10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2015; 11(7):762-771. doi:10.7150/ijbs.11978 This issue Cite
Research Paper
NQO1 Stabilizes p53 in Response to Oncogene-Induced Senescence
1. Department of Biochemistry and Molecular Biology, Peking University Health Science Center; Beijing, 100191 People's Republic of China;
2. Department of Clinical Laboratory, Peking University First Hospital, Beijing, 100034 People's Republic of China;
3. Center of Basic Medical Sciences, Navy General Hospital; Beijing, 100048 People's Republic of China
Abstract
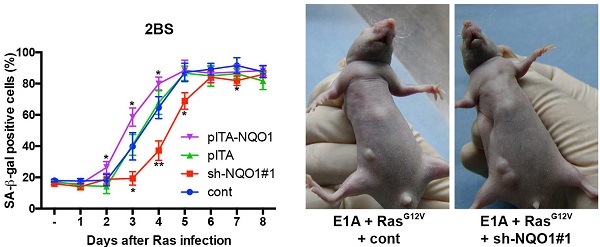
Cellular senescence is a state of permanent cellular arrest that provides an initial barrier to cell transformation and tumorigenesis. In this study, we report that expression of NAD(P)H:quinone oxidoreductase 1 (NQO1), a cytoplasmic 2-electron reductase, is induced during oncogene-induced senescence (OIS). Depletion of NQO1 resulted in the delayed onset of senescence. In contrast, ectopic expression of NQO1 enhanced the senescence phenotype. Analysis of the mechanism underlying the up-regulation of NQO1 expression during senescence identified that NQO1 promotes p53 accumulation in an MDM2 and ubiquitin independent manner, which reinforces the cellular senescence phenotype. Specifically, we demonstrated that NRF2/KEAP1 signaling regulates NQO1 expression during OIS. More importantly, we confirmed that depletion of NQO1 facilitates cell transformation and tumorigenesis, which indicates that NQO1 takes part in the senescence barrier and has anti-oncogenic properties in cell transformation.
Keywords: NQO1, p53, senescence

 Global reach, higher impact
Global reach, higher impact