10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2016; 12(9):1150-1154. doi:10.7150/ijbs.15747 This issue Cite
Review
Epithelial Sodium Channels in Pulmonary Epithelial Progenitor and Stem Cells
1. Institute of Lung and Molecular Therapy, Xinxiang Medical University, Xinxiang, Henan 453003, China;
2. School of Public Health, Xinxiang Medical University, Xinxiang, Henan 453003, China.
3. Department of Cellular and Molecular Biology, University of Texas Health Science Center at Tyler, Tyler, Texas 75708, USA;
4. Texas Lung Injury Institute, University of Texas Health Science Center at Tyler, Tyler, Texas 75708, USA.
Received 2016-4-5; Accepted 2016-7-11; Published 2016-8-15
Abstract
Regeneration of the epithelium of mammalian lungs is essential for restoring normal function following injury, and various cells and mechanisms contribute to this regeneration and repair. Club cells, bronchioalveolar stem cells (BASCs), and alveolar type II epithelial cells (ATII) are dominant stem/progenitor cells for maintaining epithelial turnover and repair. Epithelial Na+ channels (ENaC), a critical pathway for transapical salt and fluid transport, are expressed in lung epithelial progenitors, including club and ATII cells. Since ENaC activity and expression are development- and differentiation-dependent, apically located ENaC activity has therefore been used as a functional biomarker of lung injury repair. ENaC activity may be involved in the migration and differentiation of local and circulating stem/progenitor cells with diverse functions, eventually benefiting stem cells spreading to re-epithelialize injured lungs. This review summarizes the potential roles of ENaC expressed in native progenitor and stem cells in the development and regeneration of the respiratory epithelium.
Keywords: amiloride-inhibitable sodium channels, mesenchymal stem cells, proliferation, differentiation, pluripotent stem cells.
Introduction
Epithelial Na+ channels (ENaC) are of importance in Na+-absorptive epithelium, such as in the airway, the alveolus, the kidney, and the distal colon, and control the overall rate of transapical Na+ transport. ENaC proteins are mainly located in the apical membrane of polarized epithelial cells and have four homologous subunits (i.e., the α, β, γ and δ subunits) [1, 2]. The α or δ ENaC subunit, which is essential for acting as a sodium channel, forms the channel pore, whereas the β and γ ENaC subunits are critical for amplifying the efficiency of Na+ influx. In mouse, the gene scnn1d, which encodes δ ENaC, is assumed to be a pseudogene [2].
In the lungs, alveolar lining fluid is critical for efficient gas exchange, and ENaC complexes play a crucial role in alveolar fluid clearance to maintain homeostasis of the luminal liquid. Pulmonary diseases, including acute lung injury, cystic fibrosis, chronic obstructive pulmonary disease, and asthma result from or are associated with the dysfunction or dysregulation of ENaC, and the regeneration of epithelial cells and the restoration of ion transport are two key steps in recovery from those diseases. In this article, we review the progress of research on ENaC-mediated lung injury repair, in particular the role of ENaC proteins in re-epithelialization by endogenous and allogeneic stem/progenitor cells.
Stem/progenitor cells for pulmonary epithelium
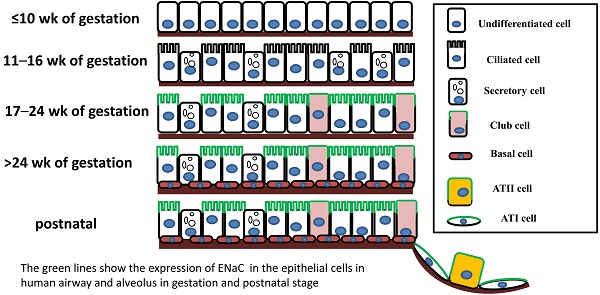
The mammalian pulmonary epithelium, a multilevel, branched network, can be functionally divided into the proximal conducting airways and the distal gas-exchange domain. The development of the airways and the lung is the result of specification and separation of a group of progenitor cells from the ventral region of the anterior foregut. In humans, the development of the airways and alveoli from fetal to adult stage was investigated by Gaillard et al [3]. Before the 10th week of gestation, the airway epithelium consists of undifferentiated columnar cells, and then ciliated cells, secretory cells, club cells, alveolar type I (ATI) and type II (ATII) cells differentiate from these progenitor cells at different stages of gestation (Figure 1). A recent study indicated that both ATI and ATII cells are derived from a lineage of bipotential progenitors during embryonic development and that ATII cells undergo a switch that functions both to self-renew and to generate ATI cells [4]. Lineage hierarchies constructed using single-cell RNA-seq unveiled the differentiation steps of these bipotential progenitors [5].
