Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2011; 7(4):410-417. doi:10.7150/ijbs.7.410 This issue Cite
Review
Fishing Pluripotency Mechanisms In Vivo
1. Fundación para la Investigación Hospital La Fe, Valencia 46009, Spain.
2. School of Biological Sciences, University of East Anglia, Norwich, NR4 7TJ, UK.
Received 2011-1-1; Accepted 2011-4-1; Published 2011-4-15
Abstract
To understand the molecular mechanisms that regulate the biology of embryonic stem cells (ESCs) it is necessary to study how they behave in vivo in their natural environment. It is particularly important to study the roles and interactions of the different proteins involved in pluripotency and to use this knowledge for therapeutic purposes. The recent description of key pluripotency factors like Oct4 and Nanog in non-mammalian species has introduced other animal models, such as chicken, Xenopus, zebrafish and medaka, to the study of pluripotency in vivo. These animal models complement the mouse model and have provided new insights into the evolution of Oct4 and Nanog and their different functions during embryonic development. Furthermore, other pluripotency factors previously identified in teleost fish such as Klf4, STAT3, Sox2, telomerase and Tcf3 can now be studied in the context of a functional pluripotency network. The many experimental advantages of fish will fuel rapid analysis of the roles of pluripotency factors in fish embryonic development and the identification of new molecules and mechanisms governing pluripotency.
Keywords: Nanog, Oct4, teleost fish, Medaka, pluripotency.
Introduction
ESCs have the potential to be induced to differentiate into almost any cell type of an embryo or adult tissue [1] presenting great therapeutic promise in regenerative medicine [2, 3]. To derive appropriate cell types for clinical use it is necessary to understand how pluripotency gene networks function in vivo. The regulation of pluripotency has been described mainly in mammals and in vivo functional analysis of Nanog, Oct4 (Pou5f1) and Sox2, which form the gene core of pluripotency, has been performed using the mouse model [4-7]. This was partly due to the lack of Nanog orthologs identified in other non-mammalian species in the past. However, Nanog and Oct4 sequences have recently been published in non-mammalian vertebrate species, such as chicken [8, 9], Xenopus (only Oct4 homologs) [10], zebrafish (Danio rerio) [11, 12] and medaka fish (Oryzias latipes) [12, 13], demonstrating that these key pluripotency factors are not exclusive to mammals. The third key pluripotency factor of the triad is Sox2, which acts as a cofactor with Oct4 to maintain pluripotency [14, 15]. Sox2 was initially described as an early neural marker and an important factor for eye development [16, 17], however its putative interaction with Oct4 in fish has not been studied in detail. Additionally, other important pluripotency factors in mammals have been studied in fish such as Klf4, Tcf3, STAT3 and telomerase (Table 1). Although their embryonic roles have been studied in different developmental contexts, little is known about their function in fish pluripotency.
Here, we will review the recent introduction and use of fish models to study early pluripotency in vivo comparing the results to those obtained in mammals. The many experimental advantages that offer teleost animal models [18, 19] allow researchers to combine embryological, molecular and genetic analyses required in the study of pluripotency. This makes them an excellent choice to study pluripotency in vivo, thus complementing the mouse model.
The introduction of non-mammalian animal models to study pluripotency
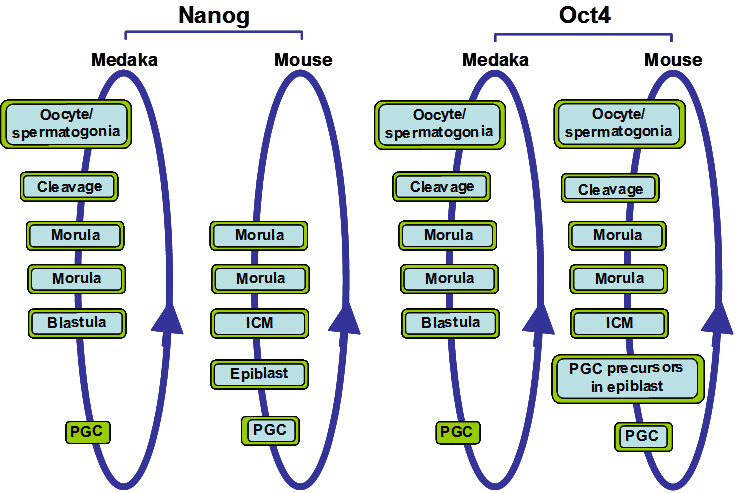
Oct4 and Nanog proteins are central to the maintenance of ESC pluripotency [4, 6, 7]. The roles of Oct4 and Nanog in pluripotency were first described in mice. Both proteins mOct4 and mNanog are expressed in the inner cell mass (ICM) and epiblast of the early mouse embryo and in mouse germ cells (Figure 1). mNanog null embryos fail to form epiblast and the ICM differentiates into parietal endoderm-like cells [7], while ICM from mOct4 null embryos is not pluripotent and differentiates into trophoblast [4].
