10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2011; 7(3):383-389. doi:10.7150/ijbs.7.383 This issue Cite
Research Paper
Tanshinone IIA Protects Against Cardiac Hypertrophy via Inhibiting Calcineurin/Nfatc3 Pathway
1. Department of Pharmacology, State-Province Key Laboratories of Biomedicine- Pharmaceutics of China, Harbin Medical University, Harbin 150081, Heilongjiang Province, China
2. Institute of Cardiovascular Research, Harbin Medical University, Harbin 150081, Heilongjiang Province, China
3. Department of Cardiology, The Forth Affiliated Hospital of Harbin Medical University, Harbin 150001, Heilongjiang Province, China
* The first two authors made equal contribution to this work.
Received 2011-2-14; Accepted 2011-3-31; Published 2011-4-7
Abstract
Pathological cardiac hypertrophy induced by adrenergic overactivation can subsequently develop to heart failure which remains as a leading cause of mortality worldwide. Tanshinone IIA is a lipid-soluble pharmacologically active compound extracted from the rhizome of the Chinese herb Salvia miltiorrhiza, a well-known traditional Chinese medicine used for the treatment of cardiovascular disorders. However, little is know about the effect of Tanshinone IIA on cardiac hypertrophy. The present study was aimed to investigate whether Tanshinone IIA prevents cardiac hypertrophy induced by isoproterenol (ISO) and to clarify its possible mechanisms. Cardiomyocytes hypertrophy was induced by ISO 10 μM for 48 h with or without Tanshinone IIA 10, 30, 100 μM pretreatment, and evaluated by determining the cell size and the expression of ANP, BNP, β-MHC, Calcineurin, and NFATc3 by real-time PCR and western blot. We found that Tanshinone IIA pretreatment attenuated the enlargement of cell surface area induced by ISO in cultured cardiomyocytes. The mRNA level of ANP, BNP and β-MHC was obviously elevated in ISO-treated cardiac cells, which was effectively inhibited by Tanshinone IIA. Moreover, we found that Tanshinone IIA pretreatment could prevent the augment of intracellular calcium transient in ISO-treated cardiomyocytes. The further study revealed that Calcineurin, NFATc3, ANP, BNP and β-MHC proteins were upregulated by ISO in ventricular myocytes, and Tanshinone IIA pretreatment significantly attenuate the increased expression of Calcineurin, NFATc3, ANP, BNP and β-MHC proteins. In summary, Tanshinone IIA attenuated cardiomyocyte hypertrophy induced by ISO through inhibiting Calcineurin/NFATc3 pathway, which provides new insights into the pharmacological role and therapeutic mechanism of Tanshinone IIA in heart diseases.
Keywords: Tanshinone IIA, Cardiac hypertrophy, Isoproterenol, Calcineurin, NFATc3
Introduction
Pathological cardiac hypertrophy is a major cause of morbidity and mortality of cardiovascular diseases all over the world [1-3]. Ventricular myocardium in response to a variety of pathologic stimuli such as adrenergic overactivation will undergo a hypertrophic growth characterized by the increased cell size and activation of numerous fetal cardiac genes so as to compensate for the decreased function of injured hearts [4]. However, sustained myocardial hypertrophy can result in the functional decompensation, electrophysiological remodeling, cardiac fibrosis, sudden death or heart failure [5]. The calcineurin/nuclear factor of activated T cells (NFATc) signaling pathway has been shown to play an essential role in pathological cardiac hypertrophy [1, 3]. Accordingly, preventing the activation of calcineurin/NFATc signal pathway is suggested as an important strategy for the treatment of myocardial hypertrophy.
