroidota and Proteobacteria in tumor tissues [32, 33, 39, 45, 55]. Notably, Actinobacteria showed marked enrichment in airway samples from synchronous multiple primary lung cancer (sMPLC) patients [37].
Respiratory microbiota diversity patterns in lung cancer patients stratified by clinical characteristics
| Year | Sample number | Smoking (%) | Sample type | Analytical Method | Histology | TNM stage | Microbiota Community Diversity Discrepancy |
|---|---|---|---|---|---|---|---|
| 2016 [22] | 196 | 94.0 | Lung tissues | 16S rRNA | Lung cancer | I-II 67.3%, III-IV 32.7% | Significantly lower α-diversity in tumor vs. adjacent normal tissues. |
| 2016 [55] | 56 | 60.7 | BALF | NGS | Lung cancer | I-II 33.3%, III-IV 66.7% | Significantly higher α-diversity in lung cancer group vs. benign mass-like lesion group. |
| 2017 [27] | 66 | 47.6 | Bronchial brushing | 16S rRNA | Lung cancer | I-II 25.0%, III-IV 66.7% | Significantly lower α-diversity in LC patients vs. healthy controls and distinct β-diversity in cancerous sites vs. paired contralateral non-cancerous sites. |
| 2018 [31] | 176 | 92.6 | Lung tissues | 16S rRNA | NSCLC | NA | Significantly higher α-diversity and distinct β-diversity in cancer vs. normal lung tissues. |
| 2018 [44] | 190 | 88.9 | Bronchial brushing BALF | 16S rRNA | Lung cancer | NA | β-Diversity differed significantly in BALF samples between healthy individuals and LC patients. |
| 2018 [32] | 40 | 100.0 | Lung tissues | 16S rRNA | Lung cancer | I-II 52.5%, III-IV 10.0% | Significantly higher α-diversity and distinct β-diversity in LC patients vs. emphysema-only patients. |
| 2019 [56] | 150 | 56.0 | BALF | MGS | Lung cancer | I-II 45.7%, III-IV 53.1% | Significantly lower LRT richness in LC patients vs. healthy controls. |
| 2019 [39] | 92 | 57.6 | BWF, sputum | 16S rRNA | NSCLC | NA | No significant differences |
| 2020 [45] | 90 | 88.9 | Saliva, BALF lung tissues | 16S rRNA | NSCLC | I-II 55.6%, III-IV 44.4% | NSCLC salivary microbiota had distinct β-diversity vs. lung specimens (BALF/tumor/adjacent/distal). |
| 2020 [36] | 125 | 78.1 | Saliva, BALF | 16S rRNA ITS | Lung cancer | NA | Significantly higher α-diversity in saliva and BALF microbiota of LC patients than non-cancer controls. Affected bronchi showed distinct β-diversity of fungi compared to the contralateral bronchi. |
| 2021 [25] | 47 | 72.3 | BALF | MG-Seq | NSCLC | I-II 71.9%, III-IV 28.1% | Significantly lower α-diversity in BALF samples from NSCLC patients vs. non-cancer controls. |
| 2021 [38] | 83 | 90.4 | Bronchial brushing | 16S rRNA | Lung cancer | I-II 26.0%, III-IV 73.0% | β-Diversity significantly differed by tumor type (SCLC vs. NSCLC), TNM stage (I-IIIA vs. IIIB-IV stage), and survival duration (short-term [<1 year] vs. long-term [>1 year] ). |
| 2022 [37] | 8 | 0.0 | Bronchial brushingBALF | 16S DNA | Lung cancer | NA | β-Diversity differed significantly between tumor regions and non-tumor regions. |
| 2022 [34] | 78 | NA | BALF | NGS | Lung cancer | NA | Significantly higher α-diversity in LC patients vs. tuberculosis and pneumonia patients. |
| 2022 [35] | 60 | 53.3 | BALF | MGS | Lung cancer | I-II 17.2%, III-IV 62.1% | Significantly higher α-diversity in BALF samples from LC patients vs. benign disease controls. |
| 2022 [29] | 85 | 47.1 | Sputum | 16S rRNA | NSCLC | I-II 26.0%, III-IV 60.0% | Significantly lower α-diversity and distinct β-diversity in advanced-stage (III-IV) vs. early-stage (I-II) NSCLC patients. |
| 2022 [23] | 162 | 60.5 | Lung tissues | 16S rRNA | NSCLC | I-II 82.5%, III-IV 17.5% | Significantly lower α-diversity and distinct β-diversity in NSCLC tissues vs. normal tissues. |
| 2022 [30] | 1306 | 90.4 | Oral swab | 16S rRNA | Lung cancer | NA | Lower α-diversity was associated with higher LC risk. |
| 2023 [46] | 38 | 15.8 | BALF | MG-Seq | NSCLC | I-II 86.4%, III-IV 13.6% | Significantly higher α-diversity and distinct β-diversity in NSCLC patients vs. non-NSCLC patients. |
| 2023 [26] | 56 | 55.6 | BALF | MGS | Lung cancer | I-II 7.4%, III-IV 66.7% | Significantly lower α-diversity and distinct β-diversity in tumor-burden segments vs. ipsilateral non-tumor sites. |
| 2023 [40] | 71 | 78.3 | Saliva, BALF | 16S rRNA | Lung cancer | NA | No significant differences |
| 2023 [24] | 52 | 19.0 | Oral swab, BALF Lung tissues | ITS, MG-Seq | NSCLC | I-II 88.0%, III-IV 12.0% | Significantly lower α-diversity in tumor tissues vs. non-tumor tissues. |
| 2024 [28] | 116 | 53.5 | Lung tissues | 16S rRNA | Lung cancer | NA | Significantly lower α-diversity and distinct β-diversity in recurrent vs. non-recurrent LC patients. |
| 2024 [33] | 369 | NA | Lung tissues | 16S rRNA | NSCLC | NA | Significantly higher bacterial α-diversity and distinct β-diversity in NSCLC tumor tissue vs. lung parenchyma tissues. |
* BALF, bronchoalveolar lavage fluid; BWF, bronchial washing fluid; ITS, internal transcribed spacer; LC, lung cancer; LRT, lower respiratory tract; MGS, shotgun metagenomics sequencing; MG-Seq, metagenomic sequence; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; sMPLC, synchronous multiple primary lung cancer; SCLC, small cell lung cancer; TNM, tumor node metastasis; WGS, whole genome sequencing.
Additionally, the lower respiratory tract (LRT) microbiota of lung cancer patients exhibits distinct genus-level alterations. Studies demonstrate significant enrichment of typical supraglottic taxa including Streptococcus, Veillonella, Prevotella, and Rothia in the lower airways, concomitant with elevated rRNA gene concentrations in BALF [27, 44, 56]. These microbial shifts correlate strongly with enhanced pulmonary inflammation, as evidenced by increased neutrophil and lymphocyte counts and exhaled nitric oxide (eNO) levels in BALF [58]. The microbial composition exhibits significant modulation by distinct clinical characteristics such as tumor node metastasis (TNM) stage, distant metastasis, pathological subtype, and epidermal growth factor receptor (EGFR) mutation status. Notably, Thermus predominates in advanced-stage (IIIB/IV) tumors, Legionella shows strong associations with metastatic progression, and Acidovorax enrichment characterizes smoking-related squamous cell carcinomas, particularly in cases with tumor protein p53 (TP53) mutations [31].
Gut microbiota diversity patterns in lung cancer patients stratified by clinical characteristics
| Year | Sample number | Smoking (%) | Sample type | Analytical Method | Histology | TNM stage | Microbiota Community Diversity Discrepancy |
|---|---|---|---|---|---|---|---|
| 2018 [48] | 82 | 53.7 | Stool | 16S rRNA | Lung cancer | I-II 56.1%, III-IV 43.9% | β-diversity significantly differed in LC patients vs. healthy controls. |
| 2019 [49] | 60 | NA | Stool | 16S rRNA | Lung cancer | I-II 16.6%, III-IV 83.4% | β-diversity significantly differed in LC patients vs. healthy controls. |
| 2019 [41] | 46 | NA | Stool | 16S rRNA | Lung cancer | NA | Significantly lower α-diversity and distinct β-diversity in LC patients vs. healthy controls. |
| 2020 [51] | 107 | 2.4 | Stool | 16S rRNA | Lung cancer | I-II 100.0% | β-diversity significantly differed in LC patients vs. healthy controls. |
| 2021 [52] | 81 | NA | Stool | 16S rRNA | Lung cancer | I-II 30.8%, III-IV 67.9% | β-diversity significantly differed in LC patients vs. healthy controls. |
| 2022 [57] | 58 | 15.5 | Stool | 16S rRNA | NSCLC | I-II 95.7%, III-IV 4.3% | No significant difference. |
| 2023 [42] | 299 | NA | Stool | ITS | LUAD | I-II 97.2%, III-IV 2.8% | Increased α- and β-diversity of gut fungal microbiome in LUAD vs. healthy controls. |
| 2023 [53] | 78 | 30.8 | Stool | 16S rRNA | NSCLC | I-II 100.0% | β-diversity significantly differed in early-stage NSCLC patients and healthy controls. |
| 2024 [43] | 83 | 18.0 | Stool | 16S rRNA | Lung cancer | I-II 90.9%, III-IV 9.1% | Significantly higher α-diversity and distinct β-diversity in LC with moderate/severe (vs. mild/normal) ventilation dysfunction.β-diversity significantly differed in LC patients vs. benign pulmonary disease patients. |
* ITS, internal transcribed spacer; LC, lung cancer; LUAD, lung adenocarcinoma; NSCLC, non-small cell lung cancer; TNM, tumor node metastasis.
Significantly, Parvimonas shows specific enrichment in EGFR-mutant lung adenocarcinoma (LUAD) [29]. Fungal taxa including Aspergillus, Agaricomycetes, and Blastomyces are also overrepresented in tumor tissues, though their mechanistic roles in carcinogenesis remain to be elucidated [59, 60]. Analysis of stool samples from lung cancer patients revealed a distinct gut microbiome signature (Table S4), characterized by significant enrichment of Bacteroidetes, Proteobacteria, Halanaerobiaeota, and Desulfobacterota concurrent with depletion of Actinobacteria. At the species level, Streptococcus anginosus was significantly enriched in rectal swabs of LUAD patients, whereas Corynebacterium aurimucosum predominated in lung squamous cell carcinoma (LUSC) cases [33, 49, 51, 52, 57, 61]. Notably, the ratio of Firmicutes/Bacteroidetes was significantly reduced in the gut microbiota of lung cancer patients [41]. Given that Firmicutes and Actinobacteria facilitate colonic short-chain fatty acids (SCFAs) production, this decline correlates with diminished circulating SCFAs levels [62, 63]. Fungal community analysis demonstrated significant enrichment of Basidiomycota, Mortierellomycota, and Chytridiomycota but depletion of Ascomycota in LUAD patients [42]. Specific bacterial genera including Bacteroides, Veillonella, Megasphaera, and Enterococcus, and fungal genera including Saccharomyces and Aspergillus, showed significantly increased abundance in stools of lung cancer patients compared to controls. Additionally, building upon previous research findings, this study identified the anaerobic bacteria Prevotella and Veillonella as shared lung cancer-associated genus across both lung (including BALF, bronchial brushing, and lung tumor tissue samples) and gut (stool samples) sites (Figure S1). These anaerobic bacteria, which demonstrate high abundance and ubiquitous colonization in humans [64, 65], may mediate trans-organ carcinogenesis through the gut-lung axis. Overall, the systematic analysis of microbial dysbiosis in the lungs and gut reveals lung cancer-specific microbial signatures. Alterations in microbiota composition, both in diversity and taxon abundance, demonstrate site-specific patterns with significant clinical associations. This microbe-host interaction contributes to the initiation and progression of lung cancer.