Pluripotency genes present in human (Homo sapiens), mouse (Mus musculus), medaka (Oryzias latipes) and zebrafish (Danio rerio). Numbers identify the Entrez Gene ID for each gene except for zebrafish Nanog that represents the NCBI reference sequence.
| Homo sapiens | Mus musculus | Oryzias latipes | Danio rerio | |
|---|---|---|---|---|
| Oct4 | 5460 | 18999 | 100049520 | 30333 |
| Nanog | 79923 | 71950 | 100301580 | NP_001091862.1 |
| Sox2 | 6657 | 20674 | 100049368 | 378723 |
| Klf4 | 9314 | 16600 | - | 65238 |
| Stat3 | 6774 | 20848 | 100049477 | 30767 |
| Tcf3 | 6929 | 21423 | - | 30310 |
Nanog and Oct4 expression during medaka and mouse development. The circular blue arrow indicates the totipotent cycle. Cells and tissues types that express Oct4 or Nanog are boxed in blue when their mRNA expression are detected and/or boxed in green when their protein expressions are detected. Both Nanog and Oct4 have similar expression patterns in medaka and mouse, although mouse Nanog is first detected at the morula stage.
In Xenopus, Nanog homologs have not been detected. On the other hand, Morrison and Brickman [10] described three Oct4 homolog genes in Xenopus laevis, XlPou25, XlPou60 and XlPou91, grouped under the name XlPouV. Depleting the levels of these three XlPouV genes using morpholino oligos caused embryonic lethality in injected Xenopus embryos. In order to further evaluate their functional conservation, all three genes were transformed into Oct4-null mouse ESCs. Only XlPou91 was able to rescue the self-renewal capability of the cells almost to the same extent as mOct4, suggesting that in Xenopus, XlPou91 may be the gene that during evolution has retained the same role in regulating pluripotency as the mammalian Oct4 gene [10]. Xenopus embryos do not form trophectoderm, therefore XlPouV genes depletion can not produce differentiation to this tissue, as observed after mOct4 depletion in the mouse embryo. However, XlPouV genes depletion induces an expansion of Xcad3 expression, which is the Xenopus homologue of mouse Cdx2, a marker of trophectoderm. XlPouV genes depletion also induces an early progression to a committed cell type, provoking an increase in the expression of early commitment markers such as Goosecoid, Chordin and Cerberus, and a decrease in genes associated with the inhibition of differentiation such as Bmp4 [10]. In view of the results obtained in Xenopus, the role of Oct4 is associated with preventing the premature commitment of pluripotent cells before and during gastrulation.
In chicken, cNanog and cOct4 sequences have been described with only one homolog of each identified to date [8, 9]. The functional conservation of these genes was analyzed by performing overexpression experiments of cNanog and cOct4 in mouse and chicken ESC cultures and comparing their effects with those of mNanog and mOct4. Both cNanog and cOct4 share some functional roles with their mouse homologous genes. mNanog or cNanog overexpression maintains proliferation of mouse ESCs in the absence of Leukaemia Inhibition Factor (LIF), while overexpression of cOct4 induces differentiation in chicken ESCs and mouse ESCs. On the other hand, inactivation of cNanog or cOct4 induces differentiation of chicken ESCs and inhibits their proliferation. Moreover, cNanog and cOct4 expression is detected in chicken primordial germ cells (PGCs) just as observed in the mouse [6, 8, 9].
In zebrafish and medaka, embryonic stem-like cells have been isolated and characterised. These ESCs from fish share the in vitro properties with murine ESCs and exhibit self-renewal capacity [20-22]. Oct4 orthologous sequences have been identified in medaka OlOct4 (Olpou5f1) [13] and zebrafish pou2/spg [11, 23]. Furthermore, in vitro analysis has demonstrated that mOct4 promoter can be activated in medaka ESCs, suggesting that the pluripotency regulatory network in mammals is conserved in fish [24]. In addition, a single Nanog orthologous gene has been found in medaka and zebrafish genomes and the medaka Nanog (OlNanog) gene has been functionally characterized [12].