Danshen, the dry root and rhizome of Salvia miltiorrhiza Bge (Labiatae), was widely used in therapeutic remedies in China and other countries [6]. Many clinical studies indicated that Danshen and its preparations could treat coronary artery diseases, myocardial infarction, liver malfunction, etc [6-9]. Tanshinone IIA (Tan IIA) is isolated from Salvia miltiorrhiza and one of the main ingredients of Danshen for cardioprotective effects [10]. Tanshinone IIA was shown to exert beneficial effects on cardiovascular system with minimal reported side effects [11-15]. For example, Tanshinone IIA prevented endothelial cell damage through its anti-oxidant effect [11-12] and protected cardiomyocytes against oxidative stress-triggered damage and apoptosis [13]. Our previous studies also uncovered that Tanshinone IIA protected rats against sudden cardiac death due to lethal arrhythmias via repression of microRNA-1. However, little is known about whether Tanshinone IIA can prevent ISO-induced cardiac hypertrophy. The present study is aimed to determine the preventive role of Tanshinone IIA in ISO-induced cardiac hypertrophy and to clarify its underlying mechanisms.
Materials and Methods
Isolation and culture of cardiomyocytes
The procedure to culture neonatal rat ventricular myocytes (NRVMs) is just as described previously [16]. Briefly, the hearts of neonatal rats were rapidly removed. Both ventricles were cut into l to 2 mm3 and dissociated in 0.25% trypsin at 37 °C for 1-2 min. Cell suspensions were shifted out and neutralized with cell culture medium. Cardiac tissues were trypsinized until the tissues disappeared and cell suspensions were collected. Then all suspensions were pelleted by centrifugation at 2000 rpm for 180 s. The isolated cells were then resuspended in DMEM (Hyclone Laboratories) supplemented with 10% fetal bovine serum (Gibco) and penicillin (100 U/ml)/streptomycin (100 U/ml), transferred into culture flask and cultured at 37 °C in humid air with 5% CO2. After 90 min for fibroblast adherence, neonatal cardiomyocytes were plated into 6-well plate at a density of 3×105 cells per well. After 48 h culture, NRVMs were treated by ISO for 48 h in the absence or presence of Tanshinone IIA.
Measurement of cell surface area
Cultured cardiomyocytes were fixed with 4% paraformaldehyde for 0.5 h. Then the cell membrane was penetrated by 0.4% Triton X-100 for 1h and blocked by goat serum for 1 h. Followed by anti-sarcomeric actin antibody (Sigma, St. Louis, MO), at 4°C overnight, subsequently incubation with FITC-conjugated goat anti-mouse antibody for 1h. Immunofluorescence was analyzed under a fluorescence microscope (Nikon 80i). Cell surface was measured by Image-Pro Plus Data Analysis Software. Quantification of cell surface area by measuring 60 random cells from three experiments, and the average value was used for analysis.
Quantitative Real-time PCR
Total RNA was extracted from cultured neonatal cardiomyocytes using TRIZOL reagent. To detect the level of ANP, BNP and β-MHC mRNAs, quantitative Real-time PCR was performed on ABI 7500 fast Real Time PCR system (Applied Biosystems, USA). The Real-time PCR primer sequences for ANP were forward: 5′-CTCCGATAGATCTGCCCTCTTGAA-3′ and reverse: 5′-GGTACCGGAAGCTGTTGCAGCCTA-3′. The primer sequences for BNP were forward 5′-TTGGGCAGAAGATAGACCGGAT-3′ and reverse 5′-GGTCTTCCTAAAACAACCTCA-3′; The primer sequences for β-MHC were forward: 5'-AACCTGTCCAAGTTCCGCAAGGTG-3′ and reverse GAGCTGGGTAGCACAAGAGCTACT-3′; The sequences of GAPDH primers were forward primer: 5′-TCTACATGTTCCAGTATGACTC-3′ and reverse: 5′-ACTCCACGACATACTCAGCACC-3′. GAPDH was used as an internal control.
Measurement of intracellular calcium transient
Cultured cells were stained by Fluo-3/AM at 37 °C for 45 min, and then washed with standard external solution contain Ca2+ for 3 times. Fluorescent changes of Fluo-3/AM-loaded cells were detected using laser scanning Confocal microscope (Olympus FV-300) with 488 nm for excitation from an argon ion laser and 530 nm for emission. The fluorescent intensities before (FI0) and after (FI) KCl 30 mM administration were both recorded. Qualitative changes in the increase of intracellular Ca2+ level were inferred from the ratio of FI/FI0.