Potential Mechanisms of Gut-Lung Microbiota in Lung Cancer Pathogenesis
Lung Microbiota Modulates Local Immune Remodeling and Oncogenic Signaling
The lung microbiota contributes to the pathogenesis of lung cancer through various mechanisms, including inducing local inflammatory responses, remodeling the immune microenvironment, disrupting cellular metabolism, and modulating gene expression [66-69] (Figure 2). Research finding has demonstrated a marked dysbiosis in the lower respiratory tract of lung cancer patients, which is prominently characterized by the abnormal colonization of supraglottic microbiota, such as Streptococcus and Veillonella [44]. Notably, Veillonella parvula shows specific enrichment in the lower airways and exhibits a significant correlation with poor prognosis in advanced-stage (TNM IIIB-IV) LUAD patients, as evidenced by notably decreased survival and increased tumor burden [38]. Additionally, advanced pulmonary adenocarcinoma correlates with an airway microbiota featuring a "high-burden, low-diversity" characteristic [70]. In this context, the elevated bacterial load positively correlates with tumor burden, further corroborating the significant association between microbial dysbiosis and lung cancer progression. Airway microbiota dysbiosis promotes lung cancer progression through multifaceted mechanisms. The dysbiotic microbiota activates the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) and phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) signaling pathways in respiratory epithelial cells, enhancing cellular proliferation, survival, differentiation, and invasive capacity to drive tumorigenesis [71]. Microbiota-derived SCFAs may function as crucial mediators, driving oncogenesis through insulin-like growth factor 1 (IGF-1) signaling and M2-like macrophage polarization. These processes synergistically upregulate the PI3K and MAPK signaling pathways [72]. Microbiota dysbiosis remodels the TIME by activating interleukin-17A (IL-17A), interleukin-1 (IL-1), interleukin-6 (IL-6), and inflammasome signaling pathways, which results in the expansion of T-helper (Th17) cells, the upregulation of PD-1⁺ T cells, and the exhaustion of CD4⁺ T cells within the tumor tissue [38, 73, 74]. Notably, airway microbiota imbalance stimulates alveolar macrophages and neutrophils to produce interleukin-1β (IL-1β) and IL-23 via Myd88-dependent myeloid signaling within tumor tissues, thereby inducing the proliferation and activation of tissue-resident Vγ6+Vδ1+ γδ T cells [70]. Activated γδ T cells serve as the principal source of IL-17 in tumor-bearing lungs. In combination with Th17 cells, they are the main producers of IL-17 [75]. The upregulated IL-17 further promotes neutrophil infiltration and chronic pulmonary inflammation [76]. Accumulating evidence suggests the functional importance of neutrophils in tumor immunity, progression, and response to immunotherapy [77].
Pulmonary microbiota dysbiosis drives lung cancer progression through dual mechanisms. Airway microbiota dysbiosis drives lung cancer progression through multiple mechanisms, including direct activation of ERK/MAPK, PI3K/AKT, and inflammasome signaling pathways in respiratory epithelial cells to promote tumorigenesis; modulation of key gene expression indicated by positive correlations between tumor bacterial burden and CTNNB1 (Wnt/β-catenin), HIF1A (hypoxia response), and VEGFA (angiogenesis), alongside negative correlations with TP73 and TLR5; and immune microenvironment remodeling evidenced by positive associations between bacterial diversity and CAF infiltration or immune exclusion traits, resulting in enrichment of neutrophils, macrophages, and NK cells in high-diversity tumors and closely linked to increased PD-1⁺ T cells and CD4⁺ T cell exhaustion. Specifically, airway dysbiosis induces Myd88-dependent IL-1β/IL-23 release from alveolar macrophages and neutrophils, activating tissue-resident Vγ6⁺Vδ1⁺ γδ T cells to secrete IL-17. This synergizes with Th17 cell responses to drive neutrophilic recruitment and perpetuate pulmonary inflammation. Lung cancer tissue-enriched Aspergillus sydowii induces IL-1β via the β-glucan/Dectin-1/CARD9 axis, promoting MDSC and Treg expansion alongside PD-1⁺CD8⁺ T cell accumulation, fostering immunosuppression. Roseburia-derived butyrate upregulates non-coding RNA H19, increasing MMPs like MMP15 to facilitate metastasis; concurrently, butyrate polarizes M2 macrophages to secrete IL-10, IL-13, and MIP3α, enhancing tumor migration and invasion. Figure 2 was created using BioRender and holds the appropriate license. Amphiregulin, Areg; Arginase-1, Arg1; Cancer-associated Fibroblast, CAF; Caspase recruitment domain-containing protein 9, CARD9; Cytotoxic T Lymphocyte, CTL; Extracellular Signal-Regulated Kinase, ERK; Interleukin, IL; Matrix Metalloproteinases, MMPs; Macrophage Inflammatory Protein-3 alpha, MIP-3α; Myeloid-Derived Suppressor Cells, MDSCs; Mitogen-Activated Protein Kinase, MAPK; Natural Killer cells, NK; Nitric Oxide, NO; Programmed Cell Death Protein 1, PD-1; Phosphoinositide 3-Kinase, PI3K; Reactive Oxygen Species, ROS; Regulatory T Cells, Tregs; Toll-Like Receptor, TLR.
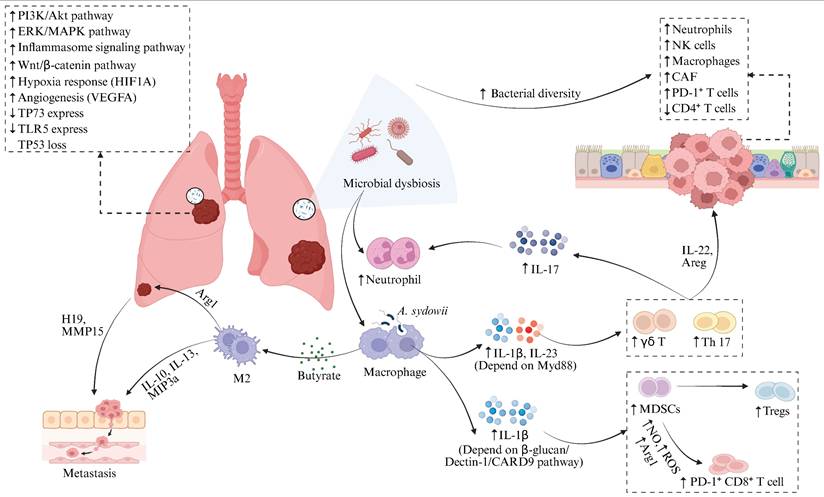
Collectively, the enhanced pulmonary inflammation and diminished antitumor immune surveillance contribute to the establishment of a local pro-tumorigenic immune environment [78]. Similarly, the intratumoral microbiome within lung cancer tissue engages in disease regulation. Bacterial burden augmented from tumor-adjacent normal lung tissue and tertiary lymphoid structures to tumor tissue and finally to the airways, implying airway-mediated invasion of intratumoral bacteria. Spatial transcriptomic analysis [79] revealed that bacteria within lung tumors were predominantly enriched in tumor cells, with their burden showing a strong positive correlation with the expression of genes involved in the Wnt/β-catenin signaling pathway (catenin beta 1, CTNNB1), hypoxia response (hypoxia-inducible factor 1 alpha, HIF1A), and angiogenesis (vascular endothelial growth factor A, VEGFA). These dysregulated gene expressions significantly contribute to tumor initiation and progression [80-83]. Moreover, the bacterial burden on these tumor cells showed a negative correlation with genes associated with cell cycle arrest and apoptosis (tumor protein 73, TP73) [84] and pattern recognition (Toll-like receptor 5, TLR5) [85]. These findings suggest that tumor-associated bacteria promote tumor growth. Nevertheless, these bacteria fail to augment the immunogenicity of tumor cells, as there exists no notable correlation between bacterial burden and the expression of genes associated with the antigen presentation pathway [79]. The fungus Aspergillus sydowii is significantly enriched in LUAD tumor tissues, which promotes cancer progression by inducing an immunosuppressive TME. This phenomenon is manifested by the expansion and activation of myeloid-derived suppressor cells (MDSCs) [86] and regulatory T cells (Tregs) [87], along with the upregulation of immunosuppressive gene signatures related to myeloid leukocyte chemotaxis, reactive oxygen species (ROS) metabolic and IL-1β production [88-90]. Aspergillus sydowii activates MDSCs through the secretion of IL-1β via the β-glucan/Dectin-1/caspase recruitment domain-containing protein 9 (CARD9) axis, thereby inhibiting the activity of cytotoxic T lymphocytes and promoting the accumulation of PD-1+CD8+ T cells [24]. This mechanism contributes to patient immunosuppression and unfavorable prognosis. Fungal enrichment (e.g., Alternaria alternata, Malassezia globosa) in pancreatic ductal adenocarcinoma (PDAC) drives KrasG12D-dependent activation of the dectin-1-mediated Src-Syk-CARD9 pathway, resulting in interleukin-33 (IL-33) secretion. This recruits and polarizes Th2 cells and innate lymphoid cells (ILC2s), ultimately accelerating tumor progression via elevated interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-13 (IL-13) production. Antifungal therapy markedly attenuates PDAC progression [91, 92]. Alternaria-derived serine protease activity induces rapid IL-33 release from lung epithelium, driving robust Th2 inflammation that contributes to early pulmonary inflammation [93]; however, its role in lung cancer remains unexplored despite reports of Alternaria enrichment in tumor tissues of NSCLC patients [46].
Emerging evidence indicates that microbiota may contribute to lung cancer recurrence and metastasis. The butyrate-excreting bacterium Roseburia could promote tumor recurrence and metastasis [28], as low butyrate levels inhibit histone deacetylase 2 (HDAC2), thereby enhancing histone H3 Lysine 27 (H3K27) acetylation and upregulating the non-coding ribonucleic acid (RNA) H19. Subsequently, H19 upregulates multiple matrix metalloproteinases (MMPs) [94, 95], including MMP15 which plays key roles in NSCLC metastasis [96]. The Cancer Genome Atlas (TCGA) database analysis reveals MMP15 overexpression is associated with advanced TNM stages and shorter recurrence-free survival (RFS) in NSCLC patients. Beyond the H19-MMP pathway, low-dose butyrate induces M2 macrophage polarization, enhancing secretion of interleukin-10 (IL-10), IL-13, and MIP-3α and thereby promoting tumor cell migration and invasion [28, 97, 98]. Microbes remodel the immune microenvironment and signaling in metastatic tumors. Specifically, higher bacterial diversity is associated with elevated signatures of cancer-associated fibroblast (CAF) infiltration and immune exclusion. Furthermore, lung metastatic with rich microbial diversity exhibit increased infiltration of innate immune cells such as neutrophils, natural killer (NK) cells, macrophages, and Tregs, along with an enhanced signaling through EGFR. Specifically, there is a positive association between Bifidobacterium abundance and NK cells (S2 state) [99]. This result aligns with previous study [100], demonstrating that Bifidobacterium restores NK cell frequency and enhances tumor immunity within the TME. Collectively, these findings demonstrate that microorganisms within lung cancer cells contribute to TME modulation, promoting tumor progression and immune evasion, underscoring their tumor-promoting role.
Gut Dysbiosis Drives Systemic Immune Modulation and Metabolic Reprogramming
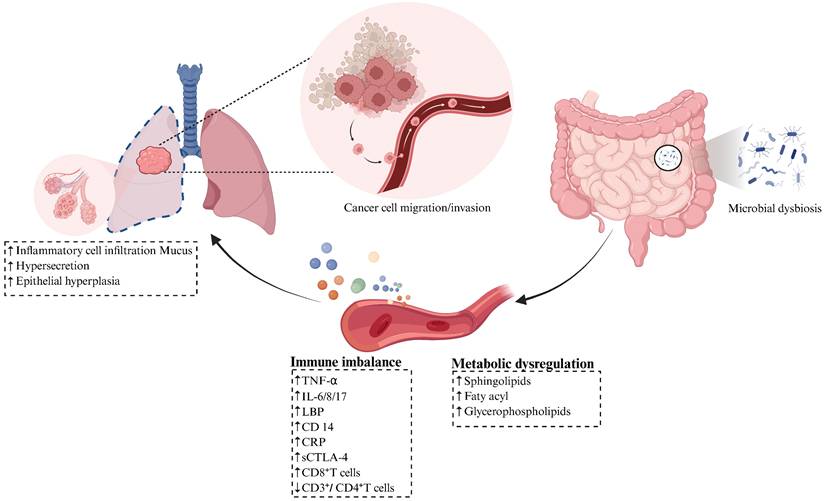
Gut dysbiosis plays a crucial role in driving the pathogenesis of lung cancer through the remodeling of systemic immunity and the reprogramming of host metabolic homeostasis, consequently accelerating tumor proliferation, invasion, and metastatic progression (Figure 3). Experimental evidence shows fecal microbiota transplantation from lung cancer patients into tumor-bearing Lewis lung carcinoma C57BL/6 mice, or monocolonization with lung cancer-enriched Prevotella copri, induces systemic immunodysregulation. This manifests through elevated serum levels of proinflammatory mediators such as tumor necrosis factor-α (TNF-α) [101] and interleukin-8 (IL-8) [102], along with endotoxin biomarkers including lipopolysaccharide binding protein (LBP) and CD14 molecule (CD14), as well as increased C-reaction protein (CRP). Concurrently, peripheral CD3⁺/CD4⁺ T cells decline, while CD8⁺ T cells rise. These inflammatory factors induced alveolar inflammatory cell infiltration, excessive mucus secretion in lung tissues, and abnormal epithelial hyperplasia. Critically, nervonic acid/all-trans retinoic acid intervention reverses these pathologies, demonstrating reversible microbiota-host pathogenic interplay [57]. Compared with healthy controls, lung cancer patients exhibit synchronous elevation of pro-inflammatory and immunosuppressive mediators, particularly IL-6, IL-17, and soluble CTLA-4 (sCTLA-4) in peripheral blood. This triad establishes a self-perpetuating 'microbiota-metabolite-immune' pathological circuit [48]. Ni et al. [53] identified significant enrichment of Agathobacter, Blautia, and Clostridium genera along with Muribaculaceae family in the gut microbiota of early-stage NSCLC patients. This dysbiosis coincided with abnormally elevated peripheral levels of sphingolipids, fatty acyl, and glycerophospholipids. Further analyses revealed positive correlations between Clostridium abundance and multiple glycerophospholipids, while Muribaculaceae abundance was associated with specific phospholipid metabolism perturbations and fatty acyl. Moreover, an independent study demonstrated that aberrantly enriched Clostridioides and Synergistes genera exhibit significant positive correlations with peripheral glycerophospholipid metabolism in lung cancer cohorts, suggesting these microbes may drive pathogenesis through lipid metabolic reprogramming [52].
