10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2018; 14(11):1599-1609. doi:10.7150/ijbs.26274 This issue Cite
Research Paper
MiR27a Promotes the Development of Macrophage-like Characteristics in 3T3-L1 Preadipocytes
1. Department of Pharmacology, College of Basic Medical Sciences, Jilin University, Changchun 130021, China
2. School of nursing, Jilin University, Changchun 130021, China
3. Department of Surgery, Hepatology Hospital of Jilin Province, Changchun, Jilin, 130021,China
4. Department of Pharmacology & Therapeutics, Center for Research and Treatment of Atherosclerosis, University of Manitoba, DREAM, Children's Hospital Research Institute of Manitoba, Winnipeg, Manitoba, Canada, R3E 3P4
Abstract
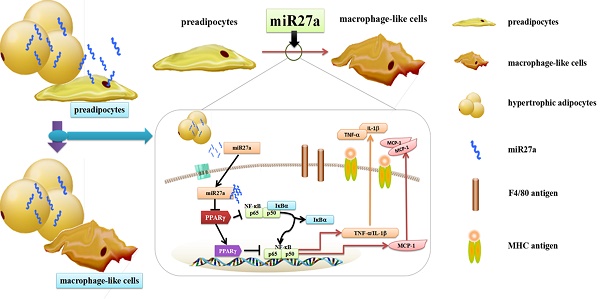
Recruitment and polarization of classically activated (M1) macrophages within adipose tissue contribute to chronic low-grade inflammation in obesity. Adipose tissue precursor cells exhibit the capacity to develop macrophage-like characteristics and adipocyte-derived miR27a is known to promote reprogramming of somatic cells. It was unknown whether exogenous addition of miR27a promote the development of macrophage-like characteristics of adipose precursor cells. We examined macrophage surface antigen, phagocytosis and migration ability in 3T3-L1 preadipocytes transfected with miR27a mimics. Transfection of 3T3-L1 preadipocytes with miR27a mimics increased phagocytosis and migration and increased the number of cells expressing the macrophage makers F4/80 and MHC compared to controls. M2 and CD206 macrophage markers were unaltered. In addition, transfection of 3T3-L1 preadipocytes with miR27a mimics reduced PPARγ expression, activated NF-κB and promoted secretion of the inflammatory cytokines MCP-1, TNF-α and IL-1β compared to controls. The level of anti-inflammatory factors Arg-1, IL-10, Ym1 and Fizz1 were unaltered. Secretion of miR27a was increased in conditioned medium prepared from palmitic acid-treated differentiated 3T3-L1 adipocytes compared to controls. Incubation of 3T3-L1 preadipocytes with this conditioned medium increased phagocytosis and migration compared to controls. Finally, conditioned medium prepared from differentiated 3T3-L1 adipocytes transfection with miR27a inhibitors reduced phagocytosis and migration in 3T3-L1 preadipocytes compared to controls. The data indicate that PPARγ agonists may reverse the activation of NF-κB pathway mediated by miR27a overexpression and reduce phagocytosis and migration of adipose precursor cells. In addition, miR27a may promote the development of macrophage-like characteristics in 3T3-L1 preadipocytes.
Keywords: obesity, miR27a, inflammation, preadipocytes, 3T3 cells, macrophage-like

 Global reach, higher impact
Global reach, higher impact