10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2017; 13(11):1458-1469. doi:10.7150/ijbs.21300 This issue Cite
Research Paper
Overexpression of selenoprotein H prevents mitochondrial dynamic imbalance induced by glutamate exposure
1. School of Basic Medical Sciences, Department of Pathology, Ningxia Medical University; Ningxia Key Laboratory of Cerebrocranial Diseases, Yinchuan, Ningxia, P. R. China.
2. Department of Pharmaceutical Sciences, Biomanufacturing Research Institute and Technological Enterprise (BRITE), North Carolina Central University, Durham, North Carolina, USA.
3. Department of Pathology, Shanxi Traditional Chinese Medicine Hospital, Xi'an, Shanxi, P. R. China.
* These authors contributed equally to this work.
Abstract
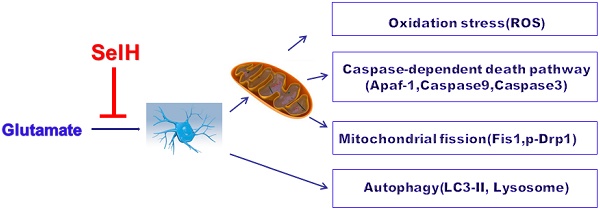
Selenium and selenoproteins play important roles in neuroprotection against glutamate‑induced cell damage, in which mitochondrial dysfunction is considered a major pathogenic feature. Recent studies have revealed that mitochondrial fission could activates mitochondrial initiated cell death pathway. The objectives of the study are to determine whether glutamate induced cell death is mediated through mitochondrial initiated cell death pathway and activation of autophagy, and whether overexpression of selenoprotein H can protect cells from glutamate toxicity by preserving mitochondrial morphology and suppressing autophagy. Vector- or human selenoprotein H (SelH)-transfected HT22 cells (V-HT22 and SelH-HT22, respectively) were exposed to glutamate. The results showed that glutamate-induced cytotoxicity was associated with increased ROS production and imbalance in mitochondrial dynamics and autophagy. These alterations were reversed and cellular integrity restored by overexpression of SelH in HT22 cells.
Keywords: Autophagy, Glutamate, Mitochondrial dynamics, Mitochondrial fission, Selenoprotein H.

 Global reach, higher impact
Global reach, higher impact