Gut Microbiota Modulates the Progression of Lung Cancer. Gut microbial dysbiosis promotes lung cancer progression by remodeling systemic immunity and metabolism. Fecal microbiota transplantation (FMT) from lung cancer patients induces systemic immune imbalance, specifically manifested by significant elevation of serum pro-inflammatory mediators (TNF-α, IL-8), endotoxin-related markers (LBP, CD14), and CRP, alongside increased peripheral blood CD8⁺ T cells and decreased CD3⁺/CD4⁺ T cells, accompanied by inflammatory cell infiltration in alveolar cavities, hyperactive mucus secretion in lung tissue, and abnormal epithelial hyperplasia. Compared to healthy controls, lung cancer patients exhibit synchronized elevation of plasma pro-inflammatory/immunosuppressive factors such as IL-6, IL-17, and sCTLA-4. Gut microbial dysbiosis in lung cancer patients further causes abnormal lipid metabolism, characterized by significantly elevated peripheral levels of sphingolipids, fatty acyl and glycerophospholipids, indicating hyperactive lipid metabolism and oxidative stress status. Figure 3 was created using BioRender and holds the appropriate license. Binding protein, LBP; CD14 molecule, CD14; C-reaction protein, CRP; Interferon-gamma, IFN-γ; Tumor necrosis factor-α, TNF-α; Soluble cytotoxic T-lymphocyte-associated protein 4, sCTLA-4.
Patients with lung cancer consistently exhibit dysregulated lipid metabolism, manifested by heightened levels of acylcarnitines and phospholipids, alongside enhanced lipid metabolic activity and oxidative stress. Notably, glycerophospholipids and sphingolipids serve as essential membrane components, facilitating transmembrane signaling and transport. Phospholipids additionally function as signaling molecules, allosteric enzyme activators, and central regulators of energy metabolism, and their dysregulation directly correlates with tumor progression [103]. Moreover, sphingolipids mediate inflammatory signals and the release of pro-inflammatory cytokines, thereby modulating intracellular complement activation. This cascade induces NOD-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasome assembly, triggering caspase-1-dependent IL-1β maturation that stimulates malignant cell dissemination [104]. Impaired functional activity of intestinal microbiota constitutes the pivotal mechanism underlying microecological dysbiosis in lung cancer. Metagenomic analyses reveal significantly diminished expression of proteins involved in amino acid transport/metabolism, coenzyme transport/metabolism, cell cycle control, and cell division within the gut microbiome of lung cancer patients [49]. Furthermore, these patients exhibit markedly reduced abundance in energy metabolism pathways and ATP-binding cassette (ABC) transporter systems compared to healthy controls [41]. This pervasive functional suppression compromises bacterial proliferation and colonization capacity, destabilizes microbial communities, and perpetuates metabolic derangement with concomitant immune dysregulation. Targeting the aforementioned mechanisms [105], modulating microbiota-host interactions demonstrates therapeutic potential. In Lewis lung cancer (LLC) tumor-bearing models, oral administration of polysaccharopeptides (PSP) significantly suppressed pro-tumorigenic mediators, specifically leukotriene B₄ (LTB₄), leukotriene D₄ (LTD₄), and leukotriene E₄ (LTE₄), through inhibition of FcεRI signaling and downstream arachidonic acid metabolism. These leukotrienes drive tumor progression through increased vascular permeability, inflammatory cascade activation, TNF-α/IL-1/IL-6/IL-2 overexpression, and amplified Th2-polarized pulmonary inflammation [106-108]. Crucially, PSP concurrently restores microbial equilibrium while inhibiting this pathway, thereby suppressing lung cancer proliferation.
Integrated Mechanisms of the Gut-Lung Axis Mediated by Metabolic Signaling and Immune Trafficking
A well-established gut-lung axis mediates bidirectional crosstalk through microbial communities and their metabolites, profoundly influencing lung cancer pathogenesis and progression. Mao et al. [109] found that the development of lung cancer is inversely correlated with the microbial diversity in the lung and gut compartments. This dysbiosis coincides with lung tissue accumulation of pro-inflammatory mediators (leukotriene B4 and prostaglandin E2) and concomitant elevation of intestinal anti-inflammatory metabolites (resolvins and isohumulones). Important evidence indicates that gut-lung axis microbial translocation plays a pivotal role in lung disease pathogenesis [110-112]. Specifically, gut microbiota dysbiosis facilitates systemic translocation of gut microbiota to lung tissues via circulatory pathways, which promotes lung cancer progression by disrupting lung microbial homeostasis and potentiating inflammatory signaling cascades [113-115]. The gut communicates with distant organs including the lungs through microbiota and their derived metabolites, establishing intimate cross-talk. Short-chain fatty acids (SCFAs), including representative molecules such as butyrate, acetate, propionate and valerate, are produced through the fermentation of dietary fibre by the intestinal microbiota. Butyrate can promote the extrathymic generation of regulatory T cells (Treg) by activating G protein-coupled receptor signaling on the cell membrane or by inhibiting histone deacetylase activity [116]. Consequently, it suppresses inflammation, modulates epithelial barrier function, and regulates mucosal and systemic immunity [117]. Acetate and propionate enhance the integrity of the intestinal barrier, reduce bacterial translocation, and alleviate systemic inflammatory responses [118]. Previous study has demonstrated that Bacteroides in the gut can enhance the anti-tumour activity of CD8+ T cells by producing the metabolite acetate, thereby inhibiting the progression of breast cancer [119]. Building on this, Luu et al. further identified that valerate can act as a novel metabolic regulatory molecule for the treatment of autoimmune diseases by reprogramming lymphocyte metabolic activity to promote the secretion of the anti-inflammatory cytokine IL-10 and suppress the differentiation of pro-inflammatory Th17 cells [120]. Similarly, gut-derived SCFAs can exert distal regulatory effects on lung diseases. Robust epidemiological evidence [121] indicates that antibiotic use in early life increases the risk of developing asthma in adulthood, a phenomenon that may be related to antibiotic-induced disruption of the gut microbiota and consequent reduction in butyrate production. Butyrate can suppress allergic Th2-type immune responses by downregulating the migratory and activation capacity of dendritic cells (DCs) [122]. Clinical studies have also shown that individuals with higher levels of butyrate-producing bacteria in the gut have a significantly reduced risk of lower respiratory tract viral infections [123]. This protective effect is closely associated with the ability of propionate and butyrate to effectively enhance the antiviral immune function of pulmonary CD8+ T cells. Beyond SCFAs, the gut microbiota also produce a variety of other metabolites, such as bile acids and amino acid derivatives, which may also influence pulmonary immunity and inflammation via the gut-lung axis [124]. For example, gut-derived indole compounds can be transported to the lung via the bloodstream, where they influence T lymphocyte differentiation and the polarization status of alveolar macrophages, thereby altering immune homeostasis and the level of inflammatory responses in lung tissue [125, 126]. Beyond microbial metabolites, immune cells mediate bidirectional gut-lung axis regulation. Virus-activated lung CD4⁺ T cells upregulate CCR9 chemokine receptors and migrate to the intestinal mucosa, where Interferon-gamma (IFN-γ) secretion disrupts microbiota homeostasis [127]. Concurrently, gut innate lymphoid cells (ILCs) can extravasate into lung tissues through lymphatic or systemic circulation, where they modulate regional inflammation and coordinate synchronized immune responses between the two organs [128]. This form of cross-organ communication not only remotely remodels the lung immune microenvironment and affects lung cancer progression but also offers novel targets for improving responses to lung cancer immunotherapy by modulating the gut microbiota.
Gut-Lung Microbiota Axis Modulates Antitumor Immunotherapy
Clinical relevance: Microbial signatures predict the efficacy of immunotherapy response
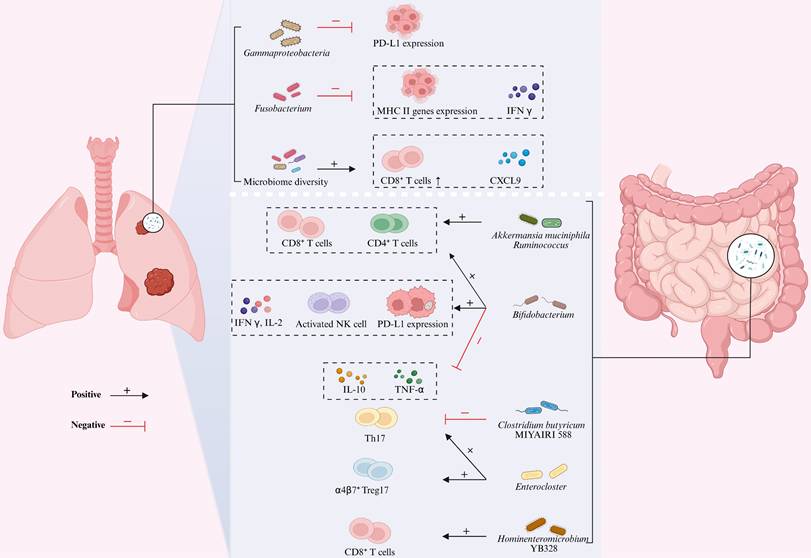
Multiple studies have demonstrated that specific gut and lung microbial signatures are closely associated with the efficacy of ICIs (Table 3). In ICI responders, the lung microbiota characterized by significantly increased α-diversity and enrichment of commensal taxa such as the Bacteroidetes and Veillonella dispar, these features are positively correlated with higher objective response rates (ORR), including complete response (CR), partial response (PR), and stable disease (SD) incidence [129, 130]. In contrast, the lung microenvironment of non-responders is predominantly occupied by Gammaproteobacteria and Fusobacterium, whose aberrant overgrowth is strongly associated with shorter progression-free survival (PFS) and overall survival (OS). Among these, the abundance of Gammaproteobacteria is negatively correlated with PD-L1 expression, whereas higher Fusobacterium abundance is associated with markedly reduced intratumoral cytotoxicity, IFN-γ levels, and expression of major histocompatibility complex (MHC) class II genes [99, 131]. The gut microbiota exhibits significant prognostic value. Characteristic microbial signatures including Akkermansia muciniphila (A. muciniphila), Clostridium butyricum MIYAIRI 588, and Bifidobacterium bifidum are significantly associated with prolonged PFS and OS [132-136]. Notably, the survival benefit conferred by A. muciniphila exhibits a distinct nonlinear relationship, with optimal therapeutic benefits observed at intermediate relative abundance (0%-4.8%). This range frequently co-occurs with other beneficial taxa such as Ruminococcus, Faecalibacterium, and Bifidobacterium, which have been established to correlate with favorable ICI outcomes [134, 137-139], underscoring microbiota interactions. Conversely, depletion or excessive proliferation of A. muciniphila typically coincides with enrichment of pathogenic taxa including Veillonella, Actinomyces, and Clostridium, which are associated with poorer prognosis [38, 140]. Furthermore, the strain Hominenteromicrobium YB328 has been found to be significantly enriched in the gut of responders and has been shown to act synergistically with anti-PD-1 therapy to potently inhibit tumor growth [141]. Clinical evidence indicates that antibiotic use disrupts host microbial homeostasis and may even lead to the overgrowth and recolonization of pathogenic bacteria (e.g., Enterocloster), thereby significantly reducing the efficacy of ICIs [132, 142-144]. Importantly, Clostridium butyricum MIYAIRI 588 has been shown to reverse antibiotic-induced dysbiosis and consequently restore survival in lung cancer models [133, 145]. Furthermore, gut microbial metabolism significantly influences the therapeutic efficacy of ICIs. Metabolomics studies have confirmed that in NSCLC patients, the relative abundance of SCFAs-producing gut microbiota, their metabolic products SCFAs [138, 146], as well as the levels of lysine and nicotinic acid, exhibit statistically significant positive correlations with durable clinical benefits from ICIs therapy [147]. These may potentially serve as biological markers for predicting the treatment response to ICIs.