Other genes associated with pluripotency in mammals (Sox2, Tcf3, Klf4, STAT3) have been described and analyzed in other species such as Xenopus, fish or chicken. However, the initial absence of Nanog or Oct4 orthologs in these species suggested that pluripotency might be an exclusive mammalian property and focused the study of these genes to their roles in other aspects of development. The recent description and characterization of Nanog and Oct4 in non-mammal animals paves the way for a thorough comparative analysis of the genetic networks regulating embryonic pluripotency. Furthermore, the experimental advantages provided by these species, particularly zebrafish and medaka embryos, such as the number of embryos and their transparency, ex utero development, easy gene function manipulation and transgenic generation, will further expand the field of in vivo pluripotency. For example, new in vivo genetic screens can be designed to reveal other proteins involved in pluripotency, generation of inducible transgenes or the roles of pluripotency genetic networks in adult stem cells and regenerating tissues.
Comparing the roles of Oct4 between mammals and fish
In mouse ESCs, repression of mOct4 expression causes differentiation to trophectoderm and loss of pluripotency, while overexpression produces the differentiation to primitive mesoderm and endoderm [25]. Therefore, precise levels of Oct4 control the maintenance of pluripotency. However Oct4 alone is necessary but not sufficient to support stem cell renewal in these cells [25]. On the other hand, Oct4 is one of the transcription factors needed for the reprogramming of human and mouse fibroblasts into induced pluripotent stem cells (iPSC) [26-28]. In fact, Oct4 is the only transcription factor that appears to be irreplaceable for reprogramming to occur [29], making Oct4 a potential key factor to be used in regenerative medicine.
During evolution, the ancestral Oct4 gene seems to have been duplicated once, resulting in pou5 and pou2. However pou5 was subsequently lost in teleost fish and pou2 was lost in mammals, thus, the remaining copy of the ancestral Oct4 gene in each class of vertebrate would most likely have retained Oct4 functions and/or acquired new ones [30]. Expression and functional analysis shows that Oct4 is expressed in the ICM of mouse embryos and in all cells from zygote to gastrula embryos in zebrafish and medaka (Figure 1) [31-33]. During later stages of development, medaka and zebrafish Oct4 also share an expression domain in the posterior part of the embryo. Additionally, mouse, chicken and medaka Oct4 are expressed in the PGCs [9, 31, 33] and, in mouse, mOct4 is necessary for PGC survival [34]. This suggests that Oct4 in PGCs may be maintained during PGC migration without de novo synthesis. In sum, the duplicated Oct4 gene copy which remains in teleost fish and mammals has maintained similar expression patterns before gastrulation and in PGCs throughout evolution in the two classes of vertebrates, although in zebrafish pou2/spg is not expressed in PGCs. This is a particular interesting evolutionary difference of the Oct4 homolog between medaka and zebrafish. The pou2/spg gene in zebrafish is not duplicated and the question remains of how the roles of Oct4 in PGC and gonad development in zebrafish may have been substituted. It will be interesting to analyze the expression patterns of the Oct4 homolog in fugu (Takifugu rubripres) and Tetraodon to determine how these functions may have evolved in different teleost species.
In this evolutionary context, it is interesting to note that after gastrulation, the zebrafish Oct4 homolog, spg/pou2, has a different spatial pattern of expression compared to Oct4 homologues in other vertebrates, and it has acquired other functions. One specific difference is that spg/pou2 is differentially expressed in the developing zebrafish brain where it plays an important role in regionalization [32]. Furthermore maternal and early zygotic spg/pou2 expression has been found to play an important role in endoderm development [35]. Moreover, although the presence of Spg/pou2 protein has not been analyzed, spg/pou2 expression has not been detected in the PGCs, and it has also been found not to be necessary for the correct development of PGCs in zebrafish [35] in contrast to medaka and mice. Thus, it appears that in zebrafish, Oct4 has acquired a new role during brain development and lost its function in PGC biology. Additionally, initial cross-species complementation experiments suggest that spg/pou2 cannot rescue mOct4 function and maintain ES cell renewal when transfected into mOct4 mutant mouse ESCs [10]. This indicates that at least some interactions necessary for pluripotency maintenance in mice have been lost in zebrafish, although in fish pou2 may still regulate pluripotency. On the other hand, the function of the OlOct4 homolog during medaka development and its capabilities to rescue ES cell renewal and cross-species pluripotency characteristics have not yet been examined. These studies will help to understand the evolution of Oct4 and determine the extent to which functional homology has been conserved between fish and mammals.