Western Blot
The protein samples were extracted from the whole cells with the procedures as previously described [3]. Cardiac cells were also lysed with standard sample buffer, and then to broke the cells by ultrasound. For deliver protein sufficiently, the mixed liquor was kept on the ice for 30 min. The mixed liquor was centrifuged and then the supernatant was collected. Protein concentration was determined in the supernatant using ABC as recommended by the manufacturer. After boiling the samples for 5 min, the protein samples were fractionated by SDS-PAGE (10%-15% polyacrylamide gels) and transferred to PVDF membrane (Millipore, Bedford, MA). The membranes were blocked with milk powder at room temperature for 2 h. The membranes were incubated with primary antibodies ANP, BNP, β-MHC (Santa Cruz Biotechnology Inc. USA), calcineurin (Santa Cruz Biotechnology Inc. USA) and NFATc3 (Santa Cruz Biotechnology Inc. USA) at 4 °C overnight, washed, and incubated with a secondary rabbit or mouse polyclonal and purchased from Santa Cruz Biotechnology for 1 h at room temperature. Western blot bands were quantified using Odyssey v1.2 software by measuring the band intensity (area×OD) for each group and normalizing to GAPDH as an internal control.
Statistical analysis
All experimental data were presented as the mean ± SEM. ANOVA or t-test was used to compare mean values using SPSS 13.0 software. Values of p < 0.05 were considered statistically significant.
Results
Tanshinone IIA attenuated ISO-induced the increase of cell surface area
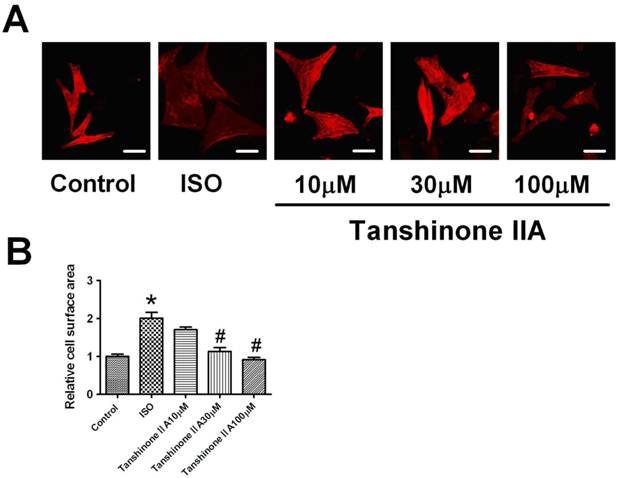
Myocardial hypertrophy was induced by exposing neonatal cardiomyocytes to ISO 10 μM for 48 h. Hypertrophic growth was assayed by the staining of a-sarcomeric actinin protein in cultured cardiomyocytes. As shown in Fig. 1A, ISO 10 μM significantly increased cell surface area of cardiac myocytes. Furthermore, Tanshinone IIA 10, 30 and 100 μM pretreatment markedly inhibited the increase of cell surface area in ISO-treated cardiomyocytes, indicating that Tanshinone IIA played a preventive role in cardiac hypertrophy induced by ISO.