Microbiota modulates response to immunotherapy in lung cancer patients.
| Year | Microbial origin | ICIs therapy | Microbes beneficial for antitumor therapy | Clinical benefit |
|---|---|---|---|---|
| 2021 [130] | Lung | PD-1/PD-L1 inhibitors | Veillonella dispar | Increased rates of CR, PR and SD. |
| 2021 [131] | Lung | PD-1/PD-L1 inhibitors | Gammaproteobacteria | Decreased PFS and OS. |
| 2022 [129] | Lung | PD-1/PD-L1 inhibitors | Bacteroidetes | Increased rates of PR and SD. |
| 2024 [99] | Lung | ICB-monotherapy | Fusobacterium | Decreased PFS and OS. |
| 2017 [132] | Gut | PD-1/PD-L1 inhibitors | Akkermansia muciniphila | Increased rates of PR, SD, and prolonged PFS. |
| 2020 [133] | Gut | PD-1/PD-L1 inhibitors | Clostridium butyricum MIYAIRI 588 | Prolonged PFS and OS. |
| 2020 [137] | Gut | PD-1/PD-L1 inhibitors | Ruminococcaceae UCG 13 and Agathobacter | Prolonged PFS and OS, improved ORR. |
| 2021 [134] | Gut | Chemo-immunotherapy combinations | Bifidobacterium bifidum | Increased rates of PR. |
| 2022 [135] | Gut | PD-1/PD-L1 inhibitors | Akkermansia muciniphila | Increased rates of CR/PR, and prolonged OS |
| 2024 [138] | Gut | PD-1/PD-L1 inhibitors | Faecalibacterium | Increased rates of CR and PR |
| 2025 [136] | Gut | PD-1/PD-L1 inhibitors, CTLA-4 inhibitors, and chemotherapy | Fusicatenibacter, Butyricicoccus and Blautia | Prolonged OS |
| 2025 [152] | Gut | PD-1/PD-L1 inhibitors | Bif.BEVs | Tumor burden reduction |
| 2025 [141] | Gut | PD-1/PD-L1 inhibitors | Hominenteromicrobium YB328 | Prolonged PFS |
| 2025 [178] | Gut | Chemo-immunotherapy combinations | Faecalibacterium and Subdoligranulum | Prolonged PFS |
* CTLA-4, cytotoxic T-lymphocyte-associated protein 4; CR, complete response; ICB, immune checkpoint blockade; ORR, objective response rates; OS, overall survival; PD-1, programmed death 1; PD-L1, programmed death-ligand 1; PR, partial response; PFS, progression-free survival; SD, stable disease.
Preclinical evidence: Microbiota and their Derivatives effectively enhance antitumor immunotherapy responses
Beyond mere clinical correlation, interventional studies have further established the causal role of specific microbial taxa and their derivatives in modulating antitumor immune responses. Previous studies have demonstrated that oral supplementation with A. muciniphila or specific Bifidobacterium bifidum strains (e.g., B. bif_K57) effectively restores the efficacy of ICIs in tumor-bearing mouse models [132, 134]. Compared to anti-PD-1 monotherapy, combination treatment with A. muciniphila promotes the recruitment of CCR9⁺CXCR3⁺CD4⁺ T cells into the TME, enhancing ICIs efficacy through an interleukin-12 (IL-12) -dependent mechanism [148]. Conversely, B. bif_K57 significantly enhances antitumor lymphocyte infiltration (including CD4+/CD8+ T cells and activated NK cells) within the TME, elevates both CD8+ T/Treg and effector CD8+ T/Treg ratios, and upregulates key antitumor cytokines such as IFN-γ and IL-2 [149], while suppressing TNF-α and IL-10, thereby mitigating chronic inflammation and restoring immune cell functionality [150,151]. Notably, the antitumor efficacy of microbiota extends beyond viable bacteria, with their bioactive components playing equally critical roles. The extracellular vesicles derived from Bifidobacterium bifidum (Bif.BEVs) can enhance the efficacy of ICIs in NSCLC patients [152]. Mechanistically, Bif.BEVs undergo dynamin-dependent endocytosis by tumor cells, activate the TLR4-NF-κB pathway, and upregulate PD-L1 expression, thereby promoting CD8⁺ T cell-dependent antitumor immunity. Additionally, similar to B. bif_K57, Bif.BEVs stimulate IFN-γ and IL-2 secretion. This functional conservation suggests that Bifidobacterium bifidum and its derivatives exert immunotherapeutic effects through convergent pathways. The probiotic Clostridium butyricum MIYAIRI 588, which is clinically used in NSCLC patients, enhances the efficacy of ICIs by promoting the colonization abundance of Bifidobacterium and suppresses tumor invasiveness by inhibiting Th17 cell differentiation, thereby exerting synergistic antitumor effects [145, 153]. Microbial metabolites also serve as a pivotal immunomodulatory axis. Experimental evidence shows that SCFAs, including butyrate and propionate, can enhance the efficacy of antitumor immunotherapy by promoting the activity of intratumoral effector T cells [154, 155]. Collectively, evidence from microbiota transplantation, administration of microbial structural components, and regulation of microbial metabolites establishes a causal role of gut microbiota and their derivatives in reshaping the TIME, highlighting their therapeutic potential as adjuvants to ICIs.
Molecular mechanisms: Deep pathways of cross-organ immune regulation
The molecular mechanisms underlying these therapeutic effects primarily involve multi-layered processes, including cross-organ migration of immune cells, activation of signaling pathways, and metabolic reprogramming (Figure 4). Research indicates that increased α-diversity of the lung microbiota shows a significant association with elevated C-X-C motif chemokine ligand 9 (CXCL9) secretion and enhanced CD8+ T cell infiltration in the tumor microenvironment (TME) [129]. As a key chemokine, CXCL9 primarily mediates the directional migration of lymphocytes to tumor sites, thereby suppressing tumor growth [156]. These findings suggest that the lung microbiota may regulate tumor sensitivity to ICIs by promoting CD8+ T cell recruitment via CXCL9-dependent mechanisms. Notably, a similar immunomodulatory mechanism exists in the gut microbiome, where patients with enrichment of Ruminococcus exhibit not only elevated frequencies of circulating effector CD4+ and CD8+ T cells but also significantly increased CD8+ T cell infiltration in tumor tissues [137]. In-depth research has revealed that the gut microbiota acts as the “central regulator” of immune cell migration. Lin et al. [141] demonstrated that Hominenteromicrobium YB328 in the gut of NSCLC patients induces maturation of intestinal CD103+CD11b- conventional dendritic cells (cDCs), enhancing their antigen-presenting capacity and costimulatory signaling, driving their migration to tumor-draining lymph nodes and subsequent infiltration into the TME. These cDCs locally activate tumor-specific CD8⁺ T cells while promoting PD-1 expression, thereby reshaping the local immune response and significantly improving PD-1 inhibitor efficacy [157]. However, when the gut microbiota balance is disrupted, it can exert completely opposite immunomodulatory effects. For example, antibiotic-induced overgrowth of Enterocloster downregulates intestinal mucosal addressin cell adhesion molecule-1 (MAdCAM-1), leading to the aberrant migration of α4(CD49d) β7+ Th17 and Treg17 cells from the circulation to the gut and tumor tissues [144]. Given the potent immunosuppressive function of these cells, their tumor infiltration significantly diminishes the therapeutic efficacy of ICIs [158].
Gut-Lung Axis Microbiota Modulates Immunotherapy Efficacy in Lung Cancer. The gut-lung axis microbiota influences lung cancer progression through multi-layered immune regulatory mechanisms. Key microbial effects in the lung are characterized by: the negative correlation between Gammaproteobacteria abundance and PD-L1 expression, while Fusobacterium significantly suppresses IFN-γ and MHC class II gene expression; increased microbial α-diversity promotes CXCL9 secretion in the tumor microenvironment and CD8⁺ T cell recruitment, thereby enhancing PD-1 blockade efficacy. Key effects of gut microbiota are manifested as follows: Akkermansia muciniphila directly promotes the recruitment of tumor-infiltrating CD4⁺/CD8⁺ T cells; at low abundance, it synergizes with Ruminococcaceae, whose enrichment significantly expands circulating effector CD4⁺/CD8⁺ T cells and enhances tumor CD8⁺ T cell infiltration; the combination of Bifidobacterium with anti-PD-1 therapy simultaneously increases the numbers of tumor-infiltrating CD4⁺ T/CD8⁺ T/NK cells and the CD8⁺ T/Treg ratio, specifically upregulating IFN-γ/IL-2 while suppressing TNF-α/IL-10; Clostridium butyricum MIYAIRI 588 reduces tumor cell invasiveness by inhibiting Th17; the recolonization of Enterocloster in the gut drives the migration of α4β7⁺ Treg17 and IL-17-producing Th17 cells to tumor tissues, forming a complete microbiota-immune regulatory network. Figure 4 was created using BioRender and holds the appropriate license. C-X-C motif chemokine ligand 9, CXCL9; Interferon-γ, IFN-γ; Major histocompatibility complex, MHC; Mucosal addressin cell adhesion molecule 1, MAdCAM-1; Natural killer, NK; Interferon-gamma, IFN-γ; Programmed death-ligand 1, PD-L1 T-helper, Th17; Regulatory T cells, Tregs.
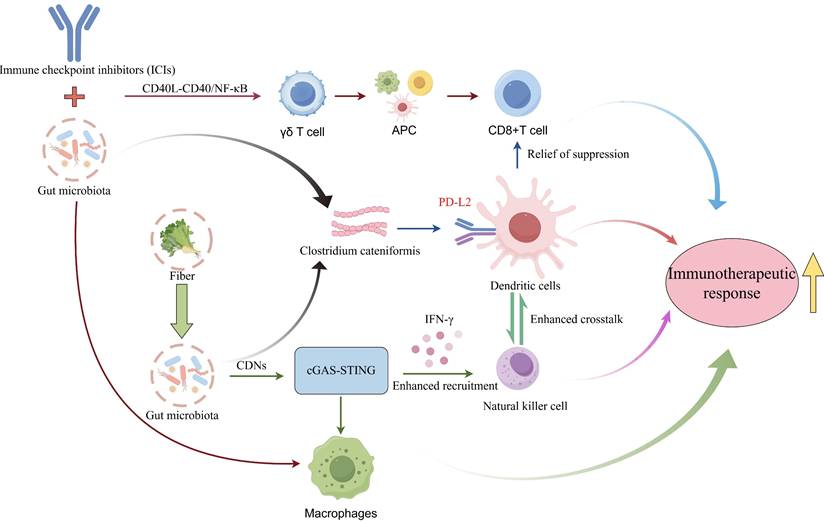
Specific microbial species and their metabolites can directly activate antitumor immune responses by modulating key immunological signaling pathways (Figure 5). As a critical bridge connecting innate and adaptive immunity, activation of the cGAS-STING (cyclic GMP-AMP synthase-stimulator of interferon genes) pathway and subsequent robust production of type I interferons (IFN-I) is essential for breaking tumor immune tolerance [159]. Research demonstrates that gut microbiota-derived cyclic dinucleotides (CDNs) under high-fiber dietary conditions serve as natural STING agonists, stimulating IFN-I production by tumor-infiltrating monocytes. This signaling axis not only recruits NK cells and reprograms macrophage polarization but also enhances crosstalk between NK cells and DCs, thereby significantly improving immune surveillance within the TME [160]. Combined intact microbiota-ICI therapy enhances effector-memory CD8+ T cell expansion while preventing terminal exhaustion, and shifts macrophages from SPP1+ protumoral to CD74+ antigen-presenting states, establishing a γδ T cell-antigen-presenting cell (APC)-CD8+ T cell activation loop via CD40L-CD40/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling [161]. Although FMT is known to influence the efficacy of immunotherapy, treatment outcomes remain inconsistent. Emerging research reveals that beneficial gut commensals such as Clostridium cateniformis can downregulate PD-L2 expression on DCs. This alteration disrupts the immunoinhibitory PD-L2/repulsive guidance molecule B (RGMb) interaction, thereby relieving T-cell suppression and enhancing antitumor immune response [162].
