10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2018; 14(8):930-937. doi:10.7150/ijbs.24582 This issue Cite
Research Paper
Computational Design of Antiangiogenic Peptibody by Fusing Human IgG1 Fc Fragment and HRH Peptide: Structural Modeling, Energetic Analysis, and Dynamics Simulation of Its Binding Potency to VEGF Receptor
1. Center for Informational Biology, University of Electronic Science and Technology of China (UESTC), Chengdu 611731, China
2. School of Life Science and Technology, University of Electronic Science and Technology of China (UESTC), Chengdu 610054, China
Abstract
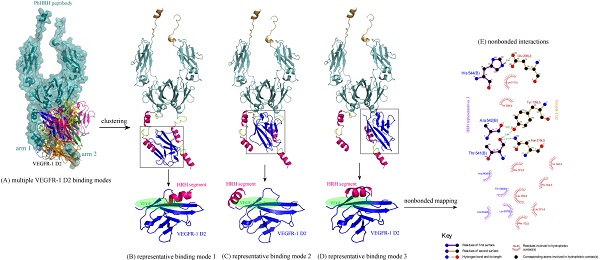
Peptibodies represent a new class of biological therapeutics with combination of peptide activity and antibody-like properties. Previously, we discovered a novel peptide HRH that exhibited a dose-dependent angiogenesis-suppressing effect by targeting vascular endothelial growth factor receptors (VEGFRs). Here, we computationally designed an antiangiogenic peptibody, termed as PbHRH, by fusing the HRH peptide to human IgG1 Fc fragment using the first approved peptibody drug Romiplostim as template. The biologically active peptide of Romiplostim is similar with HRH peptide; both of them have close sequence lengths and can fold into a α-helical conformation in free state. Molecular dynamics simulations revealed that the HRH functional domain is highly flexible, which is functionally independent of Fc fragment in the designed PbHRH peptibody. Subsequently, the intermolecular interactions between VEGFR-1 domain 2 (D2) and PbHRH were predicted, clustered and refined into three representatives. Conformational analysis and energetic evaluation unraveled that the PbHRH can adopt multiple binding modes to block the native VEGF-A binding site of VEGFR-1 D2 with its HRH functional domain, although the binding effectiveness of HRH segments in peptibody context seems to be moderately decreased relative to that of free HRH peptide. Overall, it is suggested that integrating HRH peptide into PbHRH peptibody does not promote the direct intermolecular interaction between VEGFR-1 D2 and HRH. Instead, the peptibody may indirectly help to improve the pharmacokinetic profile and bioavailability of HRH.
Keywords: peptibody, Romiplostim, PbHRH, HRH peptide, VEGF, VEGFR, antiangiogenesis

 Global reach, higher impact
Global reach, higher impact