10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2016; 12(4):359-366. doi:10.7150/ijbs.13764 This issue Cite
Review
CPT-cGMP Is A New Ligand of Epithelial Sodium Channels
1. Department of Cellular and Molecular Biology, University of Texas Health Science Center at Tyler, Tyler, Texas 75708, USA.
2. Institute of Metabolic Disease Research and Drug Development, China Medical University, Shenyang 110001, China.
3. Barrow Neurological Institute, St Joseph's Hospital and Medical Center, Phoenix, Arizona, 85013, USA.
4. Department of Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China.
5. Department of Molecular Microbiology and Immunology, Bond Life Sciences Center, University of Missouri, Columbia, MO 65211, USA.
Abstract
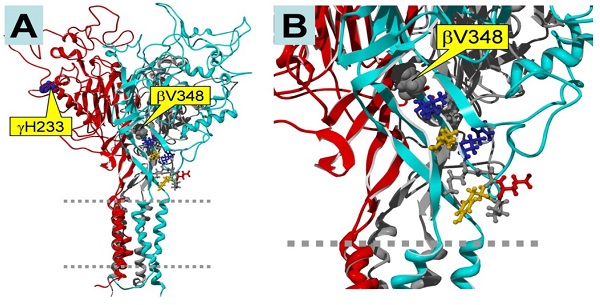
Epithelial sodium channels (ENaC) are localized at the apical membrane of the epithelium, and are responsible for salt and fluid reabsorption. Renal ENaC takes up salt, thereby controlling salt content in serum. Loss-of-function ENaC mutations lead to low blood pressure due to salt-wasting, while gain-of-function mutations cause impaired sodium excretion and subsequent hypertension as well as hypokalemia. ENaC activity is regulated by intracellular and extracellular signals, including hormones, neurotransmitters, protein kinases, and small compounds. Cyclic nucleotides are broadly involved in stimulating protein kinase A and protein kinase G signaling pathways, and, surprisingly, also appear to have a role in regulating ENaC. Increasing evidence suggests that the cGMP analog, CPT-cGMP, activates αβγ-ENaC activity reversibly through an extracellular pathway in a dose-dependent manner. Furthermore, the parachlorophenylthio moiety and ribose 2'-hydroxy group of CPT-cGMP are essential for facilitating the opening of ENaC channels by this compound. Serving as an extracellular ligand, CPT-cGMP eliminates sodium self-inhibition, which is a novel mechanism for stimulating salt reabsorption in parallel to the traditional NO/cGMP/PKG signal pathway. In conclusion, ENaC may be a druggable target for CPT-cGMP, leading to treatments for kidney malfunctions in salt reabsorption.
Keywords: amiloride-sensitive sodium channel, cyclic guanosine nucleotides, molecular docking, lung edema.

 Global reach, higher impact
Global reach, higher impact