Gut Microbiota Enhances Lung Cancer Immunotherapeutic Response via various Signaling Pathways. Gut microbiota synergistically modulates the TIME via multiple core signaling cascades, thereby potentiating immunotherapeutic responses in lung cancer. Specifically, high-fiber diet-induced gut microbiota produces CDNs, which serve as natural agonists to activate the cGAS-STING pathway, promote IFNs secretion, recruit NK cells, reprogram macrophage polarization, enhance crosstalk between NK cells and DCs, and improve immune surveillance within the tumor microenvironment. Additionally, combined therapy with intact gut microbiota and ICIs activates the CD40L-CD40/NF-κB pathway, driving the phenotypic switch of macrophages from SPP1⁺ protumoral to CD74⁺ antigen-presenting states, facilitating the expansion of effector-memory CD8⁺ T cells while preventing their terminal exhaustion, and constructing a γδ T cell-APC-CD8⁺ T cell activation loop. Furthermore, beneficial gut commensals such as Clostridium cateniformis downregulate the expression of PD-L2 on DCs, blocking the immunoinhibitory interaction between PD-L2 and RGMb to relieve T cell suppression. These pathways form functional crosstalk via core immune cells including macrophages and DCs, ultimately synergistically enhancing lung cancer immunotherapeutic response and breaking tumor immune tolerance. Cyclic dinucleotides, CDNs; cyclic GMP-AMP synthase-stimulator of interferon genes, cGAS-STING; interferon, IFN; natural killer cells, NK cells; dendritic cells, DCs; immune checkpoint inhibitor, ICI; nuclear factor kappa-light-chain-enhancer of activated B cells, NF-κB; antigen-presenting cell, APC; programmed death ligand 2, PD-L2; repulsive guidance molecule B, RGMb; tumor immune microenvironment, TIME
The gut microbiota and its metabolic byproducts act as critical immunoregulatory agents that significantly shape the TME. Research by Zhu et al. [163] demonstrates that gut-derived A. muciniphila migrates via the bloodstream to colonize lung adenocarcinoma tissue, exerting antitumor effects. This activity likely stems from its crosstalk with intratumoral microbiota, which reprograms local metabolic pathways, particularly purine, glutathione, and arginine-proline metabolism [164]. Microbial metabolites constitute deep immune regulatory mediators, and their produced small molecule signals can influence tumor immune responses through the gut-lung axis pathway [165, 166]. It is noteworthy that SCFAs in lung tissue are primarily produced in large quantities by gut microbiota through fermentation of dietary fiber, while the contribution of lung microbes is relatively limited [167]. Intestinal-derived SCFAs regulate the generation of Ly6C⁻ patrolling monocytes in the bone marrow through systemic circulation. These monocytes migrate to the lungs and differentiate into macrophages, which reduce neutrophil chemokine CXCL1 expression to blunt airway neutrophil recruitment during infection while enhancing CD8⁺ T cell responses, thereby limiting lung tissue pathology caused by inflammation [168]. Emerging evidence demonstrates that butyrate enhances the antitumor immune response of cytotoxic CD8⁺ T cells through the IL-12 signaling pathway both in vitro and in vivo [169]. Other SCFAs, such as sodium butyrate and sodium propionate, exhibit potential in suppressing lung cancer cell growth, triggering apoptosis, inducing cell cycle arrest, and modulating immune responses [154, 170]. The gut-lung microbiota axis modulates antitumor immune responses through the aforementioned mechanisms. However, it must be emphasized that current mechanistic insights predominantly rely on murine models, lacking validation in human studies. Given the immunological, microbial, and pharmacological distinctions between murine and human systems, translational applications of current findings require further clinical validation.
Clinical Translation Pathway of the Gut-Lung Microbiota Axis: From Prediction to Intervention
Clinical Translational Pathways of the Gut-Lung Microbiota Axis
The clinical translational pathway of the gut-lung microbiota involves the construction of predictive models and the application of intervention strategies. Microbial marker studies demonstrate that a lung cancer prediction model based on the Streptococcus single microbial species showed an area under the curve (AUC) of 0.693-0.897, further research revealed that a combination prediction model constructed with three characteristic operational taxonomic units (OTU19/594/645) selected from this genus exhibited significantly improved efficacy, achieving an AUC of 0.701 [27, 36]. The two-bacteria synergistic level demonstrates that the diagnostic prediction model for lung cancer combining Veillonella and Megasphaera achieves an AUC of 0.880 [55], while the more specific Veillonella and Capnocytophaga combination predicts squamous cell carcinoma (SCC) with an AUC of 0.860 [171]. Moreover, at the multi-dimensional integration level, breakthrough achievements have been presented. Zheng et al. [51] established an early diagnostic model by screening 13 gut OTUs using machine learning, achieving AUC values of 0.976 in the training set and 0.764 in the validation cohort. The Liu team developed a LUAD diagnosis model based on fungal features, ultimately incorporating 19 characteristic gut OTUs, with the predictive model achieving an AUC of 0.935 [42]. Extensive research that integrates microbial SCFAs, such as acetic acid and butyric acid [26], and tumor markers including neuron-specific enolase (NSE) [35], enhances the model's predictive performance to an AUC range of 0.959-0.993. Meanwhile, the incorporation of age and smoking factors attains an AUC of 0.882 [56]. As lung cancer prediction models undergo continuous optimization, these microbial biomarkers are being innovatively utilized for immunotherapy response prediction, thereby manifesting comprehensive clinical value spanning from diagnosis to treatment. Huang et al. [172] performed fecal microbiome sequencing before immunotherapy on 862 lung cancer patients and screened 26 characteristic fungi (primarily belonging to the Ascomycota phylum) through machine learning, establishing an immunotherapy response prediction model with AUC reaching 0.870. In responders, the abundance of 20 fungi such as Trichophyton benhamiae was significantly increased, whereas in non-responders, six fungi including Pseudocercospora musae were markedly enriched. The model predictions significantly correlated with patients' longer OS and PFS. Moreover, the model effectively distinguished the exhaustion level of CD8+ T cells and the expression of immune checkpoints, such as PD-1, CTLA-4, and T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), within the TME. These molecules are associated with the response to immunotherapy [173, 174]. Furthermore, integrating 20 fungal and 17 bacterial species, the study built a multi-kingdom microbial model with AUC of 0.890. Network analysis indicated that the core species Schizosaccharomyces octosporus modulates starch metabolic pathway, and its produced SCFAs may mediate anti-cancer effects [175-177]. Yang et al. applied a support vector machine (SVM) machine learning model to predict the response efficacy to the combined chemotherapy and anti-PD-1 immunotherapy regimen in NSCLC patients by analyzing gut microbiome composition data. When adopting the top 20 most predictive microbial features at the genus level, the AUC was 0.763; upgrading to species-level features elevated the AUC to 0.855, indicating that higher classification accuracy can optimize predictive performance [178]. Additionally, dysbiosis in the human body correlates with immune-related adverse events (irAEs). Lower gut microbial α diversity associates with increased risk of severe adverse events (≥ grade 4) [136]. Furthermore, abundances of specific microbes such as Akkermansia, Lactobacillaceae, and Raoultella associate with decreased risk of irAEs, while Agathobacter increases the risk of irAEs [21]. In conclusion, this study demonstrates that disease diagnostic and prognostic models based on multi-species microbial signatures provide greater clinical value than those relying on single microbial strains. Microbiota-metabolomics integrated prediction models demonstrate considerable potential for lung cancer diagnosis and predicting immunotherapy efficacy. Nevertheless, their clinical validation remains inadequate, as current models are primarily developed using limited-scale cohorts from single-center or public databases with population homogeneity. This fundamentally restricts their external validity. Large-scale multicenter prospective validation studies are thus critically needed to establish their real-world clinical applicability.
Clinical Intervention Potential of Microbiota
The clinical intervention potential of microbes has emerged as a key research focus in current studies. Accumulating evidence indicates that gut microbiome remodeling through FMT and probiotic supplementation can significantly enhance therapeutic response rates across various malignancies. In murine models, transplantation of fecal microbiota from either ICIs responders or non-responders among NSCLC patients into tumor-bearing mice revealed that responder-derived FMT induced substantial antitumor responses, whereas non-responder-derived FMT demonstrated limited therapeutic efficacy [20]. Clinical investigations by Wu et al. [179] identified Alistipes finegoldii as a key microbial species associated with improved immunotherapy outcomes in multiple solid tumors, including NSCLC. Based on this discovery, a phase I/II clinical trial (NCT05557696) evaluating its therapeutic supplementation has been initiated, signifying a crucial transition toward clinical translation of microbiota-targeted interventions. Notably, the therapeutic efficacy of FMT in various cancers has been substantiated by multiple studies. Baruch et al. [180] demonstrated that melanoma patients receiving FMT from immunotherapy responders achieved a 30% ORR, with all treated subjects maintaining PFS for at least six months. Subsequent studies have further validated this approach in melanoma [181-183], colorectal cancer [184], and pan-cancer cohorts [185], confirming that fecal microbiota from healthy donors or treatment responders not only enhances immunotherapy efficacy and overcomes resistance but also significantly improves ORR and prolongs both PFS and OS, thereby establishing a novel paradigm for cancer immunotherapy. The safety profile of FMT remains a critical clinical concern, as evidenced by case reports of Clostridium perfringens infections post-FMT [186]. Our systematic evaluation of adverse event (AE) data from the aforementioned studies reveals that 40% of investigations failed to report FMT-related AEs, while the remaining studies documented AE incidence rates ranging from 40% to 53.8% [182, 183, 185]. Notably, the majority of AEs comprised mild gastrointestinal manifestations, including diarrhea, abdominal distension, and emesis, with no clustering of severe adverse events observed. Recent improvements in FMT safety primarily stem from the implementation of stringent donor screening protocols: the integration of sequencing and culture techniques to exclude samples containing pathogenic microorganisms has substantially mitigated infection risks. Compared to the complexity of FMT, oral probiotics (e.g., Clostridium butyricum, Bifidobacterium) demonstrate better clinical translatability owing to scalable production. Clinical evidence confirms their ability to potentiate immunotherapy, elevating response rates and PFS in lung, renal and urothelial carcinomas [133, 187-190]. However, contradictory findings have emerged, as one retrospective melanoma study reported an unexpected association between probiotic use and impaired PFS. The discrepancy could stem from using non-specific commercial probiotics with limited immunomodulatory effects and an underpowered sample size [191]. Notably, this study further indicates that baseline gut microbiota characteristics significantly influence probiotic efficacy, underscoring the need for microbial assessment prior to probiotic intervention in clinical practice. Furthermore, existing studies have investigated the efficacy-enhancing effects of clinically established postbiotic JK5G [192] and Microecosystem Therapy 4 (MET4) [193] in combination with immunotherapy: administration of JK5G has been shown to reduce the incidence of immune-related adverse events (irAEs), while MET4 significantly improves clinical benefit rates. Notably, no adverse effects associated with probiotic or postbiotic supplementation have been reported to date, demonstrating their favorable safety profile. In summary, modulating gut microbiota through FMT and specific probiotic supplementation can effectively enhance tumor immunotherapy response rates, overcome drug resistance, and prolong patient survival. Comparatively, probiotic formulations show greater clinical translational potential due to their favorable safety profile. However, significant limitations remain including inadequate FMT research in lung cancer, considerable variability in probiotic efficacy, insufficient sample sizes, and unclear strain specificity, along with unresolved safety concerns regarding FMT and incomplete mechanistic understanding of probiotic actions. Future research should prioritize screening high-efficacy bacterial strains with expanded validation efforts while establishing standardized microbiota assessment protocols to facilitate standardized clinical application of microbial-based therapies.