In adults, the lung not only undergoes a slow turnover, but also repairs itself rapidly, indicating the existence of a subpopulation of stem cells or progenitors with preserved differentiation potential. The stem/progenitor cells able to repair injured lungs include club cells, bronchioalveolar stem cells (BASCs), and ATII cells. In addition, submucosal gland duct stem cells and neuroendocrine cells have the potential to differentiate into club, basal, serous, or ciliated cells, as well as into distal airway epithelium.
Club cells, BASCs, and ATII cells are dominant in the pulmonary epithelium and have been generally well studied. Club cells are able to self-renew, differentiate into ciliated epithelial cells, and contribute to the replenishment of both ATI and ATII cells in lungs injured by bleomycin or infection with H1N1 influenza [6-8]. BASCs are located in bronchioalveolar duct junctions, and following catastrophic alveolar epithelial injury, can replenish cell lineages in the alveolus [8]. In addition to club cells, distal airway stem cells (DASCs) and basal cells contribute to replenishing other pulmonary epithelial cells. ATII cells are involved in the regeneration of alveoli, maintaining a slow self-renewal in normal lungs and then differentiating into ATI cells during lung injury repair. Additionally, a subpopulation of alveolar epithelial cells expressing integrin α6/β4 and DASCs has the potential to differentiate into ATII and club cells [9, 10]. Recently, a new type of basally located DASCs that express Trp63 and keratin 5 was also reported to be crucial for epithelium regeneration in airways and alveoli [11-13].
Expression of ENaC in pulmonary epithelial stem/progenitor cells
The expression of ENaC in the human respiratory system was confirmed in a development-dependent manner [3]. At the early stage of embryonic development (≤ 16 wk of gestation), the β- and γ-ENaC subunits were not detected in human airways [3]. Near birth, increased ENaC activity was present on the apical surface of lung epithelial cells, and active Na+ transport was promoted [14]. In adult airways, the expression pattern of ENaC was similar to that in the canalicular period (17-24 wk) (Figure 1). Transcripts of α-ENaC were expressed in club cells throughout fetal lung development [15], and all four subunits (α, β, γ and δ) were detected in adult club cells [1, 16]. ENaC mRNA was distributed in ATII cells after 28 wk of gestation [17], and ENaC subunits were also expressed in ATII cells (Figure 1) [2, 18, 19].
The expression of ENaC in respiratory epithelial cells at different stages of human fetal development and after birth. The distribution of ENaC is shown by the green line.
Differentiation potential and ENaC expression in pulmonary epithelial cells
| Cell type | Differentiation potential | Expression of ENaC |
|---|---|---|
| Ciliated cell | - | + |
| Club cell | + | + |
| Goblet cell | - | - |
| Submucosal glands duct stem cell | + | + |
| Neuroendocrine cell | + | ? |
| Basal cell | + | - |
| Bronchioalveolar stem cell | + | ? |
| Distal airway stem cell | + | ? |
| Serous cell | - | + |
| Alveolar type I cell | - | + |
| Alveolar type II cell | + | + |
+, have differentiation potential or express ENaC; -, do not have differentiation potential or do not express ENaC; ?, status is unclear.
Regulation of ENaC in pulmonary epithelial stem/progenitor cells
In postnatal pulmonary epithelium, Na+ ions flow into epithelial cells via apically located ENaC proteins and are actively pumped out of the cells by Na+-K+-ATPase at the basolateral membrane. The subsequent osmotic gradients prompt transepithelial liquid re-absorption. ENaC has been functionally detected in both human club cells and ATII cells. Apical fluid volume regulated the activity and abundance of ENaC in H441 cells originally derived from human club cells. Dexamethasone, a corticosteroid, regulated ENaC activity in club- and ATII-like cell cultures, by promoting the expression of ENaC [20] subsequent to activating the SGK1, PI3K and cAMP/PKA signaling pathways [21]. Treatment with dexamethasone and cAMP-elevating agents can lead to the differentiation of the aforementioned native stem cells to club cells or alveolar cells [22, 23]. SGK1 and PKA can phosphorylate the ubiquitin ligase Nedd4-2, which mediates the internalisation and degradation of ENaC by binding to the proline-rich domains of ENaC [24]. Moreover, a number of signaling molecules, such as hydrogen sulfide, nitric oxide, UTP, and CPT-cGMP regulate ENaC activity in H441 cells [25-27], and respiratory syncytial virus can inhibit ENaC-mediated alveolar fluid clearance by upregulating the synthesis of UTP and nitric oxide [28].