Effect of Tanshinone IIA on ISO-induced the hypertrophic growth in cardiomyocytes. (A) Tanshinone IIA 10, 30 and 100 μM attenuated ISO-induced hypertrophic growth evaluated by the visualization of sarcomere organization; (B) ISO-induced the enlargement of cell surface area was inhibited by Tanshinone IIA 10, 30 and 100 μM. Data are expressed as mean ± SEM for examination of 60 random cells from four experiments. * P < 0.05 vs. Control, # P < 0.05 vs. ISO. (Scale bar, 20μm)
Tanshinone IIA reversed ISO-induced the increase of ANP, BNP and β-MHC at mRNA and protein levels
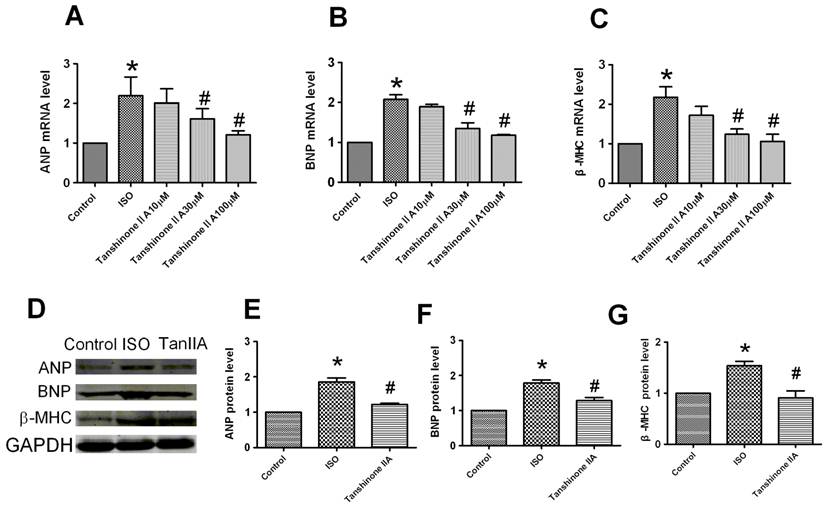
To confirm the inhibitory effect of Tanshinone IIA on cardiomyocytes hypertrophy, we further investigated the effects of Tanshinone IIA on ISO-induced the expression of hypertrophic markers atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and β-myosin heavy chain (β-MHC) in cultured cardiomyocytes. Real-time quantitative RT-PCR analysis showed ISO induced a significant increase of ANP, BNP and β-MHC mRNAs by over 2-fold in cardiac cells (Fig. 2A-2C). Tanshinone IIA 30 and 100 μM obviously inhibited ISO-induced the upregulation of these hypertrophic markers.
To further confirm the preventive effect of Tanshinone IIA on ISO-induced cardiac hypertrophy, we next evaluated effect of Tanshinone IIA on ISO-induced the changes of ANP, BNP and β-MHC proteins (Fig. 2D). The expression of ANP, BNP and β-MHC proteins was also upregulated in the presence of ISO 10 μM for 48 h (Fig. 2E-2G). Tanshinone IIA obviously reversed the upregulation of ANP, BNP and β-MHC proteins in ISO-treated cardiac cells.
Effects of Tanshinone on ISO-induced enhancement of intracellular calcium transient
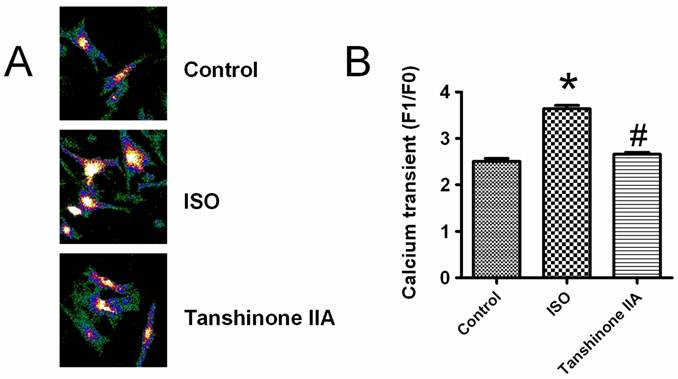
It is well known that cardiac hypertrophic responses are triggered by the elevation of intracellular Ca2+ level. To reveal the detailed mechanism for the preventive effects of Tanshinone IIA on heart hypertrophy, we observed whether intracellular calcium transient was involved in the beneficial actions of Tanshinone IIA. A typical elevation of intracellular Ca2+ level after KCl (30 mM)-induced membrane depolarization is shown in Fig.3A. As illustrated in Fig.3B, ISO incubation significantly enhanced the amplitude of intracellular calcium transient, which was obviously abolished by Tanshinone IIA 100 μM.