Conclusion
As the leading cause of cancer incidence worldwide, lung cancer presents significant challenges in early prevention and prognosis improvement. The gut-lung microbiota axis has emerged as a pivotal hub in cross-organ communication, playing a critical role in disease progression and treatment response. Current evidence underscores that lung dysbiosis drives tumorigenesis through chronic inflammation and mucosal barrier disruption, whereas gut homeostasis and its metabolites can remodel the systemic immune microenvironment and regulate the bone marrow-lung axis, thereby enhancing antitumor surveillance and sensitivity to ICIs. However, the translation of these findings faces a critical bottleneck. The core regulatory mechanisms are predominantly derived from animal models, while direct human validation remains scarce. Although clinical studies have observed correlations between the abundance of specific microbiota (e.g., Akkermansia muciniphila) and therapeutic responses or irAEs, direct evidence remains lacking regarding how these functional microbes precisely modulate human immunity and how their metabolites directly activate CD8⁺ T cells in vivo. Consequently, future research must focus on "bridging the clinical evidence gap". Efforts should prioritize the integration of large-scale clinical cohorts, multi-omics approaches, and patient-derived organoid models to systematically decode the gut-lung interaction rules within the human context. Furthermore, developing high-sensitivity and high-specificity predictive models based on distinct microbiome signatures is essential. Ultimately, these advancements will pave the way for precise, microbiome-targeted intervention strategies, moving the field from correlative studies to clinical translation and offering innovative solutions to optimize ICIs regimens and improve patient outcomes in lung cancer.
Supplementary Material
Supplementary figure and tables.
Acknowledgements
The figures were created by FigDraw (https://www.figdraw.com/#/) and BioRender (https://app.biorender.com/).
Funding
This study was supported by Natural Science Foundation of China (No. 82200113, No.82170105, No.82300123, No.82370095, No.82400119).
Author contributions
YL and SW drafted the manuscript. XX and YD designed and prepared the figures and tables. QX and WP carried out literature review and related research. YN, LY, and QZ assisted in reviewing and polishing the manuscript and submitted the final version.
Competing Interests
The authors have declared that no competing interests exist.
References
1. GBD 2023 Cancer Collaborators. The global, regional, and national burden of cancer, 1990-2023, with forecasts to 2050: a systematic analysis for the Global Burden of Disease Study 2023. Lancet. 2025;406(10512):1565-1586
2. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10-45
3. Wang SJ, Dougan SK, Dougan M. Immune mechanisms of toxicity from checkpoint inhibitors. Trends Cancer. 2023;9(7):543-553
4. Reck M, Remon J, Hellmann MD. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J Clin Oncol. 2022;40(6):586-597
5. Memon D, Schoenfeld AJ, Ye D. et al. Clinical and molecular features of acquired resistance to immunotherapy in non-small cell lung cancer. Cancer Cell. 2024;42(2):209-224.e9
6. Rizvi NA, Hellmann MD, Snyder A. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124-128
7. Gavish A, Tyler M, Greenwald AC. et al. Hallmarks of transcriptional intratumour heterogeneity across a thousand tumours. Nature. 2023;618(7965):598-606
8. Goodman AM, Kato S, Bazhenova L. et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther. 2017;16(11):2598-2608
9. Wu S, Rhee KJ, Albesiano E. et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15(9):1016-1022
10. Viaud S, Saccheri F, Mignot G. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971-976
11. Iida N, Dzutsev A, Stewart CA. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967-970
12. Vétizou M, Pitt JM, Daillère R. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079-1084
13. Dickson RP, Erb-Downward JR, Martinez FJ, Huffnagle GB. The Microbiome and the Respiratory Tract. Annu Rev Physiol. 2016;78:481-504
14. Budden KF, Shukla SD, Rehman SF. et al. Functional effects of the microbiota in chronic respiratory disease. Lancet Respir Med. 2019;7(10):907-920
15. Nejman D, Livyatan I, Fuks G. et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368(6494):973-980
16. Wang L, Cai Y, Garssen J, Henricks PAJ, Folkerts G, Braber S. The Bidirectional Gut-Lung Axis in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2023;207(9):1145-1160
17. Sencio V, Machado MG, Trottein F. The lung-gut axis during viral respiratory infections: the impact of gut dysbiosis on secondary disease outcomes. Mucosal Immunol. 2021;14(2):296-304
18. Budden KF, Gellatly SL, Wood DL. et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15(1):55-63
19. Wang Y, He F, Liu B. et al. Interaction between intestinal mycobiota and microbiota shapes lung inflammation. Imeta. 2024;3(5):e241
20. Shaikh FY, Gills JJ, Mohammad F. et al. Murine fecal microbiota transfer models selectively colonize human microbes and reveal transcriptional programs associated with response to neoadjuvant checkpoint inhibitors. Cancer Immunol Immunother. 2022;71(10):2405-2420
21. Zhang H, Xu Z. Gut-lung axis: role of the gut microbiota in non-small cell lung cancer immunotherapy. Front Oncol. 2023;13:1257515
22. Yu G, Gail MH, Consonni D. et al. Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol. 2016;17(1):163
23. Kim OH, Choi BY, Kim DK. et al. The microbiome of lung cancer tissue and its association with pathological and clinical parameters. Am J Cancer Res. 2022;12(5):2350-2362
24. Liu NN, Yi CX, Wei LQ. et al. The intratumor mycobiome promotes lung cancer progression via myeloid-derived suppressor cells. Cancer Cell. 2023;41(11):1927-1944.e9
25. Zheng L, Sun R, Zhu Y. et al. Lung microbiome alterations in NSCLC patients. Sci Rep. 2021;11(1):11736
26. Zhang Y, Chen X, Wang Y. et al. Alterations of lower respiratory tract microbiome and short-chain fatty acids in different segments in lung cancer: a multiomics analysis. Front Cell Infect Microbiol. 2023;13:1261284
27. Liu HX, Tao LL, Zhang J. et al. Difference of lower airway microbiome in bilateral protected specimen brush between lung cancer patients with unilateral lobar masses and control subjects. Int J Cancer. 2018;142(4):769-778
28. Ma Y, Chen H, Li H. et al. Intratumor microbiome-derived butyrate promotes lung cancer metastasis. Cell Rep Med. 2024;5(4):101488
29. Huang DH, He J, Su XF. et al. The airway microbiota of non-small cell lung cancer patients and its relationship to tumor stage and EGFR gene mutation. Thorac Cancer. 2022;13(6):858-869
30. Vogtmann E, Hua X, Yu G. et al. The Oral Microbiome and Lung Cancer Risk: An Analysis of 3 Prospective Cohort Studies. J Natl Cancer Inst. 2022;114(11):1501-1510
31. Greathouse KL, White JR, Vargas AJ. et al. Interaction between the microbiome and TP53 in human lung cancer. Genome Biol. 2018;19(1):123
32. Liu Y, O'Brien JL, Ajami NJ. et al. Lung tissue microbial profile in lung cancer is distinct from emphysema. Am J Cancer Res. 2018;8(9):1775-1787
33. Ankudavicius V, Nikitina D, Lukosevicius R. et al. Detailed Characterization of the Lung-Gut Microbiome Axis Reveals the Link between PD-L1 and the Microbiome in Non-Small-Cell Lung Cancer Patients. Int J Mol Sci. 2024;25(4):2323
34. Xia X, Chen J, Cheng Y. et al. Comparative analysis of the lung microbiota in patients with respiratory infections, tuberculosis, and lung cancer: A preliminary study. Front Cell Infect Microbiol. 2022;12:1024867
35. Chen Q, Hou K, Tang M. et al. Screening of potential microbial markers for lung cancer using metagenomic sequencing. Cancer Med. 2023;12(6):7127-7139
36. Bello S, Vengoechea JJ, Ponce-Alonso M. et al. Core Microbiota in Central Lung Cancer With Streptococcal Enrichment as a Possible Diagnostic Marker. Arch Bronconeumol. 2021;57(11):681-689
37. Qian K, Deng Y, Krimsky WS. et al. Airway Microbiota in Patients With Synchronous Multiple Primary Lung Cancer: The Bacterial Topography of the Respiratory Tract. Front Oncol. 2022;12:811279
38. Tsay JJ, Wu BG, Sulaiman I. et al. Lower Airway Dysbiosis Affects Lung Cancer Progression. Cancer Discov. 2021;11(2):293-307
39. Huang D, Su X, Yuan M. et al. The characterization of lung microbiome in lung cancer patients with different clinicopathology. Am J Cancer Res. 2019;9(9):2047-2063
40. Sun Y, Liu Y, Li J. et al. Characterization of Lung and Oral Microbiomes in Lung Cancer Patients Using Culturomics and 16S rRNA Gene Sequencing. Microbiol Spectr. 2023;11(3):e0031423
41. Liu F, Li J, Guan Y. et al. Dysbiosis of the Gut Microbiome is associated with Tumor Biomarkers in Lung Cancer. Int J Biol Sci. 2019;15(11):2381-2392
42. Liu Q, Zhang W, Pei Y. et al. Gut mycobiome as a potential non-invasive tool in early detection of lung adenocarcinoma: a cross-sectional study. BMC Med. 2023;21(1):409
43. Luan J, Zhang F, Suo L. et al. Analyzing lung cancer risks in patients with impaired pulmonary function through characterization of gut microbiome and metabolites. BMC Pulm Med. 2024;24(1):1
44. Tsay JJ, Wu BG, Badri MH. et al. Airway Microbiota Is Associated with Upregulation of the PI3K Pathway in Lung Cancer. Am J Respir Crit Care Med. 2018;198(9):1188-1198
45. Bingula R, Filaire E, Molnar I. et al. Characterisation of microbiota in saliva, bronchoalveolar lavage fluid, non-malignant, peritumoural and tumour tissue in non-small cell lung cancer patients: a cross-sectional clinical trial. Respir Res. 2020;21(1):129
46. Zhao Y, Yi J, Xiang J. et al. Exploration of lung mycobiome in the patients with non-small-cell lung cancer. BMC Microbiol. 2023;23(1):81
47. Han W, Wang N, Han M, Liu X, Sun T, Xu J. Identification of microbial markers associated with lung cancer based on multi-cohort 16 s rRNA analyses: A systematic review and meta-analysis. Cancer Med. 2023;12(18):19301-19319
48. Zhang WQ, Zhao SK, Luo JW. et al. Alterations of fecal bacterial communities in patients with lung cancer. Am J Transl Res. 2018;10(10):3171-3185
49. Zhuang H, Cheng L, Wang Y. et al. Dysbiosis of the Gut Microbiome in Lung Cancer. Front Cell Infect Microbiol. 2019;9:112
50. Guo Y, Yuan W, Lyu N. et al. Association Studies on Gut and Lung Microbiomes in Patients with Lung Adenocarcinoma. Microorganisms. 2023;11(3):546
51. Zheng Y, Fang Z, Xue Y. et al. Specific gut microbiome signature predicts the early-stage lung cancer. Gut Microbes. 2020;11(4):1030-1042
52. Zhao F, An R, Wang L, Shan J, Wang X. Specific Gut Microbiome and Serum Metabolome Changes in Lung Cancer Patients. Front Cell Infect Microbiol. 2021;11:725284
53. Ni B, Kong X, Yan Y, Fu B, Zhou F, Xu S. Combined analysis of gut microbiome and serum metabolomics reveals novel biomarkers in patients with early-stage non-small cell lung cancer. Front Cell Infect Microbiol. 2023;13:1091825
54. Luan J, Zhang F, Suo L. et al. Analyzing lung cancer risks in patients with impaired pulmonary function through characterization of gut microbiome and metabolites. BMC Pulm Med. 2024;24(1):1
55. Lee SH, Sung JY, Yong D. et al. Characterization of microbiome in bronchoalveolar lavage fluid of patients with lung cancer comparing with benign mass like lesions. Lung Cancer. 2016;102:89-95