Comparing the roles of Nanog between mammals and fish
Nanog is a homeodomain (HD) transcription factor expressed in early embryo cells and PGCs in mouse [6, 7], chicken [9] and medaka fish [12]. mNanog expression is initially detected at the morula stage of the mouse embryo [6, 7], while in medaka it is maternally inherited (Figure 1) and its expression is detected as early as the unfertilized egg [12]. In other teleost species the expression pattern of Nanog has not been described and thus we will focus on the medaka OlNanog for comparison with the mouse mNanog. Lack of mNanog in mouse embryos results in early embryonic lethality [7]. Similarly, in medaka, OlNanog inhibition using morpholino oligos causes embryos to die without completing epiboly [12]. Thus, in both mouse and medaka, Nanog plays a central role in early embryo survival.
Additionally, in mouse ESC cultures, mNanog is a necessary factor for ESC to maintain their ability to differentiate into multiple cell lineages acting as a gatekeeper of the gateway to pluripotency [36, 37]. However, mNanog protein is expressed in mouse ESC in a mosaic pattern and cells which do not express mNanog in these cell cultures still retain expression of pluripotency markers and possess the ability to self-renew [38]. Moreover, mouse chimeras formed by implanting mNanog knock-out cells into wild type embryos develop normally and demonstrate that mNanog depleted cells can differentiate into all tissues except the gonads [38]. Thus, in the mouse, mNanog seems to be necessary to maintain pluripotency only during a short period of time since loss of mNanog does not irremediably provoke cell differentiation.
A similar finding is also observed in medaka, where OlNanog depleted embryos maintain the expression levels of pluripotency markers such as Oct4, Tert, and Tcf3 [12]. Moreover, in these medaka OlNanog morphant embryos, in which OlNanog may not have been removed completely, the expression levels of differentiation markers associated with early lineage commitment such as Bra, Sox17, Gata3 or Sox2 were not significantly changed [12]. These results suggest that OlNanog function in OlNanog-depleted embryonic cells can be rescued by neighbouring OlNanog positive cells, indicating a non-cell autonomous OlNanog-mediated effect on undifferentiated cells. Thus, mouse and medaka in vivo studies suggest that the crucial role of Nanog in pluripotency may affect proliferation and survival. However, cross-species complementation experiments are necessary between medaka and mouse or human Nanog to determine the extent of functional conservation among species.
Functional characterization of pluripotency genes in teleost fish can provide new clues to understand the roles and evolution of pluripotency in mammals, particularly in humans. For example, the study of Nanog function using medaka embryos revealed that Nanog controls cellular proliferation during the S phase transition by regulating CyclinA expression [12]. These results are consistent with findings in human ESCs, where overexpression of human NANOG results in an increase in proliferation. Simultaneously to the medaka studies, Zhang and colleagues described the role of human NANOG in regulating the transition from G1 to S phase of the cell cycle in human ESCs by direct regulation of CDC25A and CDK6 expression [39]. In view of these results, the function of medaka Nanog is similar to that observed in human cells, hence validating the use of teleost fish as models to study pluripotency.
Later in development, Nanog expression is restricted to PGCs in human, mouse, chicken and medaka (Figure 1) [6, 8, 9, 12, 40, 41]. In mice, Nanog deleted ESCs were implanted in wild-type morulae to form chimeras that developed normally. In these chimeric mice, Nanog-/-cells can be detected in all tissues, but PGCs lacking Nanog do not mature [38]. In fact, Nanog depletion using shRNA induced cell death in the migratory PGCs [42]. In medaka embryos, Ol-Nanog loss-of-function experiments using morpholinos provoked an altered migration and abnormal distribution of PGCs. In fish and mice, the signaling chemokine Sdf1 and its receptor Cxcr4 are necessary for PGC migration [43-47]. In medaka, Ol-Nanog binds to the regulatory region of Cxcr4b and regulates its expression. Thus Ol-Nanog mediates correct PGC migration by directly regulating the expression of Cxcr4b [41]. The role of Nanog in controlling PGC migration and apoptosis could explain the mouse chimera phenotype where Nanog-deficient ESCs do not generate mature PGCs [38]. It would be beneficial to investigate the relationship between the apoptotic and migratory processes in that Nanog could be acting in the mouse PGCs.