Effect of Tanshinone IIA on ISO-induced the increased expression of ANP, BNP and β-MHC mRNAs and proteins. (A), (B) and (C) The mRNA levels of hypertrophic markers ANP, and BNP, and β-MHC was analyzed by qRT-PCR. The cardiomyocytes treated with Tanshinone IIA 30 and 100 μM exhibited a reduction of ANP, BNP and β-MHC mRNAs level in ISO-treated cells. (D) The expression of ANP, BNP and β-MHC proteins in ISO-treated cardiomyocytes in the absence and presence of Tanshinone IIA 100 μM. (E), (F) and (G) The expression of ANP, BNP and β-MHC proteins were significantly increased in the cardiomyocytes treated with ISO for 48h compared with control cells, which was significantly inhibited by Tanshinone IIA 100 μM pretreatment. Data are expressed as mean ± SEM for three individual experiments. * P < 0.05 vs. Control, # P < 0.05 vs. ISO.
Effects of Tanshinone IIA on ISO-induced the rise of intracellular calcium level in neonatal cardiomyocytes. ISO enhanced the intracellular calcium transient induced by KCl-induced membrane depolarization, which was effectively restricted by Tanshinone IIA 100 μM. Data are expressed as mean±SEM for four individual experiments. * P < 0.05 vs. Control, # P < 0.05 vs. ISO. Data are expressed as mean ± SEM for 60 cells from at least three individual experiments. * P < 0.05 vs. Control, # P < 0.05 vs. ISO.
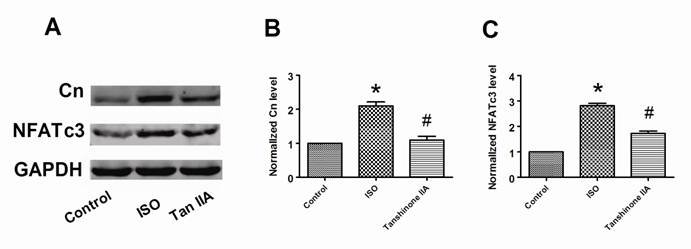
Effects of Tanshinone IIA on the expression of calcineurin and NFATc3 proteins in ISO-induced cardiac hypertrophy. (A) A representative experiment of the expression of calcineurin and NFATc3 proteins in ISO-treated cardiomyocytes in the absence and presence of Tanshinone IIA 100 μM. (B) Tanshinone IIA had inhibitory influences on ISO-induced the upregulation of calcineurin in ventricular myocytes. (C) ISO-induced the increase of NFATc3 protein in cardiac cells was obviously reversed by Tanshinone IIA. Data are expressed as mean ± SEM for four individual experiments. * P < 0.05 vs. Control, # P < 0.05 vs. ISO.
Tanshinone IIA inhibited the upregulation of calcineurin and NFATc3
Calcineurin/NFATc3 signal pathway plays a crucial role in cardiac hypertrophy. We further examined whether Tanshinone IIA prevented myocardial hypertrophy in a calcineurin/NFATc3-dependent pathway. In accordance to previous reports, we also confirmed that ISO-treated cardiomyocytes exhibited a considerably increased expression of calcineurin and NFATc3 proteins compared with control cells (Fig. 4A-4C). Tanshinone IIA 100 μM obviously reversed the upregulation of calcineurin and NFATc3 proteins in ISO-treated cardiac cells. These suggest that calcineurin/NFATc3 signal pathway also contributes to the preventive effects of Tanshinone IIA on cardiac hypertrophy.
Discussion
The present study mainly revealed that Tanshinone IIA could protect against cardiac hypertrophy induced by ISO by inhibiting calcineurin/NFATc3 signal pathway, which yield new understanding of the therapeutic effects of Tanshinone IIA on pathological myocardial remodeling.