56. Jin J, Gan Y, Liu H. et al. Diminishing microbiome richness and distinction in the lower respiratory tract of lung cancer patients: A multiple comparative study design with independent validation. Lung Cancer. 2019;136:129-135
57. Qian X, Zhang HY, Li QL. et al. Integrated microbiome, metabolome, and proteome analysis identifies a novel interplay among commensal bacteria, metabolites and candidate targets in non-small cell lung cancer. Clin Transl Med. 2022;12(6):e947
58. Segal LN, Alekseyenko AV, Clemente JC. et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome. 2013;1(1):19
59. Narunsky-Haziza L, Sepich-Poore GD, Livyatan I. et al. Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell. 2022;185(20):3789-3806.e17
60. Dohlman AB, Klug J, Mesko M. et al. A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell. 2022;185(20):3807-3822.e12
61. Brisudová A, Bielniková-Kryštofová H, Motyka O. et al. Microbiota Diversity in Non-Small Cell Lung Cancer Gut and Mouth Cavity Microbiota Diversity in Non-Small Cell Lung Cancer Patients. Pol J Microbiol. 2023;72(4):467-475
62. Li Y, Liu T, Yan C. et al. Diammonium Glycyrrhizinate Protects against Nonalcoholic Fatty Liver Disease in Mice through Modulation of Gut Microbiota and Restoration of Intestinal Barrier. Mol Pharm. 2018;15(9):3860-3870
63. Verdam FJ, Fuentes S, de Jonge C. et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity (Silver Spring). 2013;21(12):E607-E615
64. Tett A, Pasolli E, Masetti G, Ercolini D, Segata N. Prevotella diversity, niches and interactions with the human host. Nat Rev Microbiol. 2021;19(9):585-599
65. Dame-Teixeira N, de Lima AKA, Do T, Stefani CM. Meta-Analysis Using NGS Data: The Veillonella Species in Dental Caries. Front Oral Health. 2021;2:770917
66. Wang D, Cheng J, Zhang J. et al. The Role of Respiratory Microbiota in Lung Cancer. Int J Biol Sci. 2021;17(13):3646-3658
67. Goto T. Microbiota and lung cancer. Semin Cancer Biol. 2022;86(Pt 3):1-10
68. Bou Zerdan M, Kassab J, Meouchy P. et al. The Lung Microbiota and Lung Cancer: A Growing Relationship. Cancers (Basel). 2022;14(19):4813
69. Liu W, Xu J, Pi Z. et al. Untangling the web of intratumor microbiota in lung cancer. Biochim Biophys Acta Rev Cancer. 2023;1878(6):189025
70. Jin C, Lagoudas GK, Zhao C. et al. Commensal Microbiota Promote Lung Cancer Development via γδ T Cells. Cell. 2019;176(5):998-1013.e16
71. Iksen Pothongsrisit S, Pongrakhananon V. Targeting the PI3K/AKT/mTOR Signaling Pathway in Lung Cancer: An Update Regarding Potential Drugs and Natural Products. Molecules. 2021;26(13):4100
72. Prakash P, Verma S, Gupta S. Influence of microbiome in intraprostatic inflammation and prostate cancer. Prostate. 2024;84(13):1179-1188
73. Nambu A, Nakae S. IL-1 and Allergy. Allergol Int. 2010;59(2):125-135
74. Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40(7):1830-1835
75. Ritzmann F, Lunding LP, Bals R, Wegmann M, Beisswenger C. IL-17 Cytokines and Chronic Lung Diseases. Cells. 2022;11(14):2132
76. Mills KHG. IL-17 and IL-17-producing cells in protection versus pathology. Nat Rev Immunol. 2023;23(1):38-54
77. Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. 2022;22(3):173-187
78. Tan Z, Xue H, Sun Y, Zhang C, Song Y, Qi Y. The Role of Tumor Inflammatory Microenvironment in Lung Cancer. Front Pharmacol. 2021;12:688625
79. Wong-Rolle A, Dong Q, Zhu Y. et al. Spatial meta-transcriptomics reveal associations of intratumor bacteria burden with lung cancer cells showing a distinct oncogenic signature. J Immunother Cancer. 2022;10(7):e004698
80. Song P, Gao Z, Bao Y. et al. Wnt/β-catenin signaling pathway in carcinogenesis and cancer therapy. J Hematol Oncol. 2024;17(1):46
81. Liu J, Xiao Q, Xiao J. et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7(1):3
82. Bae T, Hallis SP, Kwak MK. Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer. Exp Mol Med. 2024;56(3):501-514
83. Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273(2):114-127
84. Tomasini R, Mak TW, Melino G. The impact of p53 and p73 on aneuploidy and cancer. Trends Cell Biol. 2008;18(5):244-252
85. Seo B, Lim MY. Balancing harm and harmony: Evolutionary dynamics between gut microbiota-derived flagellin and TLR5-mediated host immunity and metabolism. Virulence. 2025;16(1):2512035
86. Lasser SA, Ozbay Kurt FG, Arkhypov I, Utikal J, Umansky V. Myeloid-derived suppressor cells in cancer and cancer therapy. Nat Rev Clin Oncol. 2024;21(2):147-164
87. Tay C, Tanaka A, Sakaguchi S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell. 2023;41(3):450-465
88. Vonderheide RH, Bear AS. Tumor-Derived Myeloid Cell Chemoattractants and T Cell Exclusion in Pancreatic Cancer. Front Immunol. 2020;11:605619
89. Sahoo BM, Banik BK, Borah P, Jain A. Reactive Oxygen Species (ROS): Key Components in Cancer Therapies. Anticancer Agents Med Chem. 2022;22(2):215-222
90. Sharma BR, Kanneganti TD. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22(5):550-559
91. Alam A, Levanduski E, Denz P. et al. Fungal mycobiome drives IL-33 secretion and type 2 immunity in pancreatic cancer. Cancer Cell. 2022;40(2):153-167.e11
92. Bernstein ZJ, Shenoy A, Chen A, Heller NM, Spangler JB. Engineering the IL-4/IL-13 axis for targeted immune modulation. Immunol Rev. 2023;320(1):29-57
93. Snelgrove RJ, Gregory LG, Peiró T. et al. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134(3):583-592.e6
94. Hu XT, Xing W, Zhao RS. et al. HDAC2 inhibits EMT-mediated cancer metastasis by downregulating the long noncoding RNA H19 in colorectal cancer. J Exp Clin Cancer Res. 2020;39(1):270
95. Li L, Han T, Liu K, Lei CG, Wang ZC, Shi GJ. LncRNA H19 promotes the development of hepatitis B related hepatocellular carcinoma through regulating microRNA-22 via EMT pathway. Eur Rev Med Pharmacol Sci. 2019;23(12):5392-5401
96. Jia YC, Wang JY, Liu YY, Li B, Guo H, Zang AM. LncRNA MAFG-AS1 facilitates the migration and invasion of NSCLC cell via sponging miR-339-5p from MMP15. Cell Biol Int. 2019;43(4):384-393
97. Li M, Yang Y, Xiong L, Jiang P, Wang J, Li C. Metabolism, metabolites, and macrophages in cancer. J Hematol Oncol. 2023;16(1):80
98. Wang S, Liu G, Li Y, Pan Y. Metabolic Reprogramming Induces Macrophage Polarization in the Tumor Microenvironment. Front Immunol. 2022;13:840029
99. Battaglia TW, Mimpen IL, Traets JJH. et al. A pan-cancer analysis of the microbiome in metastatic cancer. Cell. 2024;187(9):2324-2335.e19
100. Rizvi ZA, Dalal R, Sadhu S. et al. High-salt diet mediates interplay between NK cells and gut microbiota to induce potent tumor immunity. Sci Adv. 2021;7(37):eabg5016
101. Jang DI, Lee AH, Shin HY. et al. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int J Mol Sci. 2021;22(5):2719
102. Vilotić A, Nacka-Aleksić M, Pirković A, Bojić-Trbojević Ž, Dekanski D, Jovanović Krivokuća M. IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. Int J Mol Sci. 2022;23(23):14574
103. Sakuragi T, Nagata S. Regulation of phospholipid distribution in the lipid bilayer by flippases and scramblases. Nat Rev Mol Cell Biol. 2023;24(8):576-596
104. Janneh AH, Atkinson C, Tomlinson S, Ogretmen B. Sphingolipid metabolism and complement signaling in cancer progression. Trends Cancer. 2023;9(10):782-787
105. Lu Y, Peng B, Lin Y. et al. Spirulina polysaccharide induces the metabolic shifts and gut microbiota change of lung cancer in mice. Curr Res Food Sci. 2022;5:1313-1319
106. Le Bel M, Brunet A, Gosselin J. Leukotriene B4, an endogenous stimulator of the innate immune response against pathogens. J Innate Immun. 2014;6(2):159-168
107. Jang JH, Park D, Park GS. et al. Leukotriene B4 receptor-2 contributes to KRAS-driven lung tumor formation by promoting interleukin-6-mediated inflammation. Exp Mol Med. 2021;53(10):1559-1568
108. Tager AM, Bromley SK, Medoff BD. et al. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol. 2003;4(10):982-990
109. Hagihara M, Kato H, Yamashita M. et al. Lung cancer progression alters lung and gut microbiomes and lipid metabolism. Heliyon. 2023;10(1):e23509
110. Zhou P, Zou Z, Wu W. et al. The gut-lung axis in critical illness: microbiome composition as a predictor of mortality at day 28 in mechanically ventilated patients. BMC Microbiol. 2023;23(1):399
111. Dickson RP, Singer BH, Newstead MW. et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol. 2016;1(10):16113
112. Sze MA, Tsuruta M, Yang SW. et al. Changes in the bacterial microbiota in gut, blood, and lungs following acute LPS instillation into mice lungs. PLoS One. 2014;9(10):e111228
113. Sulaiman I, Wu BG, Chung M. et al. Lower Airway Dysbiosis Augments Lung Inflammatory Injury in Mild-to-Moderate Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2023;208(10):1101-1114
114. Wu BG, Sulaiman I, Tsay JJ. et al. Episodic Aspiration with Oral Commensals Induces a MyD88-dependent, Pulmonary T-Helper Cell Type 17 Response that Mitigates Susceptibility to Streptococcus pneumoniae. Am J Respir Crit Care Med. 2021;203(9):1099-1111
115. Dicker AJ, Huang JTJ, Lonergan M. et al. The sputum microbiome, airway inflammation, and mortality in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2021;147(1):158-167
116. Arpaia N, Campbell C, Fan X. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451-455
117. Mann ER, Lam YK, Uhlig HH. Short-chain fatty acids: linking diet, the microbiome and immunity. Nat Rev Immunol. 2024;24(8):577-595
118. Lou X, Xue J, Shao R. et al. Fecal microbiota transplantation and short-chain fatty acids reduce sepsis mortality by remodeling antibiotic-induced gut microbiota disturbances. Front Immunol. 2023;13:1063543
119. Ye L, Hou Y, Hu W. et al. Repressed Blautia-acetate immunological axis underlies breast cancer progression promoted by chronic stress. Nat Commun. 2023;14(1):6160
120. Luu M, Pautz S, Kohl V. et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun. 2019;10(1):760
121. Lu Y, Wang Y, Wang J, Lowe AJ, Grzeskowiak LE, Hu YJ. Early-Life Antibiotic Exposure and Childhood Asthma Trajectories: A National Population-Based Birth Cohort. Antibiotics (Basel). 2023;12(2):314
122. Trompette A, Gollwitzer ES, Yadava K. et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159-166
123. Haak BW, Littmann ER, Chaubard JL. et al. Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood. 2018;131(26):2978-2986
124. Madapoosi SS, Cruickshank-Quinn C, Opron K. et al. Lung Microbiota and Metabolites Collectively Associate with Clinical Outcomes in Milder Stage Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2022;206(4):427-439
125. Yang Q, Cai Y, Guo S. et al. Decoding immune interactions of gut microbiota for understanding the mechanisms of diseases and treatment. Front Microbiol. 2023;14:1238822
126. Wiertsema SP, van Bergenhenegouwen J, Garssen J, Knippels LMJ. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients. 2021;13(3):886
127. Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. 2014;211(12):2397-2410
128. Huang Y, Mao K, Chen X. et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science. 2018;359(6371):114-119
129. Masuhiro K, Tamiya M, Fujimoto K. et al. Bronchoalveolar lavage fluid reveals factors contributing to the efficacy of PD-1 blockade in lung cancer. JCI Insight. 2022;7(9):e157915
130. Jang HJ, Choi JY, Kim K. et al. Relationship of the lung microbiome with PD-L1 expression and immunotherapy response in lung cancer. Respir Res. 2021;22(1):322