Other pluripotency genes in fish embryonic development
Several genes involved in mammalian pluripotency have been described in fish. Their analysis in fish embryos may help to resolve questions about the evolution of pluripotency in the vertebrate lineage and clarify some discrepancies that have raised in the gene functions in mice and humans. A clear example is the STAT3 gene which is essential to maintain mouse ESC in an undifferentiated state, however, in human and monkey ESC STAT3 seems to be dispensable. In medaka, analysis of STAT3 showed that STAT3 was also inactive in medaka ESC and blastula embryos, as in primates. These results suggest that the requirement of STAT3 in mouse pluripotency may be species specific, whereas in medaka, monkey and human STAT3 seems to be inactive [48]. Thus, from an evolutionary point of view, further studies in fish are providing clues about the evolution of pluripotency and which mechanisms are conserved among vertebrates.
Other important genes for mammalian pluripotency have been described in medaka, although their roles in early fish pluripotency have not been studied in detail. For example, Klf4 belongs to the Krüppel-like factor (KLF) family of transcriptional regulators and is necessary for maintaining mouse pluripotency. In zebrafish, many Klf orthologs have been identified and functional studies suggest that at least Klf1 and Klf4 play important roles in zebrafish hematopoiesis. However, other Klf orthologs, such as Klf2a and Klf2b which are expressed from 70% epiboly, may have a role in early fish pluripotency [49-52]. Finally, telomerase is necessary to maintain telomere length and is active in all stem cells. Telomerase RNA template and its catalytic subunit, Telomerase Reverse Transcriptase (TERT), have been described in medaka and zebrafish [53-55] which provide further circumstancial evidence that pluripotency mechanisms have been conserved in the vertebrate lineage. It is interesting to note that telomerase activity can not be detected in most human somatic tissues, but it is ubiquitously detected in teleost fish somatic tissues. This telomerase expression pattern and its similarity to its human homolog substantiate the use of teleost fish as a model to easily study telomerase function and molecular mechanisms in vivo [56].
Nanog and Oct4 in the gonads
Nanog and Oct4 are expressed in gonads of human, mouse, chicken and medaka suggesting that both proteins may play a role in gamete differentiation and/or gonadal stem cell maintenance [6-9, 12, 33, 40, 57-59]. Also, telomerase activity is detected in the gonads of both mammals and teleost fish [53, 54, 56]. However, experimentation in these adult tissues is difficult and it remains an important task to characterize the in vivo roles of pluripotency genes in the gonads. Nevertheless, the expression patterns of these genes provide some clues that point to a putative role in maintaining pluripotency in the gonad germ cells.
In medaka female gonads, OlNanog and OlOct4 transcript and proteins are detected in the small previtellogenic oocytes, however the signal diminishes and becomes undetectable in medium to large previtellogenic oocytes, which are arrested in meiosis. In medaka male gonads, OlNanog and OlOct4 transcript and protein are detected in the periphery of the testis, where undifferentiated spermatogonia, which constitutes the germ stem cell population of the testis, are located [12, 33].
The expression of Nanog is similar in medaka and mouse developing gonads. Mouse Nanog is expressed in germ cells and its expression is down-regulated in cells undergoing meiosis in female gonads, and at the onset of mitotic arrest in male gonads (Figure 1) [12, 42]. Thus, the initial role of Nanog may be to provide gonad stem cells with pluripotency characteristics and, therefore, its expression would be lost during differentiation. However, it is interesting to note that in mice mNanog is detected in type A spermatogonia and in pachytene spermatocytes, during haploid germ cell maturation, suggesting that it may play a role as an epigenetic modifier [59]. This putative role of mNanog as an epigenetic modifier during gamete maturation would introduce a new twist into Nanog function that may extend to its roles in pluripotency maintenance.
In the case of Oct4, medaka and mouse adult testis also share a similar Oct4 expression pattern. Both Oct4 mRNA and protein are detected in the most undifferentiated type A spermatogonia population in mice and medaka [33, 57]. Experiments in mice have shown that mOct4 is required for spermatogonia stem cell self-renewal [60]. On the other hand, mOct4 expression in mice ovaries has not been described and, in medaka, OlOct4 is detected in the most undifferentiated germ cells and in the germ plasm of oocytes, which will form the embryo germ cells [33]. Thus, the Oct4 expression pattern suggests that this transcription factor plays a role during gamete maturation. This hypothesis is supported by the results described in mice, where conditional inactivation of Oct4 in PGCs generates a sterility phenotype in the adult [34].