Pathological myocardial hypertrophy as a consequence of the maladaptive alterations in many heart diseases is an important risk factor for subsequent cardiac morbidity and mortality. Although initially beneficial, sustained cardiac hypertrophy can lead to decompensation and heart failure. Adrenergic overactivation has been used to induce cardiac hypertrophy and caused cardiac fibrosis in vivo or in vitro [17-19]. The Ca2+-activated Ser/Thr protein phosphatase calcineurin and the downstream transcriptional effectors of calcineurin, NFATcs, have been implicated as a key signaling pathway in the hypertrophic response of the myocardium [1, 3]. Targeted depletion of calcineurin activity showed an obvious reduction of myocardium hypertrophy in mice subjected to ISO infusion [20]. Furthermore, mice with targeted disruptions of NFATc3 demonstrated a significant reduction in calcineurin-induced cardiac hypertrophy, which indicated NFATc3 is required for calcineurin-regulated hypertrophic responses [21]. Recently, microRNAs were also shown involved in ISO-induced cardiac hypertrophy [22-23]. The forced expression of miR-23 could lead to cardiac hypertrophy by inhibiting MuRF1, an anti-hypertrophic protein. Though plenty of progress has been made in the understanding of molecular mechanism of pathological cardiac hypertrophy, the effective therapeutic drugs for restoring heart functions are still lacking.
Danshen is extracted from the dried root or rhizome of Salvia miltiorrhiza Bunge and has been widely used in the treatment of cardiovascular diseases such as myocardial infarction and myocarditis [11-15]. Danshen-derived compounds have many important pharmacological effects in basic experiments and clinic, such as anti-tumor, immunoloregulation and cardioprotective effects, scavenge lipid radicals and so on [7, 9-10, 24]. Recently, our studies also showed that Tanshinone IIA reversed cardiac potassium channel remodeling after heart infarction through depressing SRF-induced miR-1 overexpression [15]. Previous reports also revealed that angiotensin II-induced hypertrophic growth in cardiomyocytes was markedly attenuated in the presence of Tanshinone IIA through inhibiting the rise of intracellular calcium and c-jun expression [14], that indicates ISO and angiotensin II caused cardiac hypertrophy through different signaling pathway. Moreover, it was also demonstrated that the blockage of Ang II by losartan and quinapril could not prevent ISO-induced cardiac hypertrophy [25]. Thus, in this study, we aimed to investigate the effects and mechanisms of Tanshinone IIA on ISO-induced cardiac hypertrophy.
We found that Tanshinone IIA markedly attenuated ISO-induced the increase of cell surface area and ANP, BNP and β-MHC at both mRNA and protein levels. It indicates Tanshinone IIA plays a protective role in cardiac hypertrophy in response to pathological stimuli. It is well known that intracellular calcium level is an important hypertrophic messenger. ISO promotes the release of calcium ion from the sarcoplasmic reticulum, thereby increasing intracellular calcium level. The subsequent increase in intracellular calcium level plays a potentiating role in cardiac hypertrophic growth by activating several signalling pathways [26]. Previous studies have shown that intracellular Ca2+/CaM-dependent signalling stimulated the expression of hypertrophic genes in cardiomyocytes through a variety of effectors such as the protein phosphatase calcineurin, Ca2+/CaM-dependent kinase II, and NFATcs (nuclear factor of activated T-cells) [27]. Therefore, the decrease in intracellular calcium level is an important approach to prevent hypertrophic growth of cardiomyocytes in response to pathological stimuli. In this study, we found that Tanshinone IIA inhibited the increase of intracellular calcium level caused by ISO in cardiac cells. The calcineurin/NFATc3 pathway plays an important role in conveying the signals for a variety of hypertrophic stimuli. Increased cystolic Ca2+ activates calcineurin and thereby induces nuclear translocation of NFAT, which is involved in the induction of pathological cardiac hypertrophy signaling. In this study we demonstrated that Tanshinone IIA inhibited the increased expression of calcineurin and NFATc3 induced by ISO, which indicates that Tanshinone IIA protects against cardiac hypertrophy in a Calcineurin/NFATc3-dependent pathway.