Bacterial impairment of ENaC activity is controlled by the phosphorylation of ERK. Phosphorylation of ERK1/2 results in a decrease in the expression and function of ENaC in ATII cells [29]. Nedd4-2 facilitates the effects of PKC on ENaC activity in ATII [29] and club cells [24], and PKC may also play a role in ENaC-PIP2-MARCKS complexes, which regulate the open probability of ENaC and can be stabilized by binding with TNF [18], another factor that may also enhance ENaC activity in ATII cells [30, 31]. PKC, cAMP/PI3K, PKA and cGMP mediate the regulation of ENaC by LPS in ATII and club cells [32, 33], and PKC was shown to mediate Wnt signaling, which regulates the differentiation of mesenchymal stem cells (MSCs) to ATII cells and of ATII to ATI cells [34]. Additionally, cGMP levels were inversely related with the expression of ATII markers when undifferentiated lung epithelial cells were treated with inhaled nitric oxide [35]. Therefore, regulation of ENaC by ERK, PKC, cAMP, PKA, and TNF may play a role in the differentiation of endogenous progenitor/stem cells.
ENaC-mediated epithelial repair
In postnatal lungs, epithelial stem/progenitor cells are reserved for injury repair. Whenever injury occurs, the epithelial stem/progenitor cells go through essentially the same process, including migrating to the injured region to cover the denuded airway and alveolar sac, and proliferating vigorously to provide enough cells for epithelium repair, differentiation, and remodeling, and finally the normal airways and lungs are regenerated structurally and functionally. For example, in naphthalene-induced airway epithelial injury, BASCs exhibit highly proliferative activity in response to the injury of club cells [36].
ENaC subunits are thought to be involved in injury repair and wound healing. Methylation of ENaC is an important event while aldosterone promotes the wound healing in BeWo cells and other epithelial cells. Mechanistically, migration of cultured epithelial and nonepithelial cells occurs in an ENaC-dependent manner [37, 38], and serum- and glucocorticoid-induced kinase 1 (SGK1) regulates cell proliferation through an ENaC-associated process.
Cell migration generally undergoes several processes, including depolarization, membrane elongation, adhesion, contraction, and de-adhesion, that are regulated by interactions between cells, and between cells and the extracellular matrix. Chifflet et al. reported that actin reorganization and membrane depolarization depend on ENaC-regulated extracellular Na+ ions during wound healing of bovine corneal endothelial cells [39]. ENaC proteins are the central part of a complex that links the cytoskeleton with the extracellular matrix [40], and the binding of the C-termini of α- and β-ENaC with filamins exerts an inhibitory effect on ENaC function [41]. ENaC proteins are also a critical part of the mechanotransducer for myogenic contraction [42].
Rooj et al. found that a deficiency in ENaC caused more D54-MG cells to arrest in G0/G1 phase, with fewer cells accumulating in the S and G2/M phases [43]; it was suggested that cell division is depressed when ASIC1 and ENaC are inhibited and that phosphorylation of ERK1/2 may be an underlying mechanism [43]. This group also found that interactions between amiloride-sensitive cation channels (ASIC1 and ENaC) and integrin-β1, mediated by α-actinin, could in part regulate the proliferation and migration of glioma cells [44]. In addition, ENaC is involved in the proliferation and migration of various cancer cells [38].
The effects of ENaC on osteoblast differentiation have been studied by several groups who found that the expression of ENaC mRNA accompanied the osteoclastogenesis of rat osteoblasts. Stimulation of osteoblast differentiation by 8-pCPT-cGMP is also dependent on the expression of ENaC [45] and therefore, ENaC activity is apparently required for the differentiation of both osteoblasts and osteoclasts.
In club and ATII cells, the aberrance of ENaC expression usually leads to Na+ absorption disorder, hydropic degeneration and necrosis of club cells, goblet cell metaplasia, failure of airway mucus clearance, susceptibility to spontaneous bacterial infection, airway inflammation, and even death caused by airway obstruction and asphyxiation [24].