Conclusion
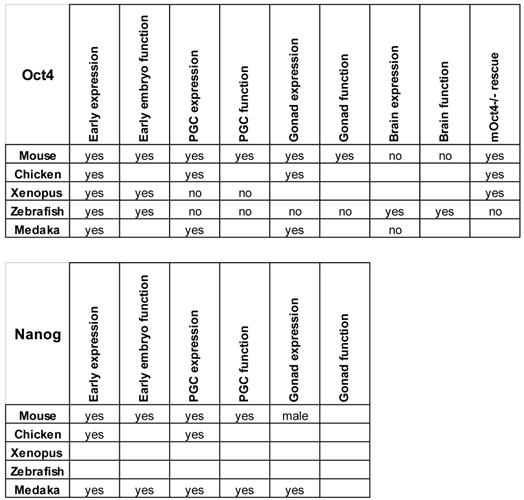
The different embryological and genetic manipulations to which zebrafish and medaka are amenable will expand the possibilities to study the roles of the different pluripotency factors that operate during vertebrate embryonic development (Table 2).
Expression and function of Nanog and Oct4 in different species. The sites of expression and roles of these genes described in the text are summarized. Empty boxes represent not determined characteristics.
The studies in medaka during early embryo development have already contributed important information on Nanog function. Furthermore, the use of medaka or zebrafish will allow researchers to design new screening strategies to identify proteins involved in pluripotency in vivo. Experiments in fish may also provide valuable information on new specific molecular interactions in vivo between Nanog, Oct4, Klf4, Sox2, TERT, STAT3 or Tcf3 which may be differentially conserved in mice or humans. The generation of transgenic animals for promoter analysis and conditional gene manipulation will further contribute to the understanding of the roles of these genes during embryonic development. Thus, the introduction of medaka and zebrafish to the study of embryonic pluripotency raises new and exciting questions and opportunities for the field.
Acknowledgements
We would like to thank Aranzazu Leal-Tassias for technical support and Deborah Burks for discussions. Our work is supported by a grant (BFU2009-10808) from the Spanish Ministry of Science and Innovation.
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Solter D. From teratocarcinomas to embryonic stem cells and beyond: a history of embryonic stem cell research. Nat Rev Genet. 2006;7:319-327
2. Prelle K, Zink N, Wolf E. Pluripotent stem cells-model of embryonic development, tool for gene targeting, and basis of cell therapy. Anat Histol Embryol. 2002;31:169-186
3. Johnson BV, Shindo N, Rathjen PD, Rathjen J, Keough RA. Understanding pluripotency-how embryonic stem cells keep their options open. Mol Hum Reprod. 2008;14:513-520
4. Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Schöler H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379-391
5. Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126-140
6. Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643-655
7. Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631-642
8. Cañón S, Herranz C, Manzanares M. Germ cell restricted expression of chick Nanog. Dev Dyn. 2006;235:2889-2894
9. Lavial F, Acloque H, Bertocchini F, Macleod DJ, Boast S, Bachelard E, Montillet G, Thenot S, Sang HM, Stern CD, Samarut J, Pain B. The Oct4 homologue PouV and Nanog regulate pluripotency in chicken embryonic stem cells. Development. 2007;134:3549-3563
10. Morrison GM, Brickman JM. Conserved roles for Oct4 homologues in maintaining multipotency during early vertebrate development. Development. 2006;133:2011-2022
11. Burgess S, Reim G, Chen W, Hopkins N, Brand M. The zebrafish spiel-ohne-grenzen (spg) gene encodes the POU domain protein Pou2 related to mammalian Oct4 and is essential for formation of the midbrain and hindbrain, and for pre-gastrula morphogenesis. Development. 2002;129:905-916
12. Camp E, Sánchez-Sánchez AV, García-España A, Desalle R, Odqvist L, Enrique O'Connor J, Mullor JL. Nanog regulates proliferation during early fish development. Stem Cells. 2009;27:2081-2091
13. Thermes V, Candal E, Alunni A, Serin G, Bourrat F, Joly JS. Medaka simplet (FAM53B) belongs to a family of novel vertebrate genes controlling cell proliferation. Development. 2006;133:1881-1890
14. Yuan H, Corbi N, Basilico C, Dailey L. Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev. 1995;9:2635-2645
15. Reményi A, Lins K, Nissen LJ, Reinbold R, Schöler HR, Wilmanns M. Crystal structure of a POU/HMG/DNA ternary complex suggests differential assembly of Oct4 and Sox2 on two enhancers. Genes Dev. 2003;17:2048-2059