We can confirm that there are some limitations of this study as followed. First, we just detected the expression of total NFATc3 and did not investigate the translocation of NFATc3 from cytoplasmic to nucleus though the Calcineurin/NFATc3-dependent pathway that has been confirmed in previous studies [28]. Second, inhibitors or siRNA technique was not used in this study that will be another evidence to demonstrate that Ca2+/Calcineurin/NFATc3 pathway is involved in the regulation of ISO-induced cardiac hypertrophy.
Taken together, this study investigated the therapeutic effects of Tanshinone IIA on myocardial hypertrophy induced by ISO. We revealed for the first time that Tanshinone IIA was capable of preventing ISO-induced hypertrophic growth in cardiomyocyte, which is attributed, at least partially, to its inhibition of Ca2+/Calcineurin/NFATc3 pathway. These findings raise the possibility of developing Tanshinone IIA as a therapeutic drug for cardiac hypertrophy.
Acknowledgements
This work was supported by the National Natural Science Fund of China (30971252), the Program for New Century Excellent Talents In Heilongjiang Provincial University (1155-NCET-010), the National Natural Science Fund of China (30900601) and the Dr. Wu Lien-Teh (Wu Lian-De) Youth Foundation of Harbin Medical University.
Conflict of Interests
The authors declare no conflict of interests.
References
1. Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93(2):215-228
2. Hunter JJ, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341(17):1276-1283
3. Dong DL, Chen C, Huo R, Wang N, Li Z, Tu YJ, Hu JT, Chu X, Huang W, Yang BF. Reciprocal repression between microRNA-133 and calcineurin regulates cardiac hypertrophy: a novel mechanism for progressive cardiac hypertrophy. Hypertension. 2010;55(4):946-952
4. Osadchii OE. Cardiac hypertrophy induced by sustained beta-adrenoreceptor activation: pathophysiological aspects. Heart Fail Rev. 2007;12(1):66-86
5. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561-1566
6. Wu B, Liu M, Zhang S. Dan Shen agents for acute ischaemic stroke. Cochrane Database Syst Rev. 2004;18:CD004295
7. Bi HC, Zuo Z, Chen X, Xu CS, Wen YY, Sun HY, Zhao LZ, Pan Y, Deng Y, Liu PQ, Gu LQ, Huang ZY, Zhou SF, Huang M. Preclinical factors affecting the pharmacokinetic behaviour of tanshinone IIA, an investigational new drug isolated from Salvia miltiorrhiza for the treatment of ischaemic heart diseases. Xenobiotica. 2008;38:185-222
8. Oh SH, Cho KH, Yang BS, Roh YK. Natural compounds from Danshen suppress the activity of hepatic stellate cells. Arch Pharm Res. 2006;29:762-767
9. Lee CY, Sher HF, Chen HW, Liu CC, Chen CH, Lin CS, Yang PC, Tsay HS, Chen JJ. Anticancer effects of tanshinone I in human non-small cell lung cancer. Mol Cancer Ther. 2008;7(11):3527-3538
10. Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Cell Biochem. 2005;45:1345-1359
11. Wu TW, Zeng LH, Fung KP, Wu J, Pang H, Grey AA, Weisel RD, Wang JY. Effect of sodium tanshinone IIA sulfonate in the rabbit myocardium and on human cardiomyocytes and vascular endothelial cells. Biochem Pharmacol. 1993:2327-2332
12. Lin R, Wang WR, ;Liu JT, Yang GD, Han CJ. Protective effect of tanshinone IIA on human umbilical vein endothelial cell injured by hydrogen peroxide and its mechanism. J Ethnopharmacol. 2006:217-222