ENaC as a biomarker for injury repair
ENaC proteins are electrically detectable as functional biomarkers of differentiated epithelial cells. Because of the role of ENaC in the migration and proliferation of stem/progenitor cells, it can be speculated that normal ENaC function would be critical in the repair of injured lungs by MSCs [46, 47]. Goolaerts and colleagues reported that impaired ENaC activity under hypoxic and cytomixic conditions was restored by co-cultured MSCs and paracrine KGF [48]. Moreover, impaired ENaC function of alveolar fluid clearance was recovered by human MSCs delivered intratracheally in a clinically related, human lung injury model [49]. The improvement of ENaC function and the contribution of ENaC to re-epithelialization potentially explain the promising results of clinical trials that show a significant reduction in lung injury score in acute respiratory distress syndrome (ARDS) and other lung injury treated with stem cells [50-53].
Future Perspective
Lung injury is associated with defective epithelium and dysfunctional ion transport, and stem cell therapy to repair injured tissue has broadened the prospects for treatment beyond supportive approaches. The functional consequences of normalizing injured epithelium are usually evaluated by detecting the expression and activity of ENaC. However, our understanding of the mechanisms by which ENaC regulates differentiation of lung stem/progenitor cells is incomplete, for example, whether ENaC contributes to the release of paracrines from allogeneic MSCs, and what are the roles of ENaC in the re-epithelialization mediated by these paracrines. Further mechanistic studies are required to address these essential issues.
Acknowledgements
This work was supported partially by institutional funds (to YL, BJJ, and HLJ) and American Heart Association grants (HLJ and RZZ, 14GRNT20130034 and 16GRNT30780002).
Abbreviations
ARDS: acute respiratory distress syndrome; ATI: alveolar type I; ATII: alveolar type II; BASCs: bronchioalveolar stem cells; DASCs: distal airway stem cells; ENaC: epithelial Na+ channels; TNF: tumor necrosis factor; KGF: keratinocyte growth factor; LPS: lipopolysaccharide; MSCs: mesenchymal stem cells; PKC: protein kinase C; SGK1: serum- and glucocorticoid-induced kinase 1
Competing Interests
The authors declare no conflict of interest.
References
1. Ji HL, Su XF, Kedar S, Li J, Barbry P, Smith PR. et al. Delta-subunit confers novel biophysical features to alpha beta gamma-human epithelial sodium channel (ENaC) via a physical interaction. J Biol Chem. 2006;281:8233-41
2. Ji HL, Zhao RZ, Chen ZX, Shetty S, Idell S, Matalon S. delta ENaC: a novel divergent amiloride-inhibitable sodium channel. Am J Physiol Lung Cell Mol Physiol. 2012;303:L1013-26
3. Gaillard D, Hinnrasky J, Coscoy S, Hofman P, Matthay MA, Puchelle E. et al. Early expression of beta- and gamma-subunits of epithelial sodium channel during human airway development. Am J Physiol Lung Cell Mol Physiol. 2000;278:L177-84
4. Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190-4
5. Treutlein B, Brownfield DG, Wu AR, Neff NF, Mantalas GL, Espinoza FH. et al. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature. 2014;509:371-5
6. Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D. et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525-38
7. Zheng D, Yin L, Chen J. Evidence for Scgb1a1(+) cells in the generation of p63(+) cells in the damaged lung parenchyma. Am J Respir Cell Mol Biol. 2014;50:595-604
8. Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD. et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156:440-55
9. Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K. et al. Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855-62
10. McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci U S A. 2010;107:1414-9
11. Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B. et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621-5
12. Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y. et al. p63(+) Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517:616-20