16. Kamachi Y, Sockanathan S, Liu Q, Breitman M, Lovell-Badge R, Kondoh H. Involvement of SOX proteins in lens-specific activation of crystallin genes. EMBO J. 1995;14:3510-3519
17. Uwanogho D, Rex M, Cartwright EJ, Pearl G, Healy C, Scotting PJ, Sharpe PT. Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development. Mech Dev. 1995;49:23-36
18. Wittbrodt J, Shima A, Schartl M. Medaka--a model organism from the far East. NatRevGenet. 2002;3:53-64
19. Eisen J. Zebrafish as a model system. Perspective. Dev Dyn. 2003;228:299-300
20. Wakamatsu Y, Ozato K, Sasado T. Establishment of a pluripotent cell line derived from a medaka (Oryzias latipes) blastula embryo. Mol Mar Biol Biotechnol. 1994;3:185-191
21. Sun L, Bradford CS, Ghosh C, Collodi P, Barnes DW. ES-like cell cultures derived from early zebrafish embryos. Mol Mar Biol Biotechnol. 1995;4:193-199
22. Hong Y, Winkler C, Schartl M. Pluripotency and differentiation of embryonic stem cell lines from the medakafish (Oryzias latipes). Mech Dev. 1996;60:33-44
23. Takeda H, Matsuzaki T, Oki T, Miyagawa T, Amanuma H. A novel POU domain gene, zebrafish pou2: expression and roles of two alternatively spliced twin products in early development. Genes Dev. 1994;8:45-59
24. Hong Y, Winkler C, Liu T, Chai G, Schartl M. Activation of the mouse Oct4 promoter in medaka embryonic stem cells and its use for ablation of spontaneous differentiation. Mech Dev. 2004;121:933-943
25. Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372-376
26. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676
27. Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318-324
28. Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, Muhlestein W, Melton DA. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269-1275
29. Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101-106
30. Frankenberg S, Pask A, Renfree MB. The evolution of class V POU domain transcription factors in vertebrates and their characterisation in a marsupial. Dev Biol. 2010;337:162-170
31. Yeom YI, Ha HS, Balling R, Schöler HR, Artzt K. Structure, expression and chromosomal location of the Oct-4 gene. Mech Dev. 1991;35:171-179
32. Belting HG, Hauptmann G, Meyer D, Abdelilah-Seyfried S, Chitnis A, Eschbach C, Söll I, Thisse C, Thisse B, Artinger KB, Lunde K, Driever W. spiel ohne grenzen/pou2 is required during establishment of the zebrafish midbrain-hindbrain boundary organizer. Development. 2001;128:4165-4176
33. Sánchez-Sánchez AV, Camp E, García-España A, Leal-Tassias A, Mullor JL. Medaka Oct4 is expressed during early embryo development, and in primordial germ cells and adult gonads. Dev Dyn. 2010;239:672-679
34. Kehler J, Tolkunova E, Koschorz B, Pesce M, Gentile L, Boiani M, Lomelí H, Nagy A, McLaughlin KJ, Schöler HR, Tomilin A. Oct4 is required for primordial germ cell survival. EMBO Rep. 2004;5:1078-1083
35. Lunde K, Belting HG, Driever W. Zebrafish pou5f1/pou2, homolog of mammalian Oct4, functions in the endoderm specification cascade. Curr Biol. 2004;14:48-55
36. Silva J, Chambers I, Pollard S, Smith A. Nanog promotes transfer of pluripotency after cell fusion. Nature. 2006;441:997-1001
37. Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722-737
38. Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L, Smith A. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230-1234
39. Zhang X, Neganova I, Przyborski S, Yang C, Cooke M, Atkinson SP, Anyfantis G, Fenyk S, Keith WN, Hoare SF, Hughes O, Strachan T, Stojkovic M, Hinds PW, Armstrong L, Lako M. A role for NANOG in G1 to S transition in human embryonic stem cells through direct binding of CDK6 and CDC25A. J Cell Biol. 2009;184:67-82
40. Perrett RM, Turnpenny L, Eckert JJ, O'Shea M, Sonne SB, Cameron IT, Wilson DI, Meyts ER, Hanley NA. The early human germ cell lineage does not express SOX2 during in vivo development or upon in vitro culture. BiolReprod. 2008;78:852-8