13. Fu J, Huang H, Liu J, Pi R, Chen J, Liu P. Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol. 2007;568(1-3):213-221
14. Yang L, Zou X, Liang Q, Chen H, Feng J, Yan L, Wang Z, Zhou D, Li S, Yao S, Zheng Z. Sodium tanshinone IIA sulfonate depresses angiotensin II-induced cardiomyocyte hypertrophy through MEK/ERK pathway. Exp Mol Med. 2007;39(1):65-73
15. Shan H, Li X, Pan Z, Zhang L, Cai B, Zhang Y, Xu C, Chu W, Qiao G, Li B, Lu Y, Yang B. Tanshinone IIA protects against sudden cardiac death induced by lethal arrhythmias via repression of microRNA-1. Br J Pharmacol. 2009;158(5):1227-1235
16. Cai BZ, Zhao LM, Wang N, Liu JQ, Zhu SL, Meng FY, Zhou HY, Lu YJ, Ai J, Yang BF. Bone marrow mesenchymal stem cells upregulate transient outward potassium currents in postnatal rat ventricular myocytes. J Mol Cell Cardiol. 2009;47(1):41-48
17. Hong HM, Song EJ, Oh E, Kabir MH, Lee C, Yoo YS. Endothelin-1- and isoproterenol-induced differential protein expression and signaling pathway in HL-1 cardiomyocytes. Proteomics. 2011;11(2):283-297
18. Liggett SB. Pharmacogenetics of beta-1 and beta-2-adrenergic receptors. Pharmacology. 2000;61(3):167-173
19. Morisco C, Marrone C, Galeotti J, Shao D, Vatner DE, Vatner SF, Sadoshima J. Endocytosis machinery is required for beta1-adrenergic receptor-induced hypertrophy in neonatal rat cardiac myocytes. Cardiovasc Res. 2008;78(1):36-44
20. Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94(1):110-118
21. Wilkins BJ, De Windt LJ, Bueno OF, Braz JC, Glascock BJ, Kimball TF, Molkentin JD. Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol Cell Biol. 2002;22(21):7603-7613
22. Lin Z, Murtaza I, Wang K, Jiao J, Gao J, Li PF. miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc Natl Acad Sci U S A. 2009;106(29):12103-12108
23. Wang K, Long B, Zhou J, Li PF. miR-9 and NFATc3 regulate myocardin in cardiac hypertrophy. J Biol Chem. 2010;285(16):11903-11912
24. Cheng TO. Cardiovascular effects of Danshen. Int J Cardiol. 2007;121:9-22
25. Leenen FH, White R, Yuan B. Isoproterenol-induced cardiac hypertrophy: role of circulatory versus cardiac renin-angiotensin system. Am J Physiol Heart Circ Physiol. 2001;281(6):H2410-H2416
26. Miller CL, Oikawa M, Cai Y, Wojtovich AP, Nagel DJ, Xu X, Xu H, Florio V, Rybalkin SD, Beavo JA, Chen YF, Li JD, Blaxall BC, Abe J, Yan C. Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ Res. 2009;105(10):956-964
27. Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589-600
28. Yeh JL, Hsu JH, Wu PJ, Liou SF, Liu CP, Chen IJ, Wu BN, Dai ZK, Wu JR. KMUP-1 attenuates isoprenaline-induced cardiac hypertrophy in rats through NO/cGMP/PKG and ERK1/2/calcineurin A pathway. Br J Pharmacol. 2010;159:1151-1160
Author contact
![]() Corresponding author: Prof. Yanjie Lu, Department of Pharmacology, Harbin Medical University, Harbin 150081, Heilongjiang province, China. E-mail: Yjlu86com; Tel: 86 451 86671354 ; Fax: 86 451 86671354.
Corresponding author: Prof. Yanjie Lu, Department of Pharmacology, Harbin Medical University, Harbin 150081, Heilongjiang province, China. E-mail: Yjlu86com; Tel: 86 451 86671354 ; Fax: 86 451 86671354.

 Global reach, higher impact
Global reach, higher impact