13. Rawlins EL. Stem cells: Emergency back-up for lung repair. Nature. 2015;517:556-7
14. Matalon S, Bartoszewski R, Collawn JF. Role of epithelial sodium channels (ENaC) in the regulation of lung fluid homeostasis. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1229-38
15. Banasikowska K, Post M, Cutz E, O'Brodovich H, Otulakowski G. Expression of epithelial sodium channel alpha-subunit mRNAs with alternative 5'-untranslated regions in the developing human lung. Am J Physiol Lung Cell Mol Physiol. 2004;287:L608-15
16. Woollhead AM, Sivagnanasundaram J, Kalsi KK, Pucovsky V, Pellatt LJ, Scott JW. et al. Pharmacological activators of AMP-activated protein kinase have different effects on Na+ transport processes across human lung epithelial cells. Br J Pharmacol. 2007;151:1204-15
17. Smith DE, Otulakowski G, Yeger H, Post M, Cutz E, O'Brodovich HM. Epithelial Na (+) channel (ENaC) expression in the developing normal and abnormal human perinatal lung. Am J Respir Crit Care Med. 2000;161:1322-31
18. Czikora I, Alli A, Bao HF, Kaftan D, Sridhar S, Apell HJ. et al. A novel tumor necrosis factor-mediated mechanism of direct epithelial sodium channel activation. Am J Respir Crit Care Med. 2014;190:522-32
19. Zhao RZ, Nie HG, Su XF, Han DY, Lee A, Huang Y. et al. Characterization of a novel splice variant of delta ENaC subunit in human lungs. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1262-72
20. Manwani N, Gagnon S, Post M, Joza S, Muglia L, Cornejo S. et al. Reduced viability of mice with lung epithelial-specific knockout of glucocorticoid receptor. Am J Respir Cell Mol Biol. 2010;43:599-606
21. Watt GB, Ismail NA, Caballero AG, Land SC, Wilson SM. Epithelial Na(+) channel activity in human airway epithelial cells: the role of serum and glucocorticoid-inducible kinase 1. Br J Pharmacol. 2012;166:1272-89
22. Kondo H, Miyoshi K, Sakiyama S, Tangoku A, Noma T. Differential regulation of gene expression of alveolar epithelial cell markers in human lung adenocarcinoma-derived A549 clones. Stem Cells Int. 2015;2015:165867
23. Schmeckebier S, Mauritz C, Katsirntaki K, Sgodda M, Puppe V, Duerr J. et al. Keratinocyte growth factor and dexamethasone plus elevated cAMP levels synergistically support pluripotent stem cell differentiation into alveolar epithelial type II cells. Tissue Eng Part A. 2013;19:938-51
24. Kimura T, Kawabe H, Jiang C, Zhang W, Xiang YY, Lu C. et al. Deletion of the ubiquitin ligase Nedd4L in lung epithelia causes cystic fibrosis-like disease. Proc Natl Acad Sci U S A. 2011;108:3216-21
25. Althaus M, Pichl A, Clauss WG, Seeger W, Fronius M, Morty RE. Nitric oxide inhibits highly selective sodium channels and the Na+/K+-ATPase in H441 cells. Am J Respir Cell Mol Biol. 2011;44:53-65
26. Han DY, Nie HG, Su XF, Shi XM, Bhattarai D, Zhao M. et al. 8-(4-chlorophenylthio)-guanosine-3',5'-cyclic Monophosphate-Na stimulates human alveolar fluid clearance by releasing external Na+ self-inhibition of epithelial Na+ channels. Am J Respir Cell Mol Biol. 2011;45:1007-14
27. Ji H-L, Nie H-G, Chang Y, Lian Q, Liu S-L. CPT-cGMP is a new ligand of epithelial sodium channels. Int J Biol Sci. 2016;12:359-66
28. Song W, Liu G, Bosworth CA, Walker JR, Megaw GA, Lazrak A. et al. Respiratory syncytial virus inhibits lung epithelial Na+ channels by up-regulating inducible nitric-oxide synthase. J Biol Chem. 2009;284:7294-306
29. Eaton AF, Yue Q, Eaton DC, Bao HF. ENaC activity and expression is decreased in the lungs of protein kinase C-alpha knockout mice. Am J Physiol Lung Cell Mol Physiol. 2014;307:L374-85
30. Shabbir W, Scherbaum-Hazemi P, Tzotzos S, Fischer B, Fischer H, Pietschmann H. et al. Mechanism of action of novel lung edema therapeutic AP301 by activation of the epithelial sodium channel. Mol Pharmacol. 2013;84:899-910
31. Hazemi P, Tzotzos SJ, Fischer B, Andavan GS, Fischer H, Pietschmann H. et al. Essential structural features of TNF-alpha lectin-like domain derived peptides for activation of amiloride-sensitive sodium current in A549 cells. J Med Chem. 2010;53:8021-9