131. Boesch M, Baty F, Albrich WC. et al. Local tumor microbial signatures and response to checkpoint blockade in non-small cell lung cancer. Oncoimmunology. 2021;10(1):1988403
132. Routy B, Le Chatelier E, Derosa L. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91-97
133. Tomita Y, Ikeda T, Sakata S. et al. Association of Probiotic Clostridium butyricum Therapy with Survival and Response to Immune Checkpoint Blockade in Patients with Lung Cancer. Cancer Immunol Res. 2020;8(10):1236-1242
134. Lee SH, Cho SY, Yoon Y. et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat Microbiol. 2021;6(3):277-288
135. Derosa L, Routy B, Thomas AM. et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. 2022;28(2):315-324
136. Hakozaki T, Tanaka K, Shiraishi Y. et al. Gut Microbiota in Advanced NSCLC Receiving Chemoimmunotherapy: An Ancillary Biomarker Study From the Phase III Trial JCOG2007 (NIPPON). J Thorac Oncol. 2025;20(7):912-927
137. Hakozaki T, Richard C, Elkrief A. et al. The Gut Microbiome Associates with Immune Checkpoint Inhibition Outcomes in Patients with Advanced Non-Small Cell Lung Cancer. Cancer Immunol Res. 2020;8(10):1243-1250
138. Ren S, Feng L, Liu H, Mao Y, Yu Z. Gut microbiome affects the response to immunotherapy in non-small cell lung cancer. Thorac Cancer. 2024;15(14):1149-1163
139. Routy B, Gopalakrishnan V, Daillère R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol. 2018;15(6):382-396
140. Derosa L, Routy B, Fidelle M. et al. Gut Bacteria Composition Drives Primary Resistance to Cancer Immunotherapy in Renal Cell Carcinoma Patients. Eur Urol. 2020;78(2):195-206
141. Lin NY, Fukuoka S, Koyama S. et al. Microbiota-driven antitumour immunity mediated by dendritic cell migration. Nature. 2025;644(8078):1058-1068
142. Elkrief A, Derosa L, Kroemer G, Zitvogel L, Routy B. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: a new independent prognostic factor? Ann Oncol. 2019;30(10):1572-1579
143. Mohiuddin JJ, Chu B, Facciabene A. et al. Association of Antibiotic Exposure With Survival and Toxicity in Patients With Melanoma Receiving Immunotherapy. J Natl Cancer Inst. 2021;113(2):162-170
144. Fidelle M, Rauber C, Alves Costa Silva C. et al. A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers. Science. 2023;380(6649):eabo2296
145. Chen H, Ma X, Liu Y. et al. Gut Microbiota Interventions With Clostridium butyricum and Norfloxacin Modulate Immune Response in Experimental Autoimmune Encephalomyelitis Mice. Front Immunol. 2019;10:1662
146. Sun L, Wang X, Zhou H. et al. Gut microbiota and metabolites associated with immunotherapy efficacy in extensive-stage small cell lung cancer: a pilot study. J Thorac Dis. 2024;16(10):6936-6954
147. Botticelli A, Vernocchi P, Marini F. et al. Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J Transl Med. 2020;18(1):49
148. Teng MW, Bowman EP, McElwee JJ. et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21(7):719-729
149. Kureshi CT, Dougan SK. Cytokines in cancer. Cancer Cell. 2025;43(1):15-35
150. Walsh MJ, Ali LR, Lenehan P. et al. Blockade of innate inflammatory cytokines TNFα, IL-1β, or IL-6 overcomes virotherapy-induced cancer equilibrium to promote tumor regression. Immunother Adv. 2023;3(1):ltad011
151. Stewart CA, Metheny H, Iida N. et al. Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. J Clin Invest. 2013;123(11):4859-4874
152. Preet R, Islam MA, Shim J. et al. Gut commensal Bifidobacterium-derived extracellular vesicles modulate the therapeutic effects of anti-PD-1 in lung cancer. Nat Commun. 2025;16(1):3500
153. Hagihara M, Yamashita R, Matsumoto A. et al. The impact of Clostridium butyricum MIYAIRI 588 on the murine gut microbiome and colonic tissue. Anaerobe. 2018;54:8-18
154. Thome CD, Tausche P, Hohenberger K. et al. Short-chain fatty acids induced lung tumor cell death and increased peripheral blood CD4+ T cells in NSCLC and control patients ex vivo. Front Immunol. 2024;15:1328263
155. Jani CT, Edwards K, Bhanushali C. et al. Leveraging beneficial microbiome-immune interactions via probiotic use in cancer immunotherapy. Front Immunol. 2025;16:1713382
156. Gorbachev AV, Kobayashi H, Kudo D. et al. CXC chemokine ligand 9/monokine induced by IFN-gamma production by tumor cells is critical for T cell-mediated suppression of cutaneous tumors. J Immunol. 2007;178(4):2278-2286
157. Liu P, Zhao L, Kroemer G, Kepp O. Conventional type 1 dendritic cells (cDC1) in cancer immunity. Biol Direct. 2023;18(1):71
158. Young MR. Th17 Cells in Protection from Tumor or Promotion of Tumor Progression. J Clin Cell Immunol. 2016;7(3):431
159. Fan X, Song X, Chen W. et al. cGAS-STING signaling in cancer: Regulation and therapeutic targeting. MedComm - Oncology. 2023;2(3):e49
160. Lam KC, Araya RE, Huang A. et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell. 2021;184(21):5338-5356.e21
161. Cao M, Deng Y, Hao Q. et al. Single-cell transcriptomic analysis reveals gut microbiota-immunotherapy synergy through modulating tumor microenvironment. Signal Transduct Target Ther. 2025;10(1):140
162. Park JS, Gazzaniga FS, Wu M. et al. Targeting PD-L2-RGMb overcomes microbiome-related immunotherapy resistance. Nature. 2023;617(7960):377-385
163. Zhu Z, Cai J, Hou W. et al. Microbiome and spatially resolved metabolomics analysis reveal the anticancer role of gut Akkermansia muciniphila by crosstalk with intratumoral microbiota and reprogramming tumoral metabolism in mice. Gut Microbes. 2023;15(1):2166700
164. Zhang X, Yang J, Chen M. et al. Metabolomics profiles delineate uridine deficiency contributes to mitochondria-mediated apoptosis induced by celastrol in human acute promyelocytic leukemia cells. Oncotarget. 2016;7(29):46557-46572
165. Cheng TY, Chang CC, Luo CS. et al. Targeting Lung-Gut Axis for Regulating Pollution Particle-Mediated Inflammation and Metabolic Disorders. Cells. 2023;12(6):901
166. Corrêa RO, Castro PR, Moser R. et al. Butyrate: Connecting the gut-lung axis to the management of pulmonary disorders. Front Nutr. 2022;9:1011732
167. Mirzaei R, Afaghi A, Babakhani S. et al. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed Pharmacother. 2021;139:111619
168. Trompette A, Gollwitzer ES, Pattaroni C. et al. Dietary Fiber Confers Protection against Flu by Shaping Ly6c- Patrolling Monocyte Hematopoiesis and CD8+ T Cell Metabolism. Immunity. 2018;48(5):992-1005.e8
169. He Y, Fu L, Li Y. et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 2021;33(5):988-1000.e7
170. Kim K, Kwon O, Ryu TY. et al. Propionate of a microbiota metabolite induces cell apoptosis and cell cycle arrest in lung cancer. Mol Med Rep. 2019;20(2):1569-1574
171. Yan X, Yang M, Liu J. et al. Discovery and validation of potential bacterial biomarkers for lung cancer. Am J Cancer Res. 2015;5(10):3111-3122
172. Huang X, Hu M, Sun T. et al. Multi-kingdom gut microbiota analyses define bacterial-fungal interplay and microbial markers of pan-cancer immunotherapy across cohorts. Cell Host Microbe. 2023;31(11):1930-1943.e4
173. Thommen DS, Koelzer VH, Herzig P. et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. 2018;24(7):994-1004
174. Bod L, Kye YC, Shi J. et al. B-cell-specific checkpoint molecules that regulate anti-tumour immunity. Nature. 2023;619(7969):348-356
175. Zheng J, Guinter MA, Merchant AT. et al. Dietary patterns and risk of pancreatic cancer: a systematic review. Nutr Rev. 2017;75(11):883-908
176. Bingham SA. Mechanisms and experimental and epidemiological evidence relating dietary fibre (non-starch polysaccharides) and starch to protection against large bowel cancer. Proc Nutr Soc. 1990;49(2):153-171
177. Lu H, Xu X, Fu D. et al. Butyrate-producing Eubacterium rectale suppresses lymphomagenesis by alleviating the TNF-induced TLR4/MyD88/NF-κB axis. Cell Host Microbe. 2022;30(8):1139-1150.e7
178. Yang Y, Ye M, Song Y. et al. Gut microbiota and SCFAs improve the treatment efficacy of chemotherapy and immunotherapy in NSCLC. NPJ Biofilms Microbiomes. 2025;11(1):146
179. Wu ZY, Wu QW, Han Y. et al. Alistipes finegoldii augments the efficacy of immunotherapy against solid tumors. Cancer Cell. 2025;43(9):1714-1730.e12
180. Baruch EN, Youngster I, Ben-Betzalel G. et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371(6529):602-609
181. Davar D, Dzutsev AK, McCulloch JA. et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371(6529):595-602
182. Routy B, Lenehan JG, Miller WH Jr. et al. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: a phase I trial. Nat Med. 2023;29(8):2121-2132
183. Hadi DK, Baines KJ, Jabbarizadeh B. et al. Improved survival in advanced melanoma patients treated with fecal microbiota transplantation using healthy donor stool in combination with anti-PD1: final results of the MIMic phase 1 trial. J Immunother Cancer. 2025;13(8):e012659
184. Zhao W, Lei J, Ke S. et al. Fecal microbiota transplantation plus tislelizumab and fruquintinib in refractory microsatellite stable metastatic colorectal cancer: an open-label, single-arm, phase II trial (RENMIN-215). EClinicalMedicine. 2023;66:102315
185. Kim Y, Kim G, Kim S. et al. Fecal microbiota transplantation improves anti-PD-1 inhibitor efficacy in unresectable or metastatic solid cancers refractory to anti-PD-1 inhibitor. Cell Host Microbe. 2024;32(8):1380-1393.e9
186. Azimirad M, Yadegar A, Asadzadeh Aghdaei H, Kelly CR. Enterotoxigenic Clostridium perfringens Infection as an Adverse Event After Faecal Microbiota Transplantation in Two Patients With Ulcerative Colitis and Recurrent Clostridium difficile Infection: A Neglected Agent in Donor Screening. J Crohns Colitis. 2019;13(7):960-961
187. Dizman N, Meza L, Bergerot P. et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med. 2022;28(4):704-712
188. Arai J, Niikura R, Hayakawa Y. et al. Association of probiotic use with nivolumab effectiveness against various cancers: A multicenter retrospective cohort study. Cancer Med. 2023;12(16):16876-16880
189. Arima J, Yoshino H, Tatarano S. et al. Prognostic Impact of Clostridium butyricum MIYAIRI (CBM588) in Combination With Pembrolizumab for Advanced Urothelial Carcinoma: A Retrospective Cohort Study. Cancer Rep (Hoboken). 2025;8(8):e70308
190. Wang JS, Arrowsmith ER, Beck JT. et al. Phase 1/2, open-label study of oral bacterial supplementation (EDP1503) plus pembrolizumab in participants with advanced or metastatic microsatellite-stable colorectal cancer, triple-negative breast cancer, and checkpoint inhibitor-relapsed tumors. Invest New Drugs. 2025;43(4):894-903
191. Spencer CN, McQuade JL, Gopalakrishnan V. et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374(6575):1632-1640
192. Chen M, Ma L, Yu H. et al. JK5G postbiotics attenuate immune-related adverse events in NSCLC patients by regulating gut microbiota: a randomized controlled trial in China. Front Oncol. 2023;13:1155592
193. Spreafico A, Heirali AA, Araujo DV. et al. First-in-class Microbial Ecosystem Therapeutic 4 (MET4) in combination with immune checkpoint inhibitors in patients with advanced solid tumors (MET4-IO trial). Ann Oncol. 2023;34(6):520-530
Author contact
![]() Corresponding author: Qiong Zhou, PhD, MD, Department of Respiratory and Critical Care Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 430000, Email: zhouqiongtjcom.
Corresponding author: Qiong Zhou, PhD, MD, Department of Respiratory and Critical Care Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 430000, Email: zhouqiongtjcom.

 Global reach, higher impact
Global reach, higher impact