41. Sánchez-Sánchez AV, Camp E, Leal-Tassias A, Atkinson S, Armstrong L, Llopis MD, Mullor JL. Nanog regulates primordial germ cell migration through Cxcr4b. Stem Cell. 2010 In press
42. Yamaguchi S, Kurimoto K, Yabuta Y, Sasaki H, Nakatsuji N, Saitou M, Tada T. Conditional knockdown of Nanog induces apoptotic cell death in mouse migrating primordial germ cells. Development. 2009;136:4011-4020
43. Doitsidou M, Reichman-Fried M, Stebler J, Köprunner M, Dörries J, Meyer D, Esguerra CV, Leung T, Raz E. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111:647-659
44. Ara T, Nakamura Y, Egawa T, Sugiyama T, Abe K, Kishimoto T, Matsui Y, Nagasawa T. Impaired colonization of the gonads by primordial germ cells in mice lacking a chemokine, stromal cell-derived factor-1 (SDF-1). Proc Natl Acad Sci U S A. 2003;100:5319-5323
45. Kurokawa H, Aoki Y, Nakamura S, Ebe Y, Kobayashi D, Tanaka M. Time-lapse analysis reveals different modes of primordial germ cell migration in the medaka Oryzias latipes. Dev Growth Differ. 2006;48:209-221
46. Knaut H, Werz C, Geisler R, Nüsslein-Volhard C; Tübingen 2000 Screen Consortium. A zebrafish homologue of the chemokine receptor Cxcr4 is a germ-cell guidance receptor. Nature. 2003;421:279-282
47. Molyneaux KA, Zinszner H, Kunwar PS, Schaible K, Stebler J, Sunshine MJ, O'Brien W, Raz E, Littman D, Wylie C, Lehmann R. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development. 2003Sep;130(18):4279-86
48. Wagner TU, Kraeussling M, Fedorov LM, Reiss C, Kneitz B, Schartl M. STAT3 and SMAD1 signaling in Medaka embryonic stem-like cells and blastula embryos. Stem Cells Dev. 2009;18(1):151-60
49. Kawahara A, Dawid IB. Critical role of biklf in erythroid cell differentiation in zebrafish. Curr Biol. 2001;11:1353-1357
50. Oates AC, Pratt SJ, Vail B, Yan Yl, Ho RK, Johnson SL, Postlethwait JH, Zon LI. The zebrafish klf gene family. Blood. 2001;98:1792-1801
51. Gardiner MR, Gongora MM, Grimmond SM, Perkins AC. A global role for zebrafish klf4 in embryonic erythropoiesis. Mech Dev. 2007;124:762-774
52. Gardiner MR, Daggett DF, Zon LI, Perkins AC. Zebrafish KLF4 is essential for anterior mesendoderm/pre-polster differentiation and hatching. Dev Dyn. 2005;234:992-996
53. Lau BW, Wong AO, Tsao GS, So KF, Yip HK. Molecular cloning and characterization of the zebrafish (Danio rerio) telomerase catalytic subunit (telomerase reverse transcriptase, TERT). J Mol Neurosci. 2008;34:63-75
54. Pfennig F, Kind B, Zieschang F, Busch M, Gutzeit HO. Tert expression and telomerase activity in gonads and somatic cells of the Japanese medaka (Oryzias latipes). Dev Growth Differ. 2008;50:131-141
55. Xie M, Mosig A, Qi X, Li Y, Stadler PF, Chen JJ. Structure and function of the smallest vertebrate telomerase RNA from teleost fish. J Biol Chem. 2008;283:2049-2059
56. Au DW, Mok HO, Elmore LW, Holt SE. Japanese medaka: a new vertebrate model for studying telomere and telomerase biology. Comp Biochem Physiol C Toxicol Pharmacol. 2009;149:161-167
57. Pesce M, Schöler HR. Oct-4: Control of totipotency and germline determination. Mol Reprod Dev. 2000;55:452-457
58. Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PT. Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev Biol. 2007;7:136
59. Kuijk EW, de Gier J, Chuva de Sousa Lopes SM, Chambers I, van Pelt AM, Colenbrander B, Roelen BA. A Distinct Expression Pattern in Mammalian Testes Indicates a Conserved Role for NANOG in Spermatogenesis. PLoS One. 2010;5:e10987
60. Dann CT, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, Porteus MH. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 2008Nov;26(11):2928-37
Author contact
![]() Corresponding author: jlmullororg; Tel: +34 961 973 400; Fax: +34 963 494 416.
Corresponding author: jlmullororg; Tel: +34 961 973 400; Fax: +34 963 494 416.

 Global reach, higher impact
Global reach, higher impact