32. Boncoeur E, Tardif V, Tessier MC, Morneau F, Lavoie J, Gendreau-Berthiaume E. et al. Modulation of epithelial sodium channel activity by lipopolysaccharide in alveolar type II cells: involvement of purinergic signaling. Am J Physiol Lung Cell Mol Physiol. 2010;298:L417-26
33. Wang Q, Lian QQ, Li R, Ying BY, He Q, Chen F. et al. Lipoxin A (4) activates alveolar epithelial sodium channel, Na, K-ATPase, and increases alveolar fluid clearance. Am J Respir Cell Mol Biol. 2013;48:610-8
34. Rieger ME, Zhou B, Solomon N, Sunohara M, Li C, Nguyen C. et al. p300/Beta-Catenin interactions regulate adult progenitor cell differentiation downstream of WNT5a/protein kinase C (PKC). J Biol Chem. 2016;291:6569-82
35. Johnston LC, Gonzales LW, Lightfoot RT, Guttentag SH, Ischiropoulos H. Opposing regulation of human alveolar type II cell differentiation by nitric oxide and hyperoxia. Pediatr Res. 2010;67:521-5
36. Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S. et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823-35
37. Kapoor N, Bartoszewski R, Qadri YJ, Bebok Z, Bubien JK, Fuller CM. et al. Knockdown of ASIC1 and epithelial sodium channel subunits inhibits glioblastoma whole cell current and cell migration. J Biol Chem. 2009;284:24526-41
38. Xu S, Liu C, Ma Y, Ji HL, Li X. Potential Roles of Amiloride-Sensitive Sodium Channels in Cancer Development. Biomed Res Int. 2016;2016:2190216 [Epub ahead of print]
39. Chifflet S, Hernandez JA, Grasso S. A possible role for membrane depolarization in epithelial wound healing. Am J Physiol Cell Physiol. 2005;288:C1420-30
40. Welsh MJ, Price MP, Xie J. Biochemical basis of touch perception: mechanosensory function of degenerin/epithelial Na+ channels. J Biol Chem. 2002;277:2369-72
41. Wang Q, Dai XQ, Li Q, Tuli J, Liang G, Li SS. et al. Filamin interacts with epithelial sodium channel and inhibits its channel function. J Biol Chem. 2013;288:264-73
42. Simon A, Shenton F, Hunter I, Banks RW, Bewick GS. Amiloride-sensitive channels are a major contributor to mechanotransduction in mammalian muscle spindles. J Physiol. 2010;588:171-85
43. Rooj AK, McNicholas CM, Bartoszewski R, Bebok Z, Benos DJ, Fuller CM. Glioma-specific cation conductance regulates migration and cell cycle progression. J Biol Chem. 2012;287:4053-65
44. Rooj AK, Liu Z, McNicholas CM, Fuller CM. Physical and functional interactions between a glioma cation channel and integrin-beta1 require alpha-actinin. Am J Physiol Cell Physiol. 2015;309:C308-19
45. Chen J, Zhang H, Zhang X, Yang G, Lu L, Lu X. et al. Epithelial sodium channel enhanced osteogenesis via cGMP/PKGII/ENaC signaling in rat osteoblast. Mol Biol Rep. 2014;41:2161-9
46. Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ. et al. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303:L967-77
47. Gotts JE, Matthay MA. Mesenchymal stem cells and the stem cell niche: a new chapter. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1147-9
48. Goolaerts A, Pellan-Randrianarison N, Larghero J, Vanneaux V, Uzunhan Y, Gille T. et al. Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. Am J Physiol Lung Cell Mol Physiol. 2014;306:L975-85
49. Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106:16357-62
50. Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X. et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24-32
51. Liu WW, Yu W, Chen JY, Ye GX, Liu YM, Chen LZ. et al. Effects of human umbilical cord mesenchymal stem cells in the treatment of paraquat-induced lung injury. Chin J Ind Hyg Occp Dis. 2012;30:811-5
52. Chang Y, Park SH, Huh JW, Lim CM, Koh Y, Hong SB. Intratracheal administration of umbilical cord blood-derived mesenchymal stem cells in a patient with acute respiratory distress syndrome. J Korean Med Sci. 2014;29:438-40
53. Liang ZD, Yin XR, Cai da S, Zhou H, Pei L. Autologous transplantation of adipose-derived stromal cells ameliorates ventilator-induced lung injury in rats. J Transl Med. 2013;11:179
Author contact
![]() Corresponding author: Hong-Long Ji, james.jiedu.
Corresponding author: Hong-Long Ji, james.jiedu.

 Global reach, higher impact
Global reach, higher impact