10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2018; 14(2):217-227. doi:10.7150/ijbs.22811 This issue Cite
Research Paper
Trichosanthin enhances sensitivity of non-small cell lung cancer (NSCLC) TRAIL-resistance cells
1. Department of Radiation and Medical Oncology, Zhongnan Hospital of Wuhan University, Wuhan, China
2. Department of Pathology, China Three Gorges University Medical College, Yichang, China
3. Department of Pathology, Lombardi Comprehensive Cancer Center, Georgetown University Medical School, Washington DC, USA
4. Department of Urology, Zhongnan Hospital of Wuhan University, Wuhan, China
5. Department of Biological Repositories, Zhongnan Hospital of Wuhan University, Wuhan, China
6. Hubei Key Laboratory of Tumour Biological Behaviors, Zhongnan Hospital of Wuhan University, Wuhan, China
7. Hubei Cancer Clinical Study Center, Zhongnan Hospital of Wuhan University, Wuhan, China
*These authors contributed equally to this work.
Abstract
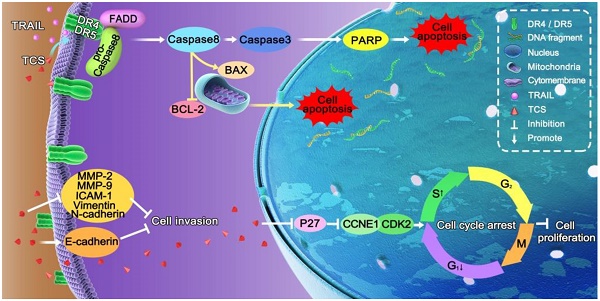
Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) has a specific antitumour activity against many malignant tumours. However, more than half of lung cancer cells are resistant to TRAIL-relevant drugs. Trichosanthin (TCS) is a traditional Chinese medicine with strong inhibitive effects on various malignancies. Nevertheless, its function on TRAIL resistance has not been revealed in non-small cell lung cancer (NSCLC). To examine the molecular mechanisms of TCS-induced TRAIL sensitivity, we administrated TCS to TRAIL-resistance NSCLC cells, and found that the combination treatment of TCS and TRAIL inhibited cancer cell proliferation and invasion, and induced cell apoptosis and S-phase arrest. This combined therapeutic method regulated the expression levels of extrinsic apoptosis-associated proteins Caspase 3/8 and PARP; intrinsic apoptosis-associated proteins BCL-2 and BAX; invasion-associated proteins E-cadherin, N-cadherin, Vimentin, ICAM-1, MMP-2 and MMP-9; and cell cycle-associated proteins P27, CCNE1 and CDK2. Up-expression and redistribution of death receptors (DRs) on the cell surface were also observed in combined treatment. In conclusion, our results indicated that TCS rendered NSCLC cells sensitivity to TRAIL via upregulating and redistributing DR4 and DR5, inducing apoptosis, and regulating invasion and cell cycle related proteins. Our results provided a potential therapeutic method to enhance TRAIL-sensitivity.
Keywords: TRAIL, NSCLC, Trichosanthin, TRAIL-resistance, death receptor

 Global reach, higher impact
Global reach